Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombia Médica
On-line version ISSN 1657-9534
Colomb. Med. vol.46 no.3 Cali July/Sept. 2015
Original Article
Sodium bicarbonate versus isotonic saline solution to prevent contrast-induced nephropathy: a systematic review and meta-analysis
El bicarbonato de sodio en comparación con solución salina isotónica para prevenir la nefropatía inducida por contraste: una revisión sistemática y meta-análisis
Carlos Andres Zapata-Chica1, Diana Bello Marquez1, Lina Maria Serna-Higuita2, John Fredy Nieto-Ríos3, Fabian David Casas-Arroyave4, Jorge Hernando Donado-Gómez5
1 Pediatric Nephrologist, The University of Antioquia, Medellin-Colombia.
2 Pediatric Nephrologist, Pablo Tobón Uribe Hospital, The University of Antioquia, Medellin, Colombia.
3 Nephrologist, Pablo Tobón Uribe Hospital, The University of Antioquia, Medellin, Colombia.
4 Anesthesiologist, Masters Student in Clinical Sciences, The University of Antioquia Medellin, Colombia.
5 Clinical Epidemiologist, MS, Pablo Tobón Uribe Hospital, Medellin-Colombia.
Zapata-Chica CA, Bello MD, Serna-Higuita LM, Nieto-Ríos JF, Casas-Arroyave FD, Donado-Gómez JH. Sodium bicarbonate versus isotonic saline solution to prevent contrastinduced nephropathy: a systematic review and meta-analysis. Colomb Med. 2015; 46(3): 90-103.
© 2015. Universidad del Valle. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Article history: Received: 08 April 2015 Revised: 24 June 2015 Accepted: 20 August 2015
Corresponding author:
Lina Maria Serna Higuita. Pediatric Nephrologist, Pablo Tobón Uribe Hospital, The University of Antioquia, Medellin, Colombia. E-mail: lm.serna@hotmail.com
Abstract
Introduction: Contrast-induced nephropathy is one of the main causes of acute kidney injury and increased hospital-acquired morbidity and mortality. The use of sodium bicarbonate for nephroprotection has emerged as a preventative strategy; however, its efficacy is controversial compared to other strategies, such as hydration using 0.9% saline solution.
Objective: To compare the effectiveness of sodium bicarbonate vs. hydration using 0.9% saline solution to prevent contrast-induced acute kidney injury.
Methods: A systematic review of studies registered in the COCHRANE, PUBMED, MEDLINE, LILACS, SCIELO and EMBASE databases was conducted. Randomized controlled studies that evaluated the use of 0.9% saline solution vs. sodium bicarbonate to prevent contrast-induced nephropathy were included.
Results: A total of 22 studies (5,686 patients) were included. Sodium bicarbonate did not decrease the risk of contrast-induced nephropathy (RD= 0.00; 95% CI= -0.02 to 0.03; p= 0.83; I2= 0%). No significant differences were found in the demand for renal replacement therapy (RD= 0.00; 95% CI= -0.01 to 0-01; I2= 0%; p= 0.99) or in mortality (RD= -0.00; 95% CI= -0.001 to 0.001; I2= 0%; p= 0.51).
Conclusions: Sodium bicarbonate administration is not superior to the use of 0.9% saline solution for preventing contrast-induced nephropathy in patients with risk factors, nor is it better at reducing mortality or the need for renal replacement therapy.
Keywords:
Contrast induced nephropathy, acute kidney injury, sodium bicarbonate, sodium chloride.
Resumen
Introducción: La nefropatía inducida por medio de contraste es una de las causas principales de lesión renal aguda, lo cual incrementa la morbilidad y mortalidad intrahospitalaria. La nefroprotección con bicarbonato de sodio ha surgido como una estrategia preventiva, sin embargo su eficacia es controversial cuando se compara con estrategias como la hidratación con solución salina al 0.9%.
Objetivo: Comparar la efectividad del bicarbonato de sodio versus la hidratación con solución salina al 0.9% en la prevención de la lesión renal aguda inducida por contraste.
Métodos: Se realizó una revisión sistemática de los estudios registrados en COCHRANE, PUBMED, MEDLINE, LILACS, SCIELO y EMBASE. Se incluyeron estudios aleatorizados, controlados donde se evaluó el uso de solución salina al 0.9% versus bicarbonato de sodio para prevenir la nefropatía por medio de contraste.
Resultados: Se incluyeron 22 estudios (5,686 pacientes). El bicarbonato de sodio no disminuyó el riesgo de nefropatía inducida por contraste (DR= 0.00 IC 95%= -0.02-0.03; p= 0.83, I2=0%). Tampoco se encontró diferencia significativa en la necesidad de terapia de reemplazo renal (DR= 0.00 IC 95%= -0.01-0-01, I2= 0%, p= 0.99); ni en la mortalidad (DR= -0.00, IC 95%= -0.001-0.001, I2= 0%, p= 0.51).
Conclusiones: La administración de bicarbonato de sodio no es superior al suministro de solución salina al 0.9% en la prevención de nefropatía inducida por medio de contraste en pacientes con factores de riesgo. Su uso tampoco es superior en la reducción de mortalidad y el requerimiento de terapia de reemplazo renal.
Palabras clave:
Nefropatía inducida por medio de contraste, lesión renal aguda, bicarbonato de sodio, cloruro de sodio.
Introduction
Contrast-induced nephropathy (CIN) is a usually reversible form of acute kidney injury (AKI) that occurs after the intravenous or intra-arterial administration of contrast media. CIN is the third most common cause of de novo AKI among hospitalized patients; it is associated with an increased risk of complications such as acute myocardial infarction, longer hospital stays and higher costs, especially when its management requires the use of renal replacement therapy1,2.
Contrast-induced nephropathy CIN is diagnosed according to some of the following criteria: a) an absolute increase in serum creatinine of >0.5 mg/dL, b) a relative increase in serum creatinine of >25% with respect to baseline or c) an estimated glomerular filtration rate (GFR) of less than 30-60 mL/min/1.73 calculated using the recommended equations within the first 24 to 72 h after exposure to contrast media in the absence of an alternative explanation for the impairment3,4. Other definitions published in the literature include a serum creatinine increase of ≥0.3 mg/ dL or to 1.5 times baseline within the previous 7 days or a urine volume of <0.5 mL/kg/h for 6 h after exposure5; however, the first two definitions are currently supported by the highest consensus.
The exact pathogenesis of CIN is uncertain. It has been postulated that hypoxic injury and the generation of free radicals induced by exposure to contrast media plays an important role6. At present, prevention measures are the best option for all patients at risk of developing CIN, and different preventive strategies have been proposed, including periprocedural hydration with 0.9% Normal saline solution (NSS)7,8 and the administration of sodium bicarbonate (SB)9,10. These therapies appear to have a protective effect against CIN; however, the results of multiple trials that have used these measures have been controversial and have not clarified the best management strategy11. Various systematic reviews and meta analyses have shown that SB was beneficial for preventing CIN but did not improve other clinical outcomes, such as death, heart failure and the need for renal replacement therapy (RRT); additionally, these meta-analyses also showed publication bias and significant heterogeneity11,12. The aim of this study was to determine the effectiveness of SB compared to 0.9% NSS for preventing CIN in patients older than 18 yrs who were exposed to contrast media.
Materials and Methods
Protocol
This review and meta-analysis was performed according to the Cochrane Collaboration13 and PRISMA-P14 guidelines for the development of systematic review protocols.
