Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Universitas Psychologica
Print version ISSN 1657-9267
Univ. Psychol. vol.15 no.spe5 Bogotá Dec. 2016
https://doi.org/10.11144/Javeriana.upsy15-5.dsct
Developing a Social Cognition Task for fMRI in Patients with Mild Traumatic Brain Injury*
Desarrollo de una tarea de Cognición Social para IRMf en pacientes con trauma craneoencefálico leve
Pablo Reyes
Pontificia Universidad Javeriana, Colombia
Diana Matallananaa
San Ignacio University Hospital, Colombia
Giselle Santiago
Fundación Santa Fe de Bogotá University Hospital, Colombia
Carlos Filizzola
Pontificia Universidad Javeriana, Colombia
Aníbal Morillo
Fundación Santa Fe de Bogotá University Hospital, Colombia
Sofia Velasco
Fundación Santa Fe de Bogotá University Hospital, Colombia
Sonia Bermúdez
Fundación Santa Fe de Bogotá University Hospital, Colombia
Notes
*Research article.
aCorresponding autor. E-mail: dianamat@javeriana.edu.co
Received: 15 August 2016 | Accepted: 28 November 2016
Para citar este artículo
Reyes, P., Matallana, D., Saniago, G., Filizzola, C., Morillo, A., Velasco, S., & Bermúdez, S. (2016). Developing a Social Cognition Task for fMRI in Patients with Mild Traumatic Brain Injury. Universitas Psychologica, 15(5). http://dx.doi.org/10.11144/Javeriana.upsy15-5.dsct
Abstract
Social cognition impairments are frequently found in patients with mild traumatic brain injury (TBI) when structural lesions may not reveal the severity of the injury. Though instruments used to assess social behavior are thought to be sensitive, the absence of structural damage in TBI patients may lead to underscore such problems. The aim of this study was to develop a complementary diagnostic tool such as a paradigm for functional Magnetic resonance Imaging (fMRI) involving a simple task that could tell how patients understand certain complex social behavior by identifying different movements with or without social intentions where language and complex cognitive process were not required. Eleven patients with mild TBI and social cognition difficulties and twelve control subjects were matched by demographic variables. A paradigm of social fMRI was developed by using dots in movement representing human motion, human motion with social intention such as dancing or sharing, and dots moving without meaning. Patients had less activation in parietotemporal junction and bilateral middle frontal gyrus in the social perception task movement compared with control group subjects. The fMRI paradigm developed can be an additional diagnostic tool for identifying social cognition impairments in mild TBI patients. Regardless the absence of structural injury, changes in activation areas suggest a prospective use of this tool since clinical, cognitive and functional outcomes support such finding.
Keywords: social cognition; functional Magnetic Resonance Image; Traumatic Brain Injury
Resumen
Los cambios en la cognición social son encontrados frecuentemente en pacientes con trauma craneoencefálico leve (TCE) aunque no exista evidencia de lesiones estructurales. Aunque los instrumentos utilizados para evaluar la cognición social son sensibles al cambio, la ausencia de daño estructural en los pacientes con TCE puede llevar pasar por inadvertidos estos problemas. El objetivo de este estudio fue desarrollar una herramienta diagnóstica complementaria como un paradigma para resonancia magnética funcional (RMf), la cual involucra una tarea simple que pudiera explicar cómo los pacientes entienden ciertos comportamientos sociales complejos por medio de movimientos con o sin intención social sin intermediación del lenguaje. Participaron once pacientes con TCE leve y con reporte de alteraciones en cognición social, estos fueron emparejados con doce sujetos control por variables demográficas. Un paradigma de RMf fue desarrollado por medio de la animación puntos blancos sobre una pantalla negra que representan el movimiento humano, el movimiento humano con la intención social como el baile o el compartir, y puntos que se mueven sin significado. Los pacientes tuvieron menos activación en la unión parietotemporal y giro frontal medio bilateral frente al movimiento social en comparación con los sujetos del grupo de control. El paradigma de fMRI desarrollado puede ser una herramienta de diagnóstico adicional para identificar las alteraciones cognitivas sociales en pacientes con TCE leve. Independientemente de la ausencia de lesión estructural, los cambios en las áreas de activación sugieren la posibilidad de usar esta herramienta como pronóstico dado que los resultados clínicos, cognitivos y funcionales soportan este hallazgo.
