Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Colombia Médica
versão On-line ISSN 1657-9534
Colomb. Med. vol.43 no.1 Cali jan./mar. 2012
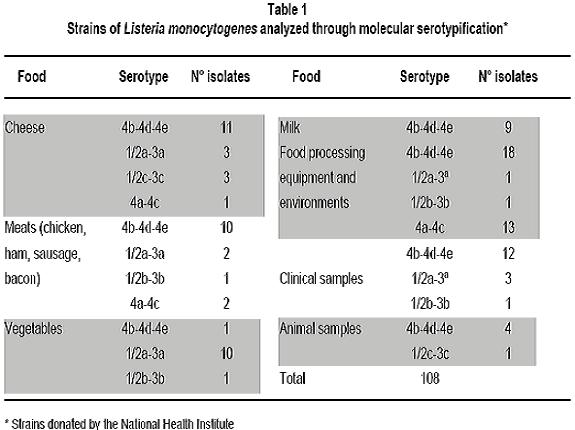
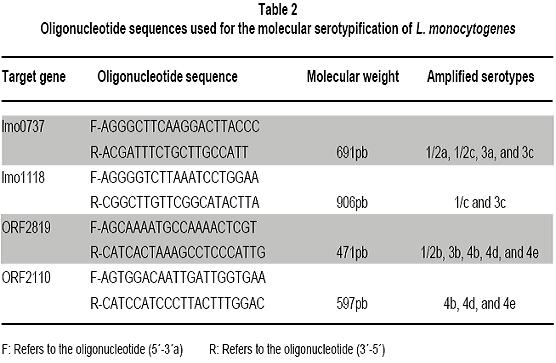
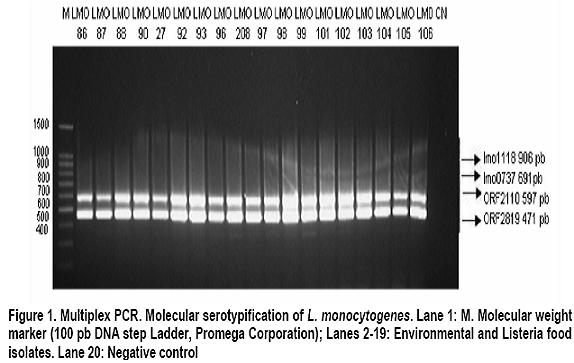
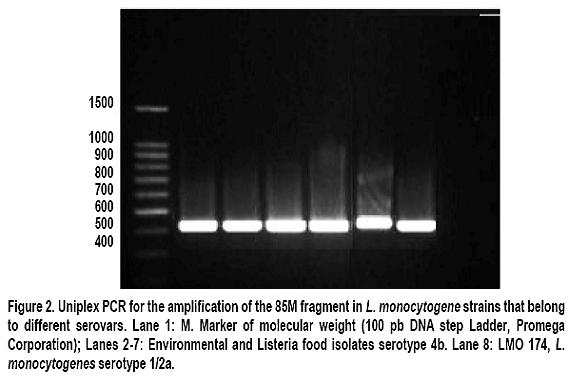
Molecular serotyping and identification of the 85M gragment in different Colombian isolates of Listeria monocytogenes strains: A descriptive study Serotipificación molecular e identificación del fragmento 85M de aislamientos colombianos de Listeria monocytogenes: Un estudio descriptivo María Consuelo Vanegas, MSc,1, Mayra Viviana Medrano, MSc,2, 1Director of the Laboratory for Microbial Ecology of Food (LEMA), Department of Biological Sciences, Universidad de los Andes, Bogotá, Colombia. e-mail: mvanegas@uniandes.edu.co Received for publication March 30, 2010 Accepted for publication March 11, 2011 Summary Introduction: Listeria monocytogenes is a pathogen acquired through the consumption of contaminated foods. Thirteen serotypes have been reported, of which 1/2a, 1/2b, and 4b are responsible for 98% of human listeriosis cases. This study examines the association between serotypes and virulent clones, offering greater information and providing tools to prevent and control diseases caused by L. monocytogenes serotype 4b. Keywords: Listeria monocytogenes; Listeriosis; Serotypes; 85M fragment; Isolation; Food. Resumen Introducción: Listeria monocytogenes es un patógeno adquirido por el consumo de alimentos contaminados. Se han reportado 13 serotipos de los cuales 1/2a, 1/2b y 4b son responsables del 98% de las listeriosis humanas. Se ha estudiado la asociación entre serotipos y clones de virulencia, lo cual aporta información y brinda herramientas para la prevención y control de las enfermedades causadas por L. monocytogenes serotipo 4b. Palabras clave:Listeria monocytogenes; Listeriosis; Serotipos; Fragmento 85M; Serotipos; Aislamiento; Alimentos. The Listeria genus is found throughout nature: in soil, water, effluents, domestic animals, and a wide variety of foods (including dairy products, meats, and vegetables). Six species and two subspecies have been identified, of which L. ivanovii and L. monocytogenes are considered potentially pathogenic1. Because of the latterâs capability of contaminating multiple sources, people have historically believed all strains of this species cause diseases. Listeria monocytogenes is an intracellular pathogen for humans and animals, which is acquired through the consumption of contaminated foods. It causes listeriosis, an opportunistic invasive disease that can manifest itself in epidemic or sporadic cases. It leads to complications like meningitis, septicemia, miscarriage, and stillbirth2,3. To characterize the strains of this species that are associated with virulence, conventional serotypification has been used (based on O and H antigens) as a discriminatory method. This has led to identifying 12 serotypes (1/2a, 1/2b, 1/2c, 3a, 3b, 3c, 4a, 4b, 4c,4d, 4e, and 74,5. Given some restrictions of the serologic method itself (the presence of the same flagellin and somatic antigens in strains of distinct serotypes6 does not allow for 100% serotypification of isolates), the use of new techniques derived from molecular biology is becoming increasingly common. Recent studies based on microarrays and catheters in three complete genomes of L. monocytogenes serotype 1/2a strain EGDe, L. monocytogenes serotype 4b strain CLIP 80459, and L. innocua serotype 6a strain CLIP 11262, allowed identifying specific genetic markers for the molecular determination of Listeria serotypes. These markers are: lmo0737 associated with serotypes 1/2a, 1/2c, 3a, and 3c, lmo1118 associated with serotypes 1/2c and 3c, ORF2819 for serotypes 1/2b, 3b, 4b, 4d, 4c, and the marker ORF2110 for serotypes 4b, 4d, and 4e5. Serotypes 1/2a, 1/2b, 1/2c, and 4b are responsible for 98% of listeriosis cases in humans, where 4b is the most widely associated with epidemic outbreaks resulting from the consumption of contaminated foods. Serotypes 3a, 3b, 3c, 4a, 4c, 4e, 4d, and 7 are very infrequent