Eligibility criteria
This review included controlled clinical trials that compared SB infusion to 0.9% NSS as a prevention strategy for CIN among adults who were older than 18 yrs and had risk factors for kidney disease or a diagnosis of chronic kidney disease or had undergone coronary procedures, interventional radiology or diagnostic tests that required contrast media. Studies published in the English or Spanish language literature or databases were included, with no restriction placed on the time of publication.
Contrast-induced nephropathy CIN is defined as a glomerular filtration rate (GFR) decrease greater than 25% calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKDEPI) formula or as an increase in serum creatinine greater than 0.5 mg/dL compared to the baseline within 48 h of the procedure or an absolute increase of 25% compared to the baseline4,15. Additionally, some secondary outcomes were evaluated, including the need for renal replacement therapy (RRT), the exchange difference with basal serum creatinine, and mortality.
Information sources and search strategies
A search of studies recorded since the formation of The Renal Group of the Cochrane Collaboration using the term "contrastinduced nephropathy" was conducted. Additionally, all of the clinical trials registered in the Cochrane Central Register of Controlled Trials (CENTRAL) were searched using the terms Nephropathy, Bicarbonate, Saline Solution, and Contrast Media (the search strategy is detailed in the annexes). Various electronic databases were also searched using terms and highly sensitive strategies to identify controlled trials. For PUBMED, the following terms were employed: "contrast nephropathy", "sodium bicarbonate", "sodium chloride" and "renal failure"; for EMBASE, "sodium chloride", "acute renal failure", "contrast nephropathy", and "sodium bicarbonate" were used. Additionally, the Latin American databases LILACS and SCIELO were searched using terms "nefropatía inducida por medio de contraste", "bicarbonato de sodio", and "solución salina".
Article selection
The titles and summaries of the studies identified by the search were independently evaluated by two authors (CAZ and DB), and the full studies were examined for their potential to meet the eligibility criteria. A third author (LMS) resolved any disagreement between the two evaluating authors. After the analysis, the authors decided which studies fulfilled the inclusion criteria. The agreement among the evaluators was assessed using the Kappa formula.
Data extraction
One author (LMS) was designated to develop a standard electronic format for data collection. The other authors (CAZ and DB) evaluated and approved the format prior to data extraction; however, LMS performed double data entry to correct errors and missing data.
The following information was extracted from each study: age, reason for exposure to contrast media, diagnosis, history of kidney and/or diabetes, doses and types of contrast media used, type of intervention performed (SB dose, time before treatment); control (doses and duration of infusion); and outcomes measured (CIN, need for RRT and death).
Analysis
Risk of bias. To determine the risk of bias, the format proposed by the Cochrane Collaboration for assessing the risk of bias in primary studies was used13. For each study, the authors determined whether the subjects and treatments were randomized, how the randomization sequence was concealed, who in the study was blinded to the intervention and how the blinding occurred, data collection, the amount of missing data and missing data were managed, the type of analysis performed and whether a reporting bias was generated. Two evaluators (LMS and DB) performed this analysis separately, and disagreements were resolved by consensus with a third reviewer (CAZ). To determine the consensus, Kappa was used.
Summary of the measures and analysis plan. For each outcome and each study, a 2x2 table was generated wherein the number of patients who experienced an event or outcome in each comparison group and the total number of patients in each group were summarized. For each statistic calculated, the program Review Manager® version 5.3 was applied, with the exception of the metaregression analysis, for which the program Comprehensive Meta-Analysis® 2.0 was used. The treatment weighting was calculated throughout the study. The results are presented as risk differences (RD) with their 95% confidence intervals (CI) for dichotomous variables and mean differences with their 95% confidence intervals for continuous variables. The DerSimonian and Laird random effects model was used for all outcomes. This method was chosen to generate estimates and a conservative CI because it includes the intra- and intervariance of the studies. For all of the results, twotailed p values are shown, and p <0.05 was considered statistically significant.
To identify the potential risk of heterogeneity, the statistical tau test2, with p <0.1 indicating statistical significance, and the I2 test, in which a value greater than 50% indicates heterogeneity, were applied.
Subgroup analyses were performed based on the methodological quality standards for studies, the use of N-Acetylcysteine (NAC) and the type of contrast medium employed (iso-osmolar or hypoosmolar), given that the risk of kidney injury is greater when hypo-osmolar contrast media are used in contrast to iso-osmolar ones. Additionally, a meta-regression was performed to evaluate whether the presence of diabetes or the quantity of contrast medium used could be related to the development of CIN. In this analysis, two-tailed p values were reported, and values less than 0.05 were considered statistically significant for the interaction or the regression coefficient.
Publication bias throughout the study
A funnel plot was generated to evaluate the presence of publication bias. For this purpose, the inverse variance was plotted against the logarithm of the RR. The presence of asymmetry was evaluated; however, the evaluation may be subjective. Egger's linear regression test was conducted and was weighted by evaluating the association between the study size and the estimated treatment effect. A value of p <0.05 was considered statistically significant for publication bias.
Results
Study selection
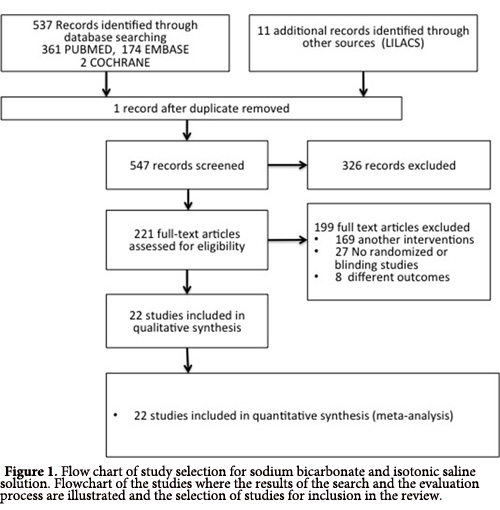
A total of 548 reports were found during the initial search of the bibliographical databases EMBASE, PUBMED, COCHRANE, SCIELO and LILACS. After the initial assessment, 327 publications were excluded; the full text of the 221 remaining reports was analyzed. Among those, 199 studies were excluded because they examined another type of intervention, were not randomized and/ or controlled or did not measure the proposed outcomes. Finally, 22 clinical randomized studies with a total of 5,686 patients in which a main outcome of CIN could be analyzed were reviewed (Fig. 1).
Methodology
The 22 studies selected for review comprised clinical randomized controlled studies published in English. However, only eight of the studies (36%) concealed the randomization sequence (Fig. 2).
Participants
The studies included a total of 5,686 patients who contributed to the primary outcomes. In all, the patients had a history of kidney disease or a high risk of developing it, which was determined using the baseline serum creatinine measurement or the GFR: In 12 of the studies, the creatinine cut-off was defined as greater than 1.1 mg/dL to 1.5 mg/dL, and in two of the studies, the GFR cutoff was less than 60 mL/min/1.73. In 13 studies, low-osmolality contrast media was used, eight were iso-osmolar and in one study both contrast medium were used.