Palabras clave: cognición social; resonancia magnética funcional; trauma craneoencefálico
Introduction
Traumatic brain injuries (TBI) generate an invisible cost to health systems, as well as to families and caregivers. This is particularly true in those cases when a patient is apparently asymptomatic in the immediate postinjury period and subsequently presents serious disorders such as those related with social cognition deficits (Martín-Rodríguez & León-Carrión, 2010; Schroeter, Ettrich, Menz, & Zysset, 2010). Since brain disorders related with mild TBI include a heterogeneous spectrum of morbidities, selective cognitive impairments as well as neuropsychiatrie symptoms are difficult to define, manage and usually, recognize.
Recent findings from neurological, neurophysiological, neuropsychological, neuroimaging, as well as electrophysiological studies in mild TBI suggest that the traditional assesment of these injuries requires serious reconsideration (Amyot et al., 2015; Arciniegas, Topkoff, & Silver, 2000). Authors propose to assess mild TBI immediately after the injury and to evaluate other cognitive domains beyond language or memory such as social cognition, one of the must common post trauma symptom.
Today, the approaches from social neuroscience allow a multilevel framework for the study of alterations in the behavior and cognition in social context (Ibanez, Kuljis, Matallana, & Manes, 2014) and a range of tasks have been created to be included in a regular neuropsychological evaluation. Defining how patients understand the social world and create sense of their social environment is then the main goal of the evaluation of the social brain. We know that to understand emotional processes as well as other social cues that allow us to be understood and tasks directed to evaluate how we represent internally our social environment are not easy to design. In fact, patients with mild TBI have serious difficulties in processing social cues such as driving decisions in a social context, having empathy with others or being able to understand others mental states as well as following social norms (Turkstra, Williams, Tonks, & Frampton, 2008). Besides, they have impairments in 'reading' a situation by not using verbal and nonverbal contextual information to make inferences in social interactions (Baez & Ibanez, 2014; Turkstra, Williams, Tonks, & Frampton, 2008) Consequently, the wide range of social cognition symptoms involves symptoms that limit autonomy and generate a lost in productive years of patients. Family and health system burden is enormous and though estimating such difficulties require sensible tests that should control variables such as education and contextual information, this type of assessment is a difficult procedure to be used in a fMRI.
Therefore, evolving procedures with this type of pathology that includes the symptoms already described should be done in TBI (Hassan, Khaw, Rosna, & Husna, 2011; Jumisko, Lexell, & Söderberg, 2007; Kreutzer, Gervasio, & Camplair, 1994; Martin-Rodriguez & Leon-Carrion, 2010; McDonald, Flanagan, Rollins, & Kinch, 2003; Turkstra, 2008).
Even though structural alterations in mild TBI are infrequently reported in studies of MRI (Belanger, Vanderploeg, Curtiss, & Warden, 2007), there is an increasing evidence of microstructural changes in mild TBI (Avants et al., 2008; Huisman et al., 2004; Metting, Cerliani, Rödiger, & van der Naalt, 2013). The search for identifying changes in cognitive functioning by methodologies as fMRI can be, therefore, promising in patients with mild TBI.
The latter considerations steered the purpose of the present research that was to proof a paradigm for an fMRI where understanding a social situation, without requiring language and complex cognition processes could be used as a complementary diagnostic tool. Though in clinical practice we have used videos of social interactions in order to register patients ability to understand and react to scenes implying pain of others or harmful situations to be judge, (TASIT or VSIT) (McDonald et al., 2003; Turkstra, 2008) , results were difficult to be interpreted since the narrative of the situation lasted some seconds.
In sum, even there is a speared range of cognitive difficulties of a mild TBI patients, this study scope was to see if the one related with a comprehension of a simple movement suggesting a social human interaction could differentiate patients with a social brain difficulties from subjects without social cognition problems.
Method
Sample
Twenty-three adult participants right-handed (11 patients with mild TBI and 12 healthy matched by age and gender subjects) where the mean age for patients was 42.18 and for healthy subjects of 38.33 and range 20-45 years old. Traumatic brain injury occurred from 6 months to 5 years earlier. The structural MRI revealed no structural alterations. Patients with mild TBI underwent to a neuropsychological evaluation and proof interview by psychiatry. This study was approved by the Board Ethics Committee. All participants provided written informed consent. All experiments were performed in accordance with the approved guidelines and regulations.