in foods, which makes them rarely mentioned as causing human listeriosis. Serotypes 4a and 4c are associated with veterinary cases, and are rarely thought to be involved in outbreaks or human diseases4,7,8. The study of the molecular serotypes of the L. monocytogenes strains responsible for massive listeriosis epidemics has represented the foundation of the idea of virulence variability; this is because different techniques have demonstrated the existence of a small number of implicated serotypes and epidemic clones9. The concept of an epidemic clone (EC) is used to refer to genetically related groups derived from the same ancestor, associated with outbreaks reported at different moments and geographic regions4. Until now, epidemic clones have been described (ECI, ECII, ECIII, and ECIV)4,10,11, and although some studies report that clonal groups Ia and II play a vital epidemiological role12, ECI is the most important and most studied, given that it has been involved in several listeriosis outbreaks in North America and Europe4. The location of strains in the EC is done by detecting specific molecular markers. To group the L. monocytogenes strains in the ECI, the cassette of the 85M region (85M, 85R, and 85S) and specific GATC sites of the modification-restriction system (which according to Kocks in 2001 is specific for this clonal group13) are amplified. Previously attained results suggest that the strains of L. monocytogenes serotypes 4b and 1/2b are grouped in this clone (ECI); this makes it possible to infer that the L. monocytogenes strains that belong to one of the serotypes responsible for the highest number of human listeriosis caused by consuming contaminated foods belongs to ECI. In Colombia, studies show that a high percentage of foods (cheeses, milk products, and meats) are contaminated with L. monocytogenes. A study carried out in Boyacá revealed the presence of this Gram positive bacteria in 26.9% of soft cheeses and in 25.9% of analyzed raw milk samples14. Another study demonstrated that it is possible to isolate this microorganism in 24% of ready-to-eat meat products sold in Bogotáâs public markets15. The pathogenicity of the L. monocytogenes strains that contaminate foods is influenced by factors like the population density of the microorganism at the moment of consumption and the type of contaminating strain (some clonal groups have greater pathogenicity than others). This suggests that only a fraction of L. monocytogenes strains are agents that cause diseases in humans16. There are few epidemiological studies available in Colombia; this is because notification of the disease is not obligatory17. However, the potential for L. monocytogenes to contaminate foods, spaces, and other sources, as well as its high mortality rate, reveals the risk consumers are being exposed to. This calls for the need to create policies for prevention and control in food processing plants, as well as the need to develop studies to determine the serotypes that circulate in our environment and that are grouped in the EC. All the information that can be gathered on this microorganism will contribute to the construction of the national panorama and provide tools to prevent and control diseases caused by L. monocytogenes. With this context in mind, the objective of this study was to determine the serotype of different L. monocytogenes strains isolated from humans, environments, and foods and to be able to associate them with the 85M cassette of the ECI. Materials and methods Bacterial isolates. This study used 108 strains of L. monocytogenes that were previously isolated and identified in the LEMA laboratory by using the protocol established by the Food and Drug Association (FDA) in its Bacteriological Analytical Manual (BAM) manual18. The confirmation of species was done through polymerase chain reaction (PCR) amplifying specific genes19. Strains were maintained at -70°C in Brain Heart Infusion broth (Oxoid, Bansistoke, United Kingdom) with glycerol at 1% up to the moment of analysis. The 108 studied strains were isolated from samples of animals, body fluids (clinical samples), foods, and food