Intervention
Among the studies that included SB administration, 18 administered SB diluted with 5% dextrose in distilled water (D5%DW), and 4 did not specify the dilution of bicarbonate used 16-19); however, stabilized bicarbonate is only achieved with the addition of dextrose, so it can be assumed that this dilution was performed. The quantity of D5%DW for dilution differed among the studies; in 16 studies, 154 mL of bicarbonate (1,000 mEq/L) was diluted in 846 mL of D5%DW (studies)8,15,20-32,33; in the remaining studies, different mixtures of bicarbonate and D5%DW were used; the infusion speed was 1 to 3 mL/k/h within 1 to 12 h before the radiological procedure was performed and then between 6 and 12 h post-procedure (Table 1). In six studies20,21,23,27,29,32, NAC was used along with the sodium bicarbonate. NAC was always administered between 6 and 12 h before the intervention. All of the trials included patients who had undergone coronary procedures, a type of coronary angiography or percutaneous coronary intervention. Furthermore, four studies included patients who underwent computerized axial tomography.
| Study (reference) | Age (years) | Serum creatinine or GFR | Diabetes N (%) | Procedure | Trial design | Dose of contrast media (DS) |
|---|---|---|---|---|---|---|
| Merten8 | >18 NaCl 69.2 (32-87) NaHCO3 66.7 (37-88) | Cr >1.1 mg/dL | NaCl: 27 (46%); NaHCO3 30 (50%) | CA. CAT | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of dextrose 5%. 3 mL/kg for 1 h before CM. followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 154 mEq/L in 5% dextrose and H2O. 1 mL/kg for 6 h before CM. followed by an infusion of 1 mL/kg/h for 12 h after | NaCl 134 mL (63) NaHCO3 130 mL (72) |
| Masud36 | >20 NaCl 76 (11) NaHCO3 75 (8) | Cr >1.1mg/dL; GFR <60 mL/min/1.73 | NaCl: 10 (35%); NaHCO3: 8 (27%) | CA. PCI | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% Dextrose and H2O versus NaCl 0.9%. 3 mL/kg for 1 h before CM. followed by an infusion of 1 mL/kg/h for 6 h after | NaCl 120 mL (61) NaHCO3 112 mL (89) |
| Briguor37 | >18 NaCl 71 (9) NaHCO3 70 (9) | Cr >2 mg/dL GFR <40 mL/min/1.73 | NaCl: 61 (55%); NaHCO3: 53 (49%) | CA. PA. PCI | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of dextrose 5% + NAC. 3 mL/kg for 1 h before CM. followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9%+NAC. 1 mL/kg for 12 h before and after CM | NaCl 179 mL (102) NaHCO3 169 mL (92) |
| Ozcan38 | >18 NaCl 70 (40-84) NaHCO3 68 (46-86) | Cr >1.2 mg/dL | NaCl: 47.7%; NaHCO3: 42% | CA. PCI | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of dextrose 5% + NAC for 1 mL/kg for 6 h before and after CM versus NaCl 0.9%+NAC. 1 mL/kg for 6 h before and after CM | NaCl 110 mL (30-270) NaHCO3 100 mL (50-300) |
| Adolph39 | >18 NaCl 72.7 (6.5) NaHCO3 70.1 (8.4) | Cr >1.2 mg/dL GFR <63 mL/min/1.73 | NaCl: 23 (28.3%); NaHCO3: 26 (36.6%) | CA | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose in H2O versus NaCl 154 mEq/L in 5% dextrose in H2O. 2 mL/kg/h for 2 h before CM followed by an infusion of 1 mL/kg/h for 6 h after | NaCl 138 mL (52) NaHCO3 141 mL (50) |
| Maioli40 | >18 NaCl 74 (70-79) NaHCO3 74 (67-79) | Cr >1.5 mg/dL GFR <60 mL/min/1.73 | NaCl: 59 (23%); NaHCO3: 62 (25%) | CA. PCI | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O + NAC 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9% + NAC 1 mL/kg for 12 h before and after CM | NaCl 167 mL (66) NaHCO3 171 mL (69) |
| Brar41 | >18 NaCl 71 65-76) NaHCO3 71(65-75) | GFR <60 mL/min/1.73 | NaCl: 81 (45.5%); NaHCO3: 76 43.4%) | CA | NaHCO3 1,000 mEq/L, 150 mL versus NaCl 0.9%. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 4 h after | NaCl 137 mL (89-247) NaHCO3 126 mL (80-214) |
| Pakfetrat42 | >18 NaCl 58.4 (11.5) NaHCO3 57.8 (11.2) | NaCl: 31 (32.3%); NaHCO3: 26 (27%) | CA | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9% 1 mL/kg for 6 h before and after | NaCl 67 mL (41.1) NaHCO3 58 mL (32.7) | |
| Cho22 | >18 NaCl 77.33 (9.39) NaHCO3 78.47(8.72) | Cr >1.1 mg/dL GFR <60mL/min/1.73 | NaCl: 8 (29.6%); NaHCO3: 9 (42.8%) | CA | NaHCO3 154 mL of 1000 mEq/L to 846 mL of 5% dextrose and H2O. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 154 mEq/L. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after | NaCl 122.59 mL NaHCO3 136.31 mL |
| Castini43 | >18 NaCl 72.7 (8.2) NaHCO3 70 (8.3) | Cr >1.2 mg/dL | NaCl: 10 (20%); NaHCO3: 18 (35%) | CA. PCI | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O. 3 mL/kg for 1 h before CM. followed by an infusion of 1mL/kg/h for 6 h after versus NaCl 0.9% 1mL/kg for 12 h before and after CM | NaCl 196.4 mL (127.7) NaHCO3 179.2 mL (125.1) |
| Lee23 | >18 NaCl 67.5 (62-72) NaHCO3 68.5 (63-73) | Cr >1.1 mg/dL GFR <60 mL/min/1.73 | NaCl: 189 (100%); NaHCO3: 193 (100%) | NaHCO3 154mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9% 1 mL/kg for 12 h before and after CM | NaCl 120 mL (79-223) NaHCO3 113 mL (80-220) | |
| Maioli34 | 18 NaCl 66 (12) NaHCO3 (13) | NaCl:11 (20.7%); NaHCO3: 31(20.7%) | CA | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O. 3 mL/kg for 1 h before CM followed by an infusion of 1mL/kg/h for 6 h after versus NaCl 0.9% 1mL/kg for 12 h before and after CM | NaCl 216 mL (101) NaHCO3 208 mL (92) | |
| Gomes44 | >18 NaCl 64.5 (12) NaHCO3 64.1 (12) | Cr >1.2 mg/dL GFR <50 mL/min/1.73 | NaCl: 45 (29.8%); NaHCO3: 43 (8.7%) | CA. PCI | NaHCO3 154 mL of 1,000mEq/L to 846 mL of 5% dextrose and H2O 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9% 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after | NaCl 125 mL (87) NaHCO3 124 mL (65) |
| Klima25 | >18 NaCl 75 (70-82) NaHCO3 78 (70-82) | Cr >1 mg/dL GFR <60 mL/min/1.73 | NaCl: 30 (34%); NaHCO3: 34 (39%) | CAT. CA. PA. PCI | NaHCO3 166 mEq/L, 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9% 1 mL/kg for 8 h before CM followed by an infusion of 1 mL/kg/h for 12 h after | NaCl 100 mL (80-163) NaHCO3 100 mL (80-143) |