Procedure
Functional images were acquired using a 1.5T General Electric MRI scanner. Blood Oxygenation Level Dependent (BOLD) sensitive functional images were acquired using a gradient echo-planar imaging (EPI) sequence (128 mm in FOV, TR=3000 ms, TE=60, 20 slices without the gap, voxel size of 3.75 x 3.75 x 7 mm3, Flip Angle 90°). A total of 84 functional volumes were collected for each participant by each task. A high-resolution volumetric Inversion Recovery T1-weighted sequence was acquired for each participant (isometric voxel 1mm) for use as an anatomical image which will be superimposed on functional images
The experimental procedure included 3 tasks in a activity/rest block design (4 blocks of 30 seconds each). During the period of activity, an animation of dots moving through the screen was presented (see Figure 1). Subjects had just to look, they do not have to verbalize anything. Subsequently, a black screen with a red dot, was presented as a rest block. Three different tasks were used. In the first one, two animations figured a biological movement: an individual was engaged in a running action like climbing stairs or walking; in the second one, two persons had a social interaction or were involved in a social intention activity such as dancing, and fighting. Finally, a third animation named not biological consisted in a series of predictable points in a circle motion.
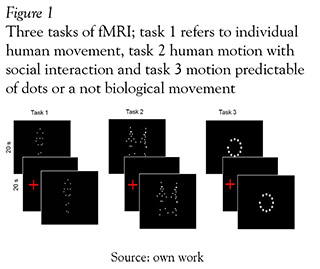
Construction of human motion animations was conducted in two stages: first, we searched and selected an individual moving, and then we had to look for two people being involved in a movement suggesting a social behavior. Selection of the images was done form the database motion capture lab at Carnegie Mellon University (http://mocap.cs.cmu.edu/). Motion capture was performed for 12 MX-40 infrared cameras and recorded at 120 Hz with images of 4-megapixel resolution. Motions were captured in a working volume of approximately 3m x 8m. The captured subject wearied 41 markers and had a stylish black garment. Second: with the coordinates of the 41 markers of movement, we proceeded to select the 15 major joint connections. These files of coordinates (x, y, z, and time series) were subsequently rendered with "DANCE" software to create an animation of white dots on a threedimensional space.
Preprocessing and analysis
Data were analyzed using the package SPM8 (Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm).
The images were realigned spatially to the first series of each subject to correct the head movement; slice timing correction ascending (Sladky et al. 2011) and the slice 10 were taken as a reference. Functional images were normalized to standard sterotaxic space Atlas Montreal Neurological Institute (MNI) (Ashburner et al., 2008; Friston, Williams, Howard, Frackowiak, & Turner, 1996). Finally, images were spatially smoothed with a three-dimensional 7-mm full-width half-maximum isotropic Gaussian kernel filter to improve S/N ratio.
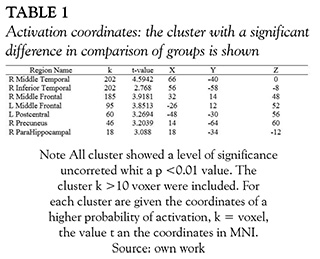
For each task we used parametrical analysis of first and second level. The model parameters were: canonical hemodynamic response function (HRF), 6 multiple regressors to reduce intra and inter-subject variability (Carp, 2012), highpass filter 128 and Robust Weighted Least Squares Estimation fMRI (RWLS) (Diedrichsen & Shadmehr, 2005). The second level analysis compared the group of patients with TBI and control subjects; a t- test was performed to establish the differences between the group of patients and controls with p-value threshold with correction by FDR. The smaller cluster with a 10 voxels were excluded. The locations of the statistical findings were reported in a space coordinate (x, y, z) developed by the Consortium of brain mapping Montreal Neurological Institute (MNI). The results of fMRI contrast were rendered by CARET (Van Essen et al., 2001) over the atlas PALS (Van Essen, 2005). Finally, we made an analysis of regions of interest with MARSBAR toolbox (Brett, Anton, Valabregue, & Poline, 2002) in order to extract the eigenvalues for the most significant cluster.