processing plant equipment and environments (Table 1). As a positive control for the molecular serotypification and amplification of the 85M fragment, L. monocytogenes F2365 serotype 4b was used, donated by the University of North Carolina. L. innocua ATCC73016 was used as a negative control. Molecular serotypification of L. monocytogenes. Oligonucleotides were used (Table 2), as well as the procedure established by Doumith et al.5 The final volume reaction was 100 µl: 1x of PCR GoTaq Green Master Mix (Promega Corporation, Madison, USA), 10 µM of each primer, nuclease-free water and 50 ng of DNA. Salmonella enteritidis ATCC 13076 was used as a negative control. The PCR products were separated by electrophoresis in agarose gel at 2% during 60 min at 80 V and 400 mA. The molecular weight marker 100 pb-step ladder (Promega Corporation, Madison, USA) was the standard to determine the molecular weight of the bands visualized in the ChemiDoc XRS System transilluminator (Bio-Rad Laboratories, Inc, USA). PCR to identify the 85M fragment. A uniplex PCR was used to amplify the 85M fragment (product of the 471-pb amplification) that belongs to the SSCS cassette of the epidemic clone I. The 85M-F indicators (AATATATTTTCAATGTTTGATGGT) and 85R (GCTAATTCAATCCCTATTCT) were used, as well as the protocol established by Yildirim et al.16 The amplification reaction contained 12.5 µl of 2X PCR master mix (Promega corporation, USA), 1 µM of each primer, and 240 ng of DNA mold. The amplification was done by using a DNA denaturalization cycle at 94°C for 15 seconds, followed by 30 amplification cycles at 94°C for 3 seconds, hybridization at 53°C for one minute and 72°C for two minutes for each elongation. The final extension was done at 74°C for two minutes. The amplification products were visualized in agarose gel at 2% dye with ethidium bromide, in the ChemiDoc XRS System transilluminator (Bio-Rad Laboratories, Inc, USA). Results The molecular weight of the multiplex PCR products obtained through electrophoresis in agarose gel was determined by using Quantity One 1-D analysis software, which uses the ChemiDoc XRS System transilluminator from Bio-Rad. The amplification of the positive control and the absence of bands in the negative control demonstrated the techniqueâs specificity (Figure 1). A total of 60.2% (65) of the molecularly serotypified strains of L. monocytogenes are serotype 4b-4d-4e, of these, 47.7% (31 strains) correspond to milk, cheese, meet, and vegetable isolates. Some 27.7% (18) are from food processing environments (drains, walls, floors) or equipment (slicers, sealers). Another 18.5% (12) are from clinical samples, specifically L. monocytogenes isolated from cerebrospinal fluid and blood cultures. The remaining 6.2% (4) correspond to veterinary samples (uterus of pregnant cows). Serotype 1/2b-3b was identified in 17.6% (19 strains) of isolates, and was particularly more frequent in food samples (78.9%, 15 of 19 strains) than in clinical samples (15.8%, 3 strains) or environmental samples (5.3%, 1 strain). There were no strains of this serotype found in L. monocytogenes isolates from veterinary samples. Sixteen strains (14.8%) belong to serotype 4a-4c, which predominated in samples taken from food processing plant equipment (81.3%, 13 strains) and 18.8% (3 strains) corresponded to artisan jam sold in public markets. For serotypes 1/2b-3b-7 and 1/2c-3c, the same number of L. monocytogenes strains (3.7%) was found for each (4). However, serotype 1/2b-3b-7 was identified in food (50%, 2 strains), food processing environments (25%, 1 strain), and clinical samples (25%, 1 strain), while serotype 1/2c-3c was found predominately in foods (75%, 3 strains) and was not found in other spaces. In regards to amplifying the 85M region, the 471pb fragment obtained for the L. monocytogenes F2365 strain (positive control) and the absence of contamination in the negative control (L. ivannovii) (Figure 2) demonstrated the specificity of this uniplex PCR. Of the 108 L. monocytogenes strains evaluated, the presence of the 85M region of the cassette that identifies the ECI (Figure 2) was determined in 63.8% (69) of