| Hafiz45 | >18 NaCl 73 (63-80) NaHCO3 74 (65-80) | Cr >1.4 mg/dL GFR <60 mL/min/1.73 | NaCl: 73 (45.3%); NaHCO3: 78 (49.1%) | CA | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O + NAC. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9%+ NAC 1 mL/kg for 12 h before and after CM | NaCl 100 mL (80-140); NaHCO3 110 mL (75-155) |
| Koc46 | >18 NaCl 62 (9) NaHCO3 62 (9) | NaCl: 100% NaHCO3: 100% | CA | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O + NAC 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9%+ NAC 1 mL/kg for 12 h before and after CM | NaCl 90 mL (85-100) NaHCO3 90 mL (90-100) | |
| Boucek47 | >18 NaCl 67 (10) NaHCO3 63 (11) | Cr >1.1 mg/dL | NaCl: 59 (100 %) NaHCO3: 61 (100%) | CA | NaHCO3 154mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O versus NaCl 5.85% 154 mL + 846 mL in 5% dextrose and H2O. 3 mL/kg for 1 h before CM followed for 1 mL/k/h for 6 h after | NaCl 104 mL (32) NaHCO3 115 mL (47) |
| Kooiamn48 | >18 NaCl 72.5 (9.5) NaHCO371.6 (9.8) | GFR <60 mL/min/1.73 | NaCl: 76 (27%); NaHCO3: 71 (26.6%) | CAT. CA | NaHCO3 1.4% 250 mL IV versus NaCl 1,000 mL before and after CM | NaCl 104.7 mL (21.6) NaHCO3 105.7 mL (21) |
| Mahmoodi49 | >18 NaCl 64.4 (11.07) NaHCO3 64.96 (10.29) | No date | CA | NaHCO3 154 mL of 1,000 mEq/L to 846 mL of 5% dextrose and H2O + NAC versus NaCl 0.9%+NAC 3 mL/kg for 6 h before and after | No date | |
| Nieto-Rios50 | >18 ; NaCl 59.8 (17.2) NaHCO3 60.7 (17.1) | Cr >1.2 mg/dL | NaCl: 39 (34.5%) NaHCO3: 43 (40.2%) | CAT. CA. PCI | NaHCO3 75 mL of 1,000 mEq/L to 425 mL of 5% dextrose and H2O. 3 mL/kg for 1 h before CM followed by an infusion of 1 mL/kg/h for 6 h after versus NaCl 0.9% 1 mL/kg for 6 h before and after | NaCl 100.6 mL (38.2) NaHCO3 99.3 mL (43.9) |
| Manari51 | >18 NaCl 65 (12.4) NaHCO3 63.9 (12.9) | No date | NaCl: 49 (16.7%); NaHCO3: 49 (16.4%) | CA. PCI | NaHCO3 77 mL 433 mL of 5% dextrose and H2O. 1 mL/kg for 12 h before CM followed by an infusion of 1 mL/kg/h for 12 h after versus NaCl 0.9% 1 mL/kg for 12 h before and after | NaCl 199 mL (77) NaHCO3 194 mL (83) |
| Yang52 | >18 NaCl 59.6 (11.08) NaHCO3 58.71 (10.9) | No date | NaCl: 37 (22.9%) NaHCO3: 27 (16.9%) | CA. PCI | NaHCO3 450 mL 433 mL of 1,050 of 5% dextrose and H2O. 1.5 mL/kg for 6 h before and after CM. versus NaCl 0.9% 1.5 mL/kg for 6 h before and after | NaCl 124 mL (63.8) NaHCO3 127 mL (48.09) |
| Cr: serum creatinine; NaCl: sodium cloruro; NaHCO3: sodium bicarbonate; NAC: N-Acetilcisteíne; CA: coronary angiography,; PCI: percutaneous coronary intervention, PA: peripheral angiography, GFR: glomerular filtration rate; CAT: computerized axial tomography; CM: contrast media. | ||||||
Control
In all of the studies, the control was performed with 0.9% NSS, usually administered between 6 and 12 h before and after the procedure. On 6 occasions, NAC was added to the treatment20,23,27,29,32 and was administered between 6 and 12 h before intervention.
Primary outcomes
The primary outcome evaluated in 13 studies was the presence of CIN, defined as a 25% elevation in serum creatinine above the baseline or a 0.5-mg/dL increase during the first 48 hours after the contrast medium was administered8,15-17,19-22,27,29-32; in seven studies, the same definition was used, but CIN was diagnosed up to 5 days post-contrast medium administration23,25,26,28,33-35; the other two studies used the maximum increase in serum creatinine as an outcome measure18,24.
Secondary effects
The need for RRT was evaluated in 15 studies and was defined across the board as the need for hemodialysis 48-72 h after exposure to contrast media secondary to acute renal failure18-23,28,30-34; in one study, the need for hemodialysis up to 30 days after contrast medium exposure was considered27. In 15 studies, mortality was defined as death by any cause within 28 days post procedure15-19,21-23,26-29,31,32,34. The difference in creatinine was assessed in 8 studies, which reported the mean difference in the creatinine level before and after exposure to contrast media in both groups. Table 1 shows the characteristics of each study included in the systematic review.
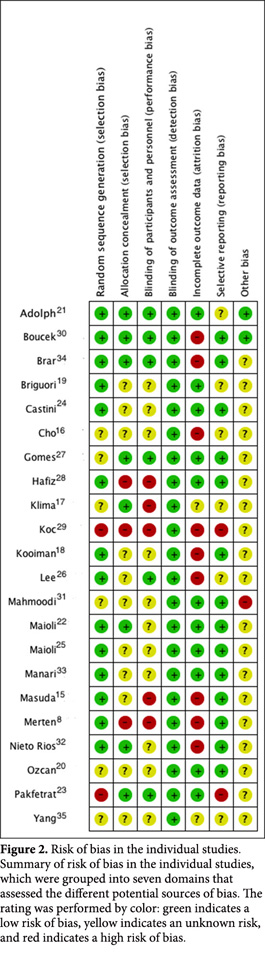
Risk of bias in the included studies
The methodological quality details of the individual studies are presented in Fig. 2. To assess the risk of bias, the studies were evaluated according to a Kappa value of 0.67.
Randomization and concealment
The random allocation sequence was judged inappropriate in two studies (9.1%)24,30 given that it was not specified and from reading it can be inferred that there was not any system of randomization. While randomization was effectively reported in 6 studies (27.3%), they failed to specify how they were conducted 17,18,21,28,32,35; in other studies, randomization was deemed adequate. In relation to the concealment of the random sequence, it was deemed adequate in 8 studies (36%)18,22-24,28,31,15,34; in 11(50.0%) it was deemed uncertain given that the type of concealment was not defined and three (13.7%) of the concealments were equally not conducted8,29,30.
Blinding
In 5 studies (9.1.%) the blinding of the patients and the doctors who conducted the intervention was rated adequate22,24,28,31,34; in 12 studies (54.5%), it was unclear how the blinding was performed; and in 5 studies (22.7%), the participants and the doctor knew the allocation of the intervention8,16,18,29,30. None of the studies specified whether those performing the study analysis were blinded; however, the outcomes assessed were not considered to have significantly increased the risk of bias.
Withdrawal and management of missing data
In 13 studies (59.1%) the data analysis performed for all of the randomized patients does not make reference to how missing data were handled; in 9 (40.9%) studies, the data analysis excluded lost data8,15,19,23,26,27,30,31,34, and in 3 (13.6%), an interim analysis was performed8,16,17.