Results
Interviews to patients were done by a psychiatrist and showed that 80% of them had personality changes. 92% of caregivers noted in them a decrease of emotional expression, as well as changes in the understanding of emotions linked to a social context (empathy). Neuropsychological assessment showed that 25% of patients had changes in targeting attention functions. Language, praxis and memory did not differ from healthy subjects.
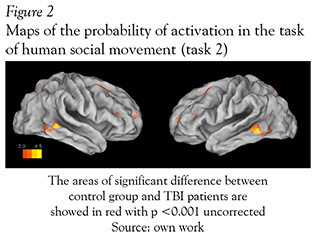
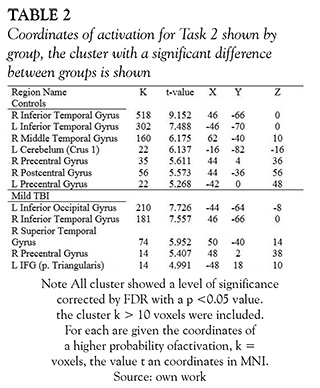
In fMRI the Mild TBI patients had a significant less activation in diverse areas when they were exposed to the task of social interaction motion (task 2) in comparison with the healthy subjects. The areas that showed significantly less activation were the middle and inferior temporal gyrus in the right hemisphere with overlapping to the parietotemporal junction, the bilateral middle frontal circumvolution and the precentral gyrus (see Figure 2 and Table 1).
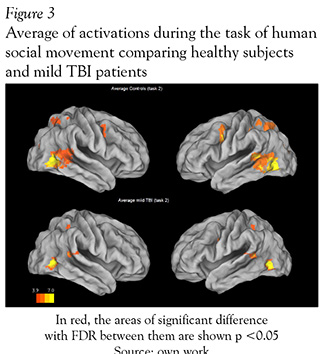
The average results of each group activation in social interaction fMRI task (task 2), compared to the baseline, showed a large area of activation in the posterior temporal lobe in control subjects; whereas TBI patients had activation in similar brain areas but in minor extent (see Figure 3 and Table 2).
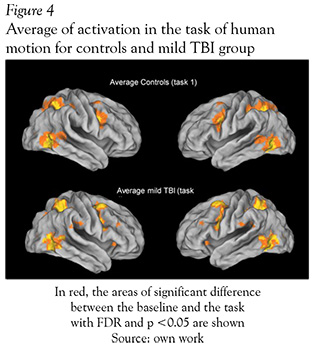
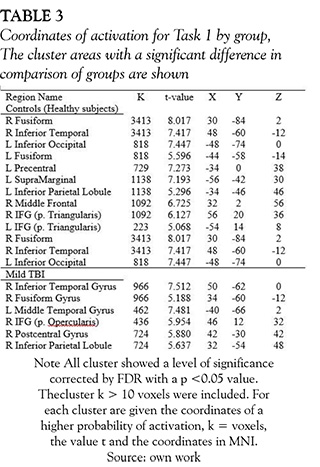
The brain areas activated with animation of a single person task, were not different between groups (healthy subjects and mild TBI patients) (see Figure 4 and Table 3). Both groups showed activations around eight clusters and the areas with activations of this task were: inferior and medial temporal gyrus, inferior parietal and inferior frontal gyrus. The last task used for perception of non-biological movement did not show differences between the two groups either, the clusters were located in bilateral occipital lobes and some regions in the prefontal lobe.
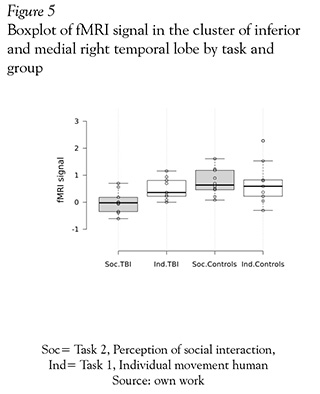
Finally, in an analysis of ROI with MARSBAR shown in Figure 5, we selected the cluster located between the right inferior and medial temporal gyri; this cluster was a area that have had significant different activation in the comparison of mild TBI patients and healthy subjects. Next the eigenvariate was extracted for each group and task (perception on individual and social interaction). This analysis showed that mild TBI group had a minor activation in this region in comparison with themselves in individual motion perception and compared with the control group.