the strains. All the isolates that had the 471 pb band associated with the 85M fragment completely corresponded with L. monocytogenes serotype 4b-4e-4d. The amplification product was not obtained in any of the molecularly serotypified isolates. Discussion Over the last several years, researchers have looked for virulence markers specific for L. monocytogenes and that can be used for epidemiological studies and the characterization of strains present in different environments13. In this descriptive study, the molecular serotypification technique was studied together with the amplification of a genetic marker that corresponds to the 85M fragment. The intention was to demonstrate that L. monocytogenes isolates identified as serotype 4b4d-4e could be associated with the presence of said fragment of the cassette that codifies for the ECI. Given the exploratory character of this research project, the presence of the three regions of the cassette was not determined, but an approximation was used to continue with the characterization of the serotypified Colombian isolates. Although serotype 4a has been widely associated with animals20,21, this study did not find it present in isolates from veterinary samples. This finding does not imply the absence of L. monocytogenes 4a-4c in this type of sample; on the contrary, it suggests that sample size should be increased to determine if this serotype is found in animals that produce foods or in food of animal origin. With the exception of the samples of animal origin, at least one strain from each source evaluated in this study (foods, clinical samples, and food processing equipment and environments) was identified as belonging to the serotype 1/2a-3a, particularly, in vegetable and milk products. These results concur with previous studies, where this serotype is associated with outbreaks caused by ingesting contaminated foods22. The results of this study show that all the strains belonging to the serotype 4b-4d-4e possess at least one of the fragments (85M) that make up the cassette used to identify the epidemic clone I. This relationship appears coherent and in accordance with previous studies where serotypes 4b-4d-4e was found grouped in clones I and II7. This information is of great importance to the food industry, public health, and risk-analysis studies; it reveals that potential highly virulent strains were found circulating in our environment, which is very significant for food safety. It is also important to highlight that most 4b-4d-4e serotypes found corresponded to food samples, coinciding with previous reports that demonstrate serotype 4b as the main cause of listeriosis cases4,23. The presence of L. monocytogenes serotype 4b-4d-4e in food products ready for human consumption and in food processing equipment and spaces represents a health risk for consumers. The number of potentially affected consumers is currently unknown and difficult to determine because of limited data and the fact that notification is not obligatory for listeriosis cases in Colombia. The use of the molecular marker 85M to identify ECI is useful as an initial method for screening and characterization of L. monocytogenes strains because it allows for the location of a specific clone depending on its genetic characteristics. It is necessary to continue conducting molecular studies that complement information on the genotypes of strains that have been identified as possibly belonging to ECI, as well as studies on the characterization of other virulent clones. Conclusions This study reports the predominant existence of serotype 4b-4d-4e strains in food, environmental, and other samples. Furthermore, it reveals the presence of one region of the SSCS cassette of the L. monocytogenes epidemic clone I in isolates belonging to the serotype (4b) most frequently associated with human listeriosis outbreaks. Further studies should focus on identifying the other regions of the cassette (85F, 85R) to determine if strains belong to the epidemic clone I. Such information would contribute in advancing our understanding of the pathogenicity and diversity of this bacterium. Conflict