Effects of the intervention
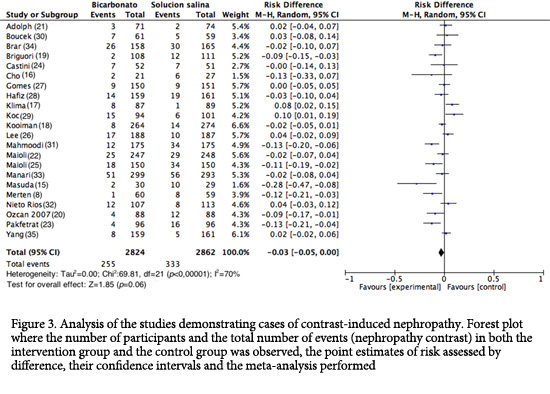
Primary outcome (CIN). The incidence of CIN varied between 1.67% and 17.06% for the SB side and between 1.12% and 34.48% for the 0.9% NSS side; an assessment of the percentage of patients with CIN in all the studies showed that 589 out of the 5,686 patients assessed developed CIN (10.36%); among the patients using SB, 9.03% (255/2,824) developed CIN, and among the 0.9% NSS group, 11.67% (334/2,862) developed CIN.
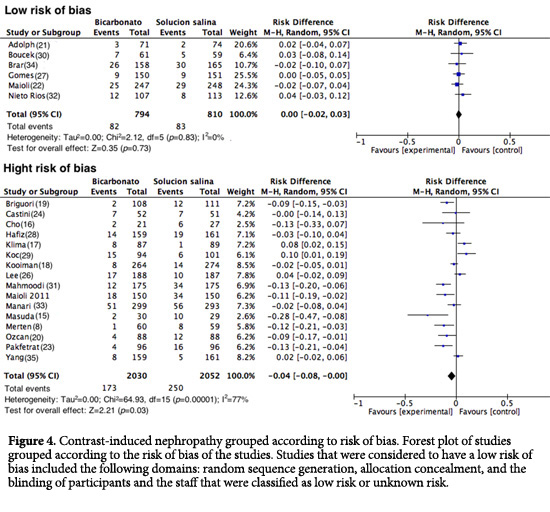
In total, 22 studies were analyzed (n= 5,686). The assessment of the primary outcome indicated that the risk of developing CIN was lower among similar group that received SB; however, high heterogeneity was observed among the studies (RD= -0.03; 95% CI= -0.05 to 0.00; I2= 70%; p <0.001; Fig. 3). Nonetheless, in the analysis of the subgroup of studies with good methodological quality (generation of randomized sequence, concealment of allocation and blinding of participants and staff), 82 of 794 patients developed CIN in the SB group vs. 83 of 810 patients in the control group (RD= 0.00; 95% CI= -0.02 to 0.03; p= 0.83; I2= 0%; Fig. 4). Upon assessing the studies with a high risk of bias, the results favored the use of SB; however, heterogeneity was high (RD= -0.04; 95%CI= -0.08 to 0.00; I2= 77%; p <0.001; Fig. 4).
Subgroup analysis
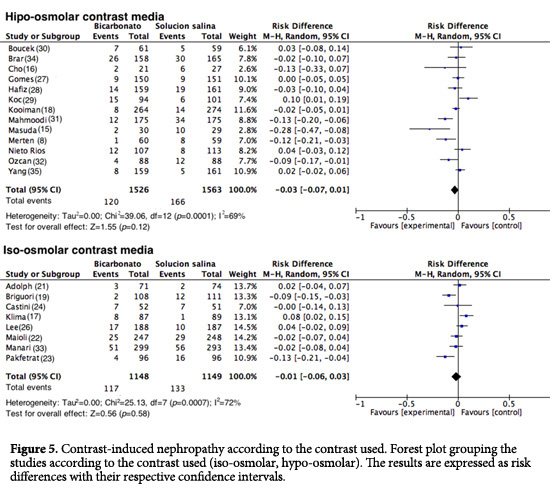
Contrast media used. For the analysis of subgroups based on the contrast media used, the studies in which high-osmolar contrast media were applied, the group that received SB experienced 120 events out of 1,526 (7.86%) vs. 166 events out of 1,563 (10.62%) in the 0.9% NSS group (RD= -0.03; 95%CI= -0.07 to 0.01; I2= 69%; p <0.001; Fig. 5). In the studies in which isoosmolar contrast media were used, 117 events out of 1,148 (10.2%) occurred in the SB group vs. 133 events out of 1,149 (11.50%) in the 0.9% NSS group (RD= -0.01; 95% CI= -0.06 to 0.03; I2= 72%; p <0.001; Fig. 5). One study was excluded from the analysis (26) because the type of contrast media used was unspecified.
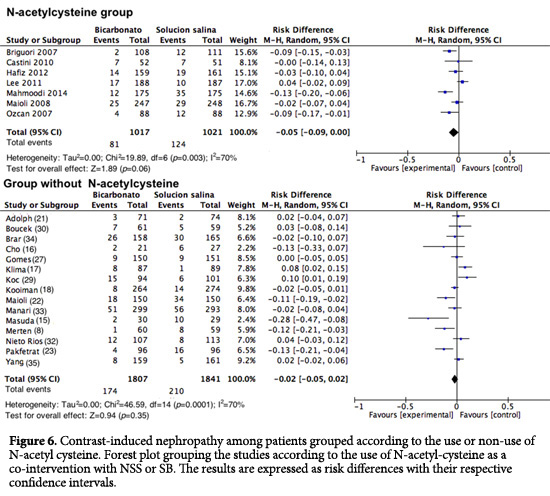
Contrast-induced nephropathy (CIN) in patients who received NAC. Upon analyzing how the studies that included NAC intervention were conducted, for CIN, an RD of 0.05 was found with a 95% CI of -0.09 to 0.00 (I2= 70%; p <0.001; Fig. 6). In the studies in which NAC was not used, no difference was found (RD= -0.02; 95% CI= -0.05 to 0.02; I2= 70%; p <0.001; Fig. 6). There was no analysis of low risk of bias among the studies because only one study could be included in the NAC group.
Meta-regression
A meta-regression analysis was performed to assess whether the quantity of contrast medium could explain the development of CIN across the primary studies. It indicated that the contrast volume did not have a statistically significant effect on the risk of CIN (p= 0.59). In contrast, a statistically significant relationship between diabetes and the risk of CIN was found (p= 0.034). Given that more than 10 studies are required to assess this variable for meta-regression, we were unable to do so with the studies with a low risk of bias because our sample included only 6 such studies.
Secondary outcomes
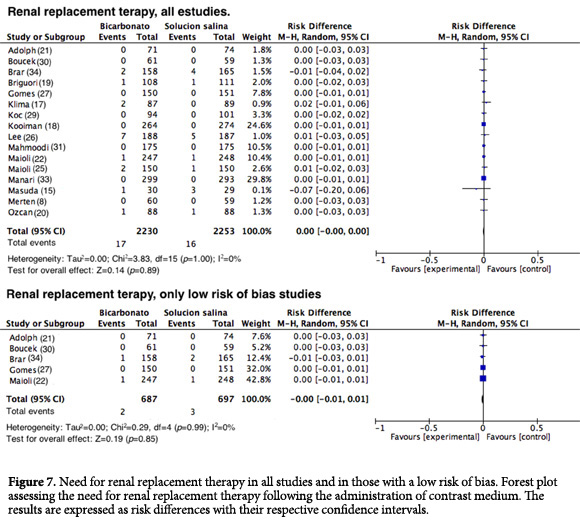
Renal replacement therapy (RRT). Sixteen studies reported the need for RRT after exposure to contrast media. However, such events were rare in all of the studies (RD= 0.00; 95% CI= -0.00 to 0.00; I2= 0%; p= 1; Fig. 7). This result has been previously noted and was expected given that in almost all of the studies that assessed this outcome, no events were reported. Similar results were observed when RRT was assessed in the studies that had a good methodology, with an RD= 0.00 (95% CI= -0001 to 0.01; I2=0%; p= 0.99; Fig. 7).