Discussion
Reports of neuropsychiatric symptoms (LoBue et al., 2016) and cognitive impairments (Stulemeijer, Vos, Bleijenberg, & van der Werf, 2007) in mild TBI usually do not require an in hospital management (Barker-Collo et al., 2015). The perception of neuropsychiatric symptoms is often only referred by a third person, usually the caregiver, some days or months after the trauma and after the patient has been discharged of the Hospital. This pioneering research allows to evaluate the perception of a social intention through plain movements situations. It shows how patients with mild TBI have a reduced activations of areas like right medial and inferior temporal gyri and medial frontal gyri. Identifying these impairments in social perception may be the most basic aspect or core in the understanding of changes occurring in social behavior.
Other researches have related the right (posterior) middle and inferior gyri of temporal lobe with the observation of human body parts (Wheaton, Thompson, Syngeniotis, Abbott, & Puce, 2004), with the affective touch sensation (Ebisch et al., 2011) and the perception of a expression in faces as well (Leslie, Johnson-Frey, & Grafton, 2004). Therefore, the perception of motion in social context can be modulated by this region according to our data; there is more evidence of the substrates of complex psychological factors like empathy associated with the sensory-motor system (de Vignemont & Singer, 2006; Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008; Rizzolatti, Fogassi, & Gallese, 2002) being then a crucial function since it modulates the behavior in social context. On the other hand, the middle frontal cortex right can be associated with a social component like mentalizing certain state of others (Raposo, Vicens, Clithero, Dobbins, & Huettel, 2011) or being able to resolve and take decisions in social contexts (Ho, Gonzalez, Abelson, & Liberzon, 2012) and in human motion (Ichikawa et al., 2011).
The use of neuroimaging to evaluate mild TBI patients is not so common, and in Latinoamerica, such procedure is used mainly to asses moderate to severe ill patients, since costs cannot be raised when computed tomography are normal. Moreover, the use of advanced neuroimages as functional resonance using tasks or rest state fMRI, is still limited to research. However, there is an initial evidence of its use in clinical setting as diagnostic method support (Belanger et al., 2007; Hulkower, Poliak, Rosenbaum, Zimmerman, & Lipton, 2013). Our results show that with a simple task or paradigm of social cognition in fMRI it is possible to discriminate between brain activations in this group of patients when compared to healthy subjects.
The limitation of this research is then the sample size. However, we implemented an analysis of mixed models and performed a match for age, gender, and age between patients and subjects; on the other hand, when comparing groups in task 1 we founded a p-value without correction to 0.001. Future research will be necessary to increase the sample size as well as to include specific tests of social cognition.
In conclusion, this research suggest that a fMRI paradigm, or task that shows human movement social interaction (represented by dots), can differentiate patients with mild TBI from healthy subjects since patients had lower activation in the parietotemporal union area and a reduced activation in the medial frontal temporal gyrus when compared with normal subjects.
Acknowledgement
We thank all patients and subjects for their participation. This work was financially supported by The Center for Studies and Health Research of Fundación Santa Fe de Bogotá.
References
Amyot, F., Arciniegas, D. B., Brazaitis, M. P, Curley, K. C., Diaz-Arrastia, R., Gandjbakhche, A., ... Stocker, D. (2015). A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. Journal of Neurotrauma, 32(22), 1693-721. http://doi.org/10.1089/neu.2013.3306 [ Links ]