of interests. The authors declare having no conflict of interest whatsoever with this studyâs sponsor, the LEMA laboratory at Universidad de los Andes. Furthermore, the strains donated by the National Health Institute used in this study had the full consent of said Institution. References 1. Callejo R PM, Martínez C, Aguerre L, Rocca F, Martínez G. Aislamiento, identificación y caracterización de Listeria monocytogenes. In: Sur CRdRdWGSSpAd, ed. Centro Regional de Referencia del WHO Global Salm Surv para América del Sur; 2008: 1-39. [ Links ] 2. Schmid MW, Ng EY, Lampidis R, et al. Evolutionary history of the genus Listeria and its virulence genes. Syst Appl Microbiol. 2005; 28: 1-18. [ Links ] 3. Unnerstad H, Romell A, Ericsson H, Danielsson-Tham ML, Tham W. Listeria monocytogenes in faeces from clinically healthy dairy cows in Sweden. Acta Vet Scand. 2000; 41: 167-71. [ Links ] 4. Kathariou S. Foodborne outbreaks of listeriosis and epidemic associated lineages of Listeria monocytogenes. In: Torrence ME, Isaacson RE (eds.). Microbial food safety in animal agriculture: Current topics. Oxford: Blackwell Publishing; 2003.p. 243-56. [ Links ] 5. Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol. 2004; 42: 3819-22. [ Links ] 6. Betancourt O, Villagrán K, Muñoz F, Gutiérrez E, Mayorga M, Melgarejo P. Serotipos de Listeria monocytogenes aislados de alimentos producidos en la provincia de Cautín, Chile. Rev Cient. 2010; 20: 529-36. [ Links ] 7. Evans MR, Swaminathan B, Graves LM, et al. Genetic markers unique to Listeria monocytogenes serotype 4b differentiate epidemic clone II (Hot Dog Outbreak Strains) from other lineages. Appl Environ Microbiol. 2004; 70: 2383 90. [ Links ] 8. Liu D, Lawrence ML, Gorski L, Mandrell RE, Ainsworth AJ, Austin FW. Listeria monocytogenes serotype 4b strains belonging to lineages I and III possess distinct molecular features. J Clin Microbiol. 2006; 44: 214-7. [ Links ] 9. López V, Suárez M, Chico-Calero I, Navas J, MartínezSuárez JV. Listeria monocytogenes en alimentos: ¿son todos los aislamientos igual de virulentos? Rev Arg Microbiol. 2006; 38: 224-34. [ Links ] 10. Chen Y, Zhang W, Knabel SJ. Multi-virulence-locus sequence typing identifies single nucleotide polymorphisms which differentiate epidemic clones and outbreak strains of Listeria monocytogenes. J Clin Microbiol. 2007; 45: 835 46. [ Links ] 11. Ducey TF, Page B, Usgaard T, Borucki MK, Pupedis K, Ward TJ. A Single-nucleotide-polymorphism-based multilocus genotyping assay for subtyping lineage isolates of Listeria monocytogenes. Appl Environ Microbiol. 2007; 73: 133-47. [ Links ] 12. Cheng Y, Kim JW, Lee S, Siletzky RM, Kathariou S. DNA probes for unambiguous identification of Listeria monocytogenes epidemic clone II strains. Appl Environ Microbiol. 2010; 76: 3061-8. [ Links ] 13. Herd M, Kocks C. Gene fragments distinguishing an epidemic-associated strain from a virulent prototype strain of Listeria monocytogenes belong to a distinct functional subset of genes and partially cross-hybridize with other Listeria species. Infect Immun. 2001; 69: 3972-9. [ Links ] 14. Rueda A. Utilización de la reacción en cadena de la polimerasa (PCR) en tiempo real para determinar la incidencia de Listeria monocytogenes en leches crudas en el departamento de Boyacá. Bogotá: Universidad de los Andes; 2005. [ Links ] 15. Vanegas M, Botina B, Martínez A. Detección por PCR de Listeria monocytogenes en productos cárnicos distribuidos en Bogotá. UDCA. 2006; 9: 149-56. [ Links ] 16. Yildirim S, Lin W, Hitchins AD, et al. Epidemic clone Ispecific genetic markers in strains of Listeria monocytogenes serotype 4b from foods. Appl Environ Microbiol. 2004; 70: 4158-64. [ Links ] 17. Crespo MP, Vélez JD, Castañeda C, Hoyos F, López ML. Aislamiento de Listeria monocytogenes en un hospital de tercer nivel. Colomb Med.1999; 30: 89-98. [ Links ] 18. FDA US Food and Drug Administration. Detection and enumeration of Listeria monocytogenes. Chapter 10. In: Bacteriological analytical manual. New Hampshire: FDA; 2002. [ Links ] 19. Medrano MV, Restrepo S, Vanegas MC. Tipificación molecular de Listeria monocytogenes aisladas de muestras clínicas y alimentos. Biomedica. 