Forest plot assessing the need for renal replacement therapy following the administration of contrast medium. The results are expressed as risk differences with their respective confidence intervals.
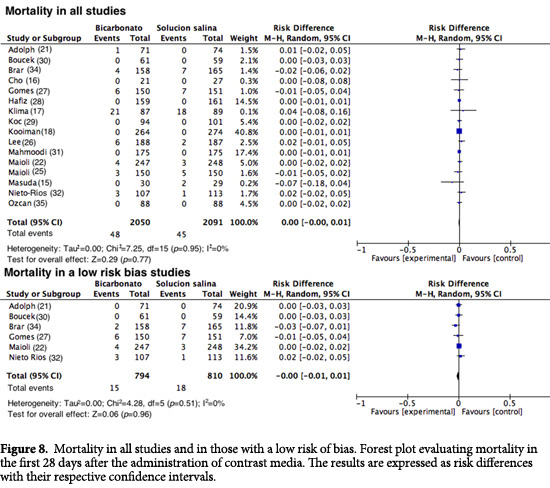
Mortality. Sixteen studies reported mortality among their outcomes. Similar to the outcome of RRT, this event was infrequent among the two groups (RD= 0.00; 95%CI= -0.00 to 0.01; I2= 0%; p= 0.95). When the studies with a good methodological quality were assessed, no statistically significant reduction in risk was found, with an RD= -0.00 (95% CI= -0.001 to 0.001; I2= 0%; p= 0.51; Fig. 8).
Publication bias
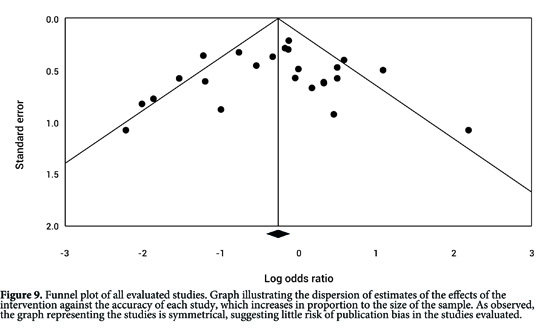
The funnel plot showed little asymmetry among the studies Fig. 9. The Egger regression test generated a value of p= 0.69 (95% CI= -2.11 to 1.44), which indicates a low risk of publication bias among the studies.
Discussion
The number of procedures that require the administration of contrast media has increased significantly in the last decade. For example, in the United States, 10 million patients per year undergo a procedure that requires contrast media36. Moreover, approximately 658,000 persons have a percutaneous coronary intervention annually, amounting to an increase of 326% between 1987 and 20045. However, the use of contrast media is not without risk, and they are categorized as nephrotoxic agents.
The global rate of CIN is close to 150,000 patients per year4. Its incidence oscillates between 0.6 to 3.0% of the general population37 and is as high as 25.0% in high-risk patients, including those with diabetes, a history of congestive heart failure, chronic kidney disease38 advanced age, malnutrition or concomitant use of nephrotoxic drugs (anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor antagonists and aminoglycosides)1,38. Other risk factors reported in the literature are the volume of contrast media and the mode of administration used (arterial vs. intravenous)1,39.
Although CIN is generally defined as a transient impairment of renal function after the administration of contrast media, it is not considered a benign complication; up to 0.8% patients may need to have temporary dialysis, and 13.0% require permanent RRT17. Additionally, the hospital stay is prolonged and medical costs are increased, as is the risk of shortand long-term morbi-mortality26,27. Therefore, studies that focus on strategies for preventing possible complications arising from the use of contrast media have great relevance.
Various pathophysiological mechanisms have been suggested to explain CIN. Under normal conditions, the renal medulla receives little oxygen despite having high metabolic activity for the reabsorption of substances in the S3 segment of the proximal tubules and the thick ascending limb of the loop of Henle. Consequently, mechanisms such as the release of prostaglandins, nitric oxide and adenosine that regulate renal blood flow and provide transtubular transport are required to prevent medullary hypoxia. Contrast media have direct and indirect effects on renal physiology: initially, they cause microvasculature disruption and hemodynamic changes that lead to prolonged intrarenal vasoconstriction, increased vascular resistance, decreased blood flow and osmotic diuresis, increased local oxygen consumption and induced medullary hypoxia. This effect induces the formation of reactive oxygen species that decrease the amount of nitric oxide, and the results are even more pronounced when reactive oxygen species combine with the superoxide anion to form the most powerful oxidizer, peroxynitrite, causing further damage to the endothelial cells. Increased renal vasoconstrictor activity (vasopressin, angiotensin II, dopamine, endothelin and adenosine) and reduced activity of the renal vasodilators (nitric oxide and prostaglandins) are also observed. Furthermore, the injection of contrast medium has a direct cytotoxic effect on the endothelium and renal tubular cells; it causes cell shrinkage, nuclear protrusion, fenestration of the endothelial layer, the formation of microvilli on the cell membrane and apoptosis3,5.
Preventive measures are the best option for all patients with risk factors for developing CIN. Different strategies have been proposed to interrupt the pathophysiology of CIN, such as the use of 1) drugs with antioxidant properties (N-acetyl cysteine [NAC], ascorbic acid, vitamin E, statins, theophylline and sodium bicarbonate), 2) vasodilators (prostaglandins, dopamine and fenoldopam), 3) alkalization (sodium bicarbonate) and 4) peri-procedural intravascular volume expansion with saline solution (NSS)5,18,40. The usefulness of efforts to expand the intravascular space with 0.9% NSS lies in the volume, which blocks the vasoconstrictive effect of contrast on the renal medulla by suppressing the vasopressin secretion that inhibits the reninangiotensin-aldosterone system and increases prostaglandin synthesis. In another way, the use of saline attenuates the direct toxic effects of contrast on the tubular epithelial cells by reducing the tubular reabsorption of salt and water, which allows the dilution of the intratubular fluid and the reduction of the intratubular viscosity, thus reducing the toxic effects. SB alkalinizes the liquid and reduces the rate of intratubular injury from hydroxyl radicals; thus, SB treatment is more beneficial than 0.9% NSS18.
Small randomized studies have shown that nephroprotection with SB initiated one hour before the administration of contrast medium can be useful for preventing CIN41. Merten et al. were the first to report a significant reduction in CIN among patients hydrated with SB (1.7% vs. 13.6% p= 0.02); however, theirs was a singlecenter study with 119 patients, and it ended prematurely with no clear justification8. Another study conducted of 7,977 patients exposed to contrast medium performed in the Rochester Mayo Clinic could not confirm Merten et al., initial finding regarding the protective effect of SB. In contrast, they found an increased rate of CIN in patients who received SB treatment42. In this regard, various systematic reviews and meta-analyses have shown that SB is beneficial in preventing CIN; however, these meta-analyses showed publication bias and significant heterogeneity11,12.
The main result of our meta-analysis, which included 22 randomized controlled clinical trials (n= 5,686 patients), suggests that the administration of SB in high-risk patients exposed to contrast media did not reduce the incidence of CIN, the need for RRT or the rate of death, compared with the use of 0.9% NSS. Additionally, no difference was found in the serum creatinine changes after the administration of contrast media.