Arciniegas, D. B., Topkoff, J., & Silver, J. M. (2000). Neuropsychiatric aspects of traumatic brain injury. Current Treatment Options in Neurology, 2 (2), 169-186. [ Links ]
Ashburner, J., Barnes, G., Chen, C., Daunizeau, J., Flandin, G., Friston, K., ... Litvak, V. (2008). SPM8 manual. Functional Imaging Laboratory, Institute of Neurology. [ Links ]
Avants, B., Duda, J. T., Kim, J., Zhang, H., Pluta, J., Gee, J. C., & Whyte, J. (2008). Multivariate (Analysis) of (Structural) and (Diffusion) (Imaging) in (Traumatic) (Brain) (Injury). Academic Radiology, 15 (11), 1360-1375. article. http://doi.Org/10.1016/j.acra.2008.07.007 [ Links ]
Barker-Collo, S., Jones, K., Theadom, A., Starkey, N., Dowell, A., McPherson, K., ... Feigin, V. (2015). Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: A population-based New Zealand study. Brain Injury, 29(13-14), 1604-1616. http://doi.org/10.3109/02699052.2015.1075143 [ Links ]
Belanger, H. G., Vanderploeg, R. D., Curtiss, G., & Warden, D. L. (2007). Recent neuroimaging techniques in mild traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 19(1), 5-20. http://doi.org/10.1176/appi.neuropsych.19.1.5 [ Links ]
Brett, M., Anton, J.-L., Valabregue, R., & Poline, J.-B. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage, 16(2), S497. [ Links ]
Carp, J. (2012). The secret lives of experiments: Methods reporting in the fMRI literature. Neurolmage, 63(1), 289-300. http://doi.org/10.1016/j.neuroimage.2012.07.004 [ Links ]
de Vignemont, F., & Singer, T. (2006). The empathic brain: how, when and why? Trends in Cognitive Sciences, 10(10), 435-441. http://doi.org/10.1016/j.tics.2006.08.008 [ Links ]
Diedrichsen, J., & Shadmehr, R. (2005). Detecting and Adjusting for Artifacts in fMRI Time Series Data. Neurolmage, 27(3), 624-634. http://doi.org/10.1016/j.neuroimage.2005.04.039 [ Links ]
Ebisch, S. J. H., Ferri, F., Salone, A., Perrucci, M. G., D'Amico, L., Ferro, F. M., ... Gallese, V. (2011). Differential Involvement of Somatosensory and Interoceptive Cortices during the Observation of Affective Touch. Journal of Cognitive Neuroscience, 23(7), 1808-1822. http://doi.org/10.1162/jocn.2010.21551 [ Links ]
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S. J., & Turner, R. (1996). Movement-Related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346-355. http://doi.org/10.1002/mrm.1910350312 [ Links ]
Hassan, S. T. S., Khaw, W F., Rosna, A. R., & Husna, J. (2011). Traumatic brain injury: caregivers' problems and needs. JNMA; Journal of the Nepal Medical Association, 51(181), 53-55. [ Links ]
Ho, S. S., Gonzalez, R. D., Abelson, J. L., & Liberzon, I. (2012). Neurocircuits underlying cognition-emotion interaction in a social decision making context. Neurolmage, 63(2), 843-57. http://doi.org/10.1016/j.neuroimage.2012.07.017 [ Links ]
Huisman, T. A. G. M., Schwamm, L. H., Schaefer, P W, Koroshetz, W J., Shetty-Alva, N., Ozsunar, Y., ... Sorensen, A. G. (2004). Diffusion Tensor Imaging as Potential Biomarker of White Matter Injury in Diffuse Axonal Injury. American Journal of Neuroradiology, 25(3), 370-376. [ Links ]
Hulkower, M. B., Poliak, D. B., Rosenbaum, S. B., Zimmerman, M. E., & Lipton, M. L. (2013). A Decade of DTI in Traumatic Brain Injury: 10 Years and 100 Articles Later. American Journal of Neuroradiology. http://doi.org/10.3174/ajnr.A3395 [ Links ]
Ibanez, A., Kuljis, R. O., Matallana, D., & Manes, F. (2014). Bridging psychiatry and neurology through social neuroscience. World Psychiatry, 13(2), 148-149. http://doi.org/10.1002/wps.20125 [ Links ]
Ichikawa, N., Siegle, G. J., Jones, N. P, Kamishima, K., Thompson, W K., Gross, J. J., & Ohira, H. (2011). Feeling bad about screwing up: emotion regulation and action monitoring in the anterior cingulate cortex. Cognitive, Affective & Behavioral Neuroscience, 11 (3), 354-71. http://doi.org/10.3758/s13415-011-0028-z [ Links ]
Jumisko, E., Lexell, J., & Soderberg, S. (2007). Living with moderate or severe traumatic brain injury: the meaning of family members' experiences. Journal of Family Nursing, 13(3), 353-369. http://doi.org/10.1177/1074840707303842 [ Links ]