2006; 26: 442-50. [ Links ] 20. Wiedmann M, Bruce JL, Keating C, Johnson AE, McDonough PL, Batt CA. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect Immun. 1997; 65: 2707-16. [ Links ] 21. Roberts A, Nightingale K, Jeffers G, Fortes E, Kongo JM, Wiedmann M. Genetic and phenotypic characterization of Listeria monocytogenes lineage III. Microbiology. 2006; 152 (Pt 3): 685-93. [ Links ] 22. Cai S, Kabuki DY, Kuaye AY, Cargioli TG, Chung MS, Nielsen R, Wiedmann M. Rational design of DNA sequence-based strategies for subtyping Listeria monocytogenes. J Clin Microbiol. 2002; 40: 3319-25. [ Links ] 23. Orsi RH dBH, Wiedmann M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int J Med Microbiol. 2011; 301: 79-96. [ Links ]
Aida Juliana Martínez, MSc,3, Stefany Alejandra Arévalo, MSc4
2Professor, Laboratory for Microbial Ecology of Food (LEMA), Department of Biological Sciences, Universidad de los Andes, Bogotá, Colombia. e-mail: mvivianablue@hotmail.com
3Technical Research Coordinator, Laboratory for Microbial Ecology of Food (LEMA), Department of Biological Sciences, Universidad de los Andes, Bogotá, Colombia. e-mail: ai-marti@uniandes.edu.co
4Private Laboratory Coordinator, Laboratory for Microbial Ecology of Food (LEMA), Department of Biological Sciences, Universidad de los Andes, Bogotá, Colombia. e-mail: stefarevalo@gmail.com
Objective: To identify the serotypes from L. monocytogene strains isolated from different samples by performing the molecular subtyping technique; to determine the 85M fragment that codifies for epidemic clone I.
Methods: 108 strains of L. monocytogenes were used, isolated from samples of animals, body fluids, foods, and food processing plant equipment and spaces. The samples were identified by following the Bacteriological Analytical Manual protocol described by the Food and Drug Administration (FDA). The strains were identified by Polymerase Chain Reaction (PCR) using primers and a standardized protocol from a previous research project. Serotype identification was performed by multiplex PCR. The determination of the 85M fragment of the SSCS cassette was done by following the protocol by Yildrim et al.
Results: Of the 108 L. monocytogenes strains analyzed, 60.2% (65 strains) belonged to the 4b-4d-4e serotype, 17.6% (19 strains) were identified as 1/2a-3a serotype, 14.8% (16 strains) were 4a-4c serotype, 3.7% (4 strains) belonged to the 1/2c-3c serotype, and (3.7%) corresponded to the 1/2b-3b-7 serotype. It was determined that the L. monocytogenes strains serotype 4b-4d-4e and 1/2a-3b have the 85M fragment of the SSCS cassette.
Conclusion: This study reports the predominant existence of L. monocytogenes strains serotype 4b-4d-4e in food, environmental, and clinical samples. The presence of an epidemic clone I region was also found in L. monocytogenes strains.
Objetivo: Identificar los serotipos de L. monocytogenes aisladas de diferentes fuentes y determinar la presencia del fragmento 85M que codifica para clon epidémico I.
Métodos: Aislamiento de L. monocytogenes a partir de muestras animales, fluidos corporales, comestibles, equipos y ambientes en plantas de alimentos, según el protocolo FDA. Identificación por reacción en cadena de la polimerasa (PCR), serotipificación molecular por PCR multiplex y determinación del fragmento 85M según Yildrim et al.
Resultados: De las 108 cepas de L. monocytogenes serotipificadas molecularmente, 60.2% (65 cepas) pertenecen al serotipo 4b-4d-4e, el 17.6% (19 cepas) fueron 1/ 2a-3a, el 14.81% (16 cepas) son serotipo 4a-4c, el 3.7% (4 cepas) corresponden a 1/2c-3c y el 3.7% fueron aislamientos serotipo 1/2b-3b-7. Se determinó que todas las cepas serotipo 4b-4d-4e y 1/2a-13b contienen el fragmento 85M del clon epidémico I.
Conclusión: Este estudio reporta la existencia predominante de cepas de L. monocytogenes serotipo 4b-4d-4e en muestras de alimentos, ambientes y otras. Asimismo, evidencia la presencia de una región del fragmento 85M del clon epidémico I de L. monocytogenes en aislamientos pertenecientes al serotipo con más frecuencia relacionado con brotes de listeriosis en humanos (4b).