When of all studies were analyzed, a summary effect in favor of the use of SB for CIN prevention was found, similar to the findings reported in other meta-analyses43-46. However, many of these studies had a high risk of bias; many did not report the proper conduct and concealment of a random allocation sequence, which may lead to systematic errors within and among studies. Additionally, many of them did not blind the patients, physicians or those assessing the outcomes. Conversely, when only the studies with a low risk of bias were analyzed, the protective effect of SB disappeared, as did the heterogeneity (RD= 0.00; 95% CI= -0.02 to 0.03; p= 0.83; I2= 0%).
Another objective of this study was to evaluate whether the type of contrast used could influence the potential nephroprotective effect of SB. Subgroup analysis showed no significant differences among the patients who received hypo-osmolar contrast (RD= -0.03; 95% CI= -0.07 to -0.01; I2= 69%; p <0.001) and those who received iso-osmolar contrast (DR= -0.01; 95% CI= -0.06 to -0.03; I2= 72%, p <0.001). This finding is consistent with recent studies that have also failed to show a significant difference in the incidence of CIN after the administration of iso-osmolar media vs. low-osmolarity media38,47
The meta-regression analysis aimed to assess whether the volume of contrast used was related to the potential protective effect of SB against the development of CIN. In the studies that reported this variable, it was not possible to establish a direct relationship with SB, while the literature indicated that the volume of contrast used increases the risk of CIN48; however, a proviso must be made that some of these studies did not use the best methodological standards. The association between a history of diabetes mellitus and the risk of CIN was also evaluated; a statistically significant relationship was found (p= 0.034), indicating that diabetes is a risk factor.
In this study, secondary outcomes, such as death, the need for RRT and changes in the creatinine level, showed no improvement with SB use compared with 0.9% NSS use. This may be related to the small number of subjects included in the tests, the design methodology, the insufficient power to detect these differences and the short-term monitoring used. Even after the outcomes were analyzed according to methodological quality, the results did not change.
Study limitations
The major limitation found in this meta-analysis was the poor methodological quality of many of the studies included, which is related to problems of randomization, concealment and blinding. These aspects negatively influenced the estimated effect of different outcomes.
Regarding the inclusion criteria, the definition of chronic kidney disease was very heterogeneous and despite being based on the creatinine value and/or GFR. The range of cutoff points for these variables was very wide and did not take gender, age and body mass into account. Most of the trials included in our study used the elevation of creatinine within 48 h after exposure to contrast medium as the definition of CIN, without considering that the elevation of serum creatinine may occur 4 to 5 days after exposure and therefore the effect of hydration protocols cannot be estimated well.
Another important limitation is the lack of uniformity in the dose and duration of therapy with SB or 0.9% NSS among the different clinical trials. Likewise, the average volume of contrast medium was variable, and none of the studies reported the patients' weights to allow an estimation of the dose per kilogram of body weight. Finally, we believe that these results cannot be generalized, and it must be remembered that the patients included were usually undergoing cardiac procedures. Furthermore, the sample size would not allow a sufficient power, and the monitoring periods of the studies were excessively short.
Conclusions
This meta-analysis of clinical trials showed that the use of SB is not superior to the use of 0.9% NSS, alone or with concomitant use of NAC, to prevent CIN among patients who are exposed to contrast media and have risk factors for CKD. Furthermore, there is no evidence to suggest that either intervention has greater beneficial effects in terms of reducing mortality and the need for RRT. These results should be considered in the context of the marked heterogeneity among the different trials. Thus, further studies with higher power and better standards and protocols are required to allow a meta-analysis of studies with a low risk of bias that can help to determine what the ideal intervention is for preventing CIN.
Conflict of interest:
None of the authors has a business relationship or other type of relationship that may pose a conflict of interest in conducting this study and the publication of its results.
Acknowledgement:
We are grateful for the support of the Pablo Tobón Uribe Hospital in the conduct of this study.
References
1. Chao C-T. Epidemiology, clinical features and diagnosis of contrast induced nephropathy: a brief review. Gen Med (Los Angel). 2013; 1: 102. [ Links ]
2. Mueller C, Buerkle G, Buettner H, Petersen J, Perruchoud A, Eriksson U, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002; 162(3): 329-36. [ Links ]
3. Andreucci M, Faga T, Pisani A, Sabbatini M, Russo D, Michael A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. Scientific World J. 2014; 2014: 823169. [ Links ]
4. Golshahi J, Nasri H, Gharipour M. Contrast-induced nephropathy; A literature review. J Nephropathol. 2014; 3(2): 51-6. [ Links ]
5. Au T, Bruckner A, Mohiuddin S, Hilleman D. The prevention of contrast-induced nephropathy. Ann Pharmacother. 2014; 48(10): 1332-42. [ Links ]
6. Silva RG, Silva NG, Lucchesi F, Burdmann EA. Prevention of contrast-induced nephropathy by use of bicarbonate solution: preliminary results and literature review. J Bras Nefrol. 2010; 32(3): 292-302. [ Links ]
7. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, et al. Prevention of contrast nephropathy by furosemide with matched hydration: The MYTHOS (induced diuresis with matched hydration compared to standard hydration for contrast induced nephropathy prevention) trial. JACC Cardiovasc Interv. 2012; 5(1): 90-7. [ Links ]
8. Merten GJ, Burgess WP, Gray L V, Holleman JH, Roush TS, Kowalchuk GJ, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004; 291(19): 2328-34. [ Links ]
9. Caulfield JL, Singh SP, Wishnok JS, Deen WM, Tannenbaum SR. Bicarbonate inhibits n -nitrosation in oxygenated nitric oxide. J Biol Chem. 1996; 271(42): 25859-63. [ Links ]
10. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Internat Suppl. 2012; 2: 1-138. [ Links ]
11. Kunadian V, Zaman A, Spyridopoulos I, Weiliang Q. Sodium bicarbonate for the prevention of contrast induced nephropathy: A meta-analysis of published clinical trials. Eur J Radiol. 2011; 79(1): 48-55. [ Links ]
12. Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim TH. Sodium bicarbonate therapy for the prevention of contrast-induced acute kidney injury.asistematic review and meta-analysis. Circ J. 2012; 76: 2255-65. [ Links ]
13. Higgins JPT, Green S. Manual Cochrane de revisiones sistemáticas de intervenciones. Version 5.1.0. The Cochrane collaboration.2011. Available from: http://es.cochrane.org/sites/es.cochrane.org/files/uploads/Manual_Cochrane_510_reduit.pdf. [ Links ]
14. Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin (Barc). 2010; 135(11): 507-11. [ Links ]