Kreutzer, J. S., Gervasio, A. H., & Camplair, P S. (1994). Patient correlates of caregivers' distress and family functioning after traumatic brain injury. Brain Injury: [BI], 8(3), 211-230. [ Links ]
Leslie, K. R., Johnson-Frey, S. H., & Grafton, S. T. (2004). Functional imaging of face and hand imitation: towards a motor theory of empathy. NeuroImage, 21(2), 601- 607. http://doi.org/10.1016/j.neuroimage.2003.09.038 [ Links ]
LoBue, C., Denney, D., Hynan, L. S., Rossetti, H. C., Lacritz, L. H., Hart, J., ... Cullum, C. M. (2016). Self-Reported Traumatic Brain Injury and Mild Cognitive Impairment: Increased Risk and Earlier Age of Diagnosis. Journal of Alzheimer's Disease, 51(3), 727-736. http://doi.org/10.3233/JAD-150895 [ Links ]
Martin-Rodriguez, J. F., & Leon-Carrion, J. (2010). Theory of mind deficits in patients with acquired brain injury: A quantitative review. Neuropsychologia, 48(5), 1181- 1191. http://doi.org/10.1016fj.neuropsychologia.2010.02.009 [ Links ]
McDonald, S., Flanagan, S., Rollins, J., & Kinch, J. (2003). TASIT: A new clinical tool for assessing social perception after traumatic brain injury. The Journal of Head Trauma Rehabilitation, 18(3), 219-238. [ Links ]
Metting, Z., Cerliani, L., Rodiger, L. A., & van der Naalt, J. (2013). Pathophysiological Concepts in Mild Traumatic Brain Injury: Diffusion Tensor Imaging Related to Acute Perfusion CT Imaging. PLoS ONE, 8(5), e64461. http://doi.org/10.1371/journal.pone.0064461 [ Links ]
Pfeifer, J. H., Iacoboni, M., Mazziotta, J. C., & Dapretto, M. (2008). Mirroring others' emotions relates to empathy and interpersonal competence in children. NeuroImage, 39(4), 2076-2085. http://doi.org/10.1016/j.neuroimage.2007.10.032 [ Links ]
Raposo, A., Vicens, L., Clithero, J. A., Dobbins, I. G., & Huettel, S. A. (2011). Contributions of frontopolar cortex to judgments about self, others and relations. Social Cognitive and Affective Neuroscience, 6(3), 260-9. http://doi.org/10.1093/scan/nsq033 [ Links ]
Rizzolatti, G., Fogassi, L., & Gallese, V. (2002). Motor and cognitive functions of the ventral premotor cortex. Current Opinion in Neurobiology, 12(2), 149-154. http://doi.org/10.1016/S0959-4388(02)00308-2 [ Links ]
Schroeter, M. L., Ettrich, B., Menz, M., & Zysset, S. (2010). Traumatic brain injury affects the frontomedian cortex- An event-related fMRI study on evaluative judgments. Neuropsychologia, 48(1), 185- 193. http://doi.org/10.10167j.neuropsychologia.2009.09.004 [ Links ]
Stulemeijer, M., Vos, P E., Bleijenberg, G., & van der Werf, S. P (2007). Cognitive complaints after mild traumatic brain injury: Things are not always what they seem. Journal of Psychosomatic Research, 63(6), 637- 645. http://doi.org/10.1016Tj.jpsychores.2007.06.023 [ Links ]
Turkstra, L. S. (2008). Conversation-based assessment of social cognition in adults with traumatic brain injury. Brain Injury#: [BI] 22(5), 397-409. http://doi.org/10.1080/02699050802027059 [ Links ]
Turkstra, L. S., Williams, W. H., Tonks, J., & Frampton, I. (2008). Measuring social cognition in adolescents: implications for students with TBI returning to school. NeuroRehabilitation, 23(6), 501-9. [ Links ]
Van Essen, D. C. (2005). A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage, 28(3), 635-662. http://doi.org/10.1016/j.neuroimage.2005.06.058 [ Links ]
Van Essen, D. C., Drury, H. a., Dickson, J., Harwell, J., Hanlon, D., & Anderson, C. H. (2001). An Integrated Software Suite for Surface-based Analyses of Cerebral Cortex. Journal of the American Medical Informatics Association, 8(5), 443-459. http://doi.org/10.1136/jamia.2001.0080443 [ Links ]
Wheaton, K. J., Thompson, J. C., Syngeniotis, A., Abbott, D. F., & Puce, A. (2004). Viewing the motion of human body parts activates different regions of premotor, temporal, and parietal cortex. Neuroimage, 22(1), 277-288. http://doi.org/10.1016/j.neuroimage.2003.12.043 [ Links ]