15. Nieto-Ríos JF, Salazar WAM, Sánchez OMS, Ortega JLJ, Caro JIG, Aristizabal JMA, et al. Prevention of contrast induced nephropathy with sodium bicarbonate (the PROMEC study). J Bras Nefrol. 2014; 36(3): 360-6. [ Links ]
16. Masuda M, Yamada T, Mine T, Morita T, Tamaki S, Tsukamoto Y, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007; 100(5): 781-6. [ Links ]
17. Cho R, Javed N, Traub D, Kodali S, Atem F, Srinivasan V. Oral hydration and alkalinization is noninferior to intravenous therapy for prevention of contrast-induced nephropathy in patients with chronic kidney disease. J Interv Cardiol. 2010; 23(5): 460-6. [ Links ]
18. Klima T, Christ A, Marana I, Kalbermatter S, Uthoff H, Burri E, et al. Sodium chloride vs. sodium bicarbonate for the prevention of contrast medium-induced nephropathy: a randomized controlled trial. Eur Heart J. 2012; 33(16): 2071-9. [ Links ]
19. Kooiman J, Sijpkens YWJ, de Vries J-PPM, Brulez HFH, Hamming JF, van der Molen AJ, et al. A randomized comparison of 1-h sodium bicarbonate hydration versus standard peri-procedural saline hydration in patients with chronic kidney disease undergoing intravenous contrast-enhanced computerized tomography.Nephrol Dial Transplant. 2014; 29(5): 1029-36. [ Links ]
20. Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, et al. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007; 115(10): 1211-7. [ Links ]
21. Ozcan EE, Guneri S, Akdeniz B, Akyildiz IZ, Senaslan O, Baris N, et al. Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy. A comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures. A single-center prospective controlled. Am Heart J. 2007; 154(3): 539-44. [ Links ]
22. Adolph E, Holdt-Lehmann B, Chatterjee T, Paschka S, Prott A, Schneider H, et al. Renal insufficiency following radiocontrast exposure trial (REINFORCE): a randomized comparison of sodium bicarbonate versus sodium chloride hydration for the prevention of contrast-induced nephropathy. Coron Artery Dis. 2008; 19(6): 413-9. [ Links ]
23. Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008; 52(8): 599-604. [ Links ]
24. Pakfetrat M, Nikoo MH, Malekmakan L, Tabandeh M, Roozbeh J, Nasab MH, et al. A comparison of sodium bicarbonate infusion versus normal saline infusion and its combination with oral acetazolamide for prevention of contrast-induced nephropathy: a randomized, double-blind trial. Int Urol Nephrol. 2009; 41(3): 629-34. [ Links ]
25. Castini D, Lucreziotti S, Bosotti L, Salerno Uriarte D, Sponzilli C, Verzoni A, et al. Prevention of contrast-induced nephropathy: a single center randomized study. Clin Cardiol. 2010; 33(3): E63-8. [ Links ]
26. Maioli M, Toso A, Leoncini M, Micheletti C, Bellandi F. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv. 2011; 4(5): 456-62. [ Links ]
27. Lee S-W, Kim W-J, Kim Y-H, Park S-W, Park D-W, Yun S-C, et al. Preventive strategies of renal insufficiency in patients with diabetes undergoing intervention or arteriography (the PREVENT Trial). Am J Cardiol. 2011;107(10):1447-52. [ Links ]
28. Gomes VO, Lasevitch R, Lima VC, Jr FSB, Perez-alva JC, Moulin B, et al. Hydration with Sodium Bicarbonate Does Not Prevent Contrast Nephropathy?: A Multicenter Clinical Trial. Arq Bras Cardiol. 2012; 99(6): 1129-34. [ Links ]
29. Hafiz AM, Jan MF, Mori N, Shaikh F, Wallach J, Bajwa T, et al. Prevention of contrast-induced acute kidney injury in patients with stable chronic renal disease undergoing elective percutaneous coronary and peripheral interventions: randomized comparison of two preventive strategies. Catheter Cardiovasc Interv. 2012; 79(6): 929-37. [ Links ]
30. Koc F, Ozdemir K, Altunkas F, Celik A, Dogdu O, Karayakali M, et al. Sodium bicarbonate versus isotonic saline for the prevention of contrast-induced nephropathy in patients with diabetes mellitus undergoing coronary angiography and/or intervention: a multicenter prospective randomized study. J Investig Med. 2013; 61(5): 872-7. [ Links ]
31. Boucek P, Havrdova T, Oliyarnyk O, Skibova J, Pecenkova V, Pucelikova T, et al. Prevention of contrast-induced nephropathy in diabetic patients with impaired renal function: a randomized, double blind trial of sodium bicarbonate versus sodium chloride-based hydration. Diabetes Res Clin Pract. 2013; 101(3): 303-8. [ Links ]
32. Mahmoodi K, Sohrabi B, Ilkhchooyi F, Malaki M, Khaniani ME, Hemmati M. The efficacy of hydration with normal saline versus hydration with sodium bicarbonate in the prevention of contrast-induced nephropathy. Heart Views. 2014; 15(2): 33-6. [ Links ]
33. Manari A, Magnavacchi P, Puggioni E, Vignali L, Fiaccadori E, Menozzi M, et al. Acute kidney injury after primary angioplasty: effect of different hydration treatments. J Cardiovasc Med (Hagerstown). 2014; 15(1): 60-7. [ Links ]
34. Brar SS, Yuh-Jer A, Jorgensen MB, Kotlewski A, Aharonian V, Desai N, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast undergoing coronary angiography. JAMA. 2008; 300(9): 1038-146. [ Links ]
35. Yang K, Liu W, Ren W, Lv S. Different interventions in preventing contrast-induced nephropathy after percutaneous coronary intervention. Int Urol Nephrol. 2014; (2):1801-7. [ Links ]
36. Sanaei-Ardekani M, Movahed M-R, Movafagh S GN. Contrast-induced nephropathy: a review. Cardiovasc Revasc Med. 2005; 6(2): 82-8. [ Links ]
37. Bouzas-Mosquera A, Vázquez-Rodríguez JM, Calviño-Santos R, Peteiro-Vázquez J, Flores-Ríos X, Marzoa-Rivas R, et al. Contrast-induced nephropathy and acute renal failure following emergent cardiac catheterization: incidence, risk factors and prognosis. Rev Esp Cardiol. 2007; 60(10): 1026-34. [ Links ]
38. Wessely R, Koppara T, Bradaric C, Vorpahl M, Braun S, Schulz S, et al. Choice of contrast medium in patients with impaired renal function undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2009; 2(5): 430-7. [ Links ]
39. Au TH, Bruckner A, Mohiuddin SM, Hilleman DE. The prevention of contrast-induced nephropathy. Ann Pharmacother. 2014; 48(10): 1332-42. [ Links ]
40. Vasheghani-farahani A, Sadigh G. Sodium bicarbonate in preventing contrast nephropathy in patients at risk for volume overload?: a randomized controlled trial. J Nephrol. 2010; 23(2): 216-23. [ Links ]
41. Sanaei-Ardekani M, Movahed M, Movafagh S, Ghahramani N. Contrast-induced nephropathy: a review. Cardiovasc Revasc Med. 2005; 6(2): 82-8. [ Links ]
42. From AM, Bartholmai BJ, Williams AW, Cha SS, Pflueger A, McDonald FS. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at mayo clinic. Clin J Am Soc Nephrol. 2008; 3: 10-8. [ Links ]
43. Kanbay M, Covic A, Coca SG, Turgut F, Akcay A, Parikh CR. Sodium bicarbonate for the prevention of contrast-induced nephropathy: a meta-analysis of 17 randomized trials. Int Urol Nephrol. 2009; 41(3): 617-27. [ Links ]
44. Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2009; 53(4): 617-27. [ Links ]
45. Meier P, Ko DT, Tamura A, Tamhane U, Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 2009; 7: 23. [ Links ]
46. Trivedi H, Nadella R, Szabo A. Hydration with sodium bicarbonate for the prevention of contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Clin Nephrol. 2010; 74(4): 288-96. [ Links ]
47. Bolognese L, Falsini G, Schwenke C, Grotti S, Limbruno U, Liistro F. Impact of iso-osmolar versus low-osmolar contrast agents on contrast-induced nephropathy and tissue reperfusion in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2012; 109: 67-74. [ Links ]
48. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008; 51: 1419-28. [ Links ]