Remark
| 1) Why was this study conducted? |
| Contact investigation is a key strategy in tuberculosis control programs. Children who are contacts of pulmonary tuberculosis cases have a higher risk of developing active tuberculosis. However, childhood tuberculosis is still a major public health issue, and there was not much population data from exposed children to tuberculosis in Colombia. |
| 2) What were the most relevant results of the study? |
| Household tuberculosis exposed children have high annual risks of infection and disease development. BCG vaccination protection is reasserted for moderate prevalence conditions, especially for the youngest. Tuberculosis transmission chain is not being cut in time and this limits control efficacy. |
| 3) What do these results contribute? |
| First study in Colombia that assessed risk of tuberculosis (infection and disease) among household contact children. Findings of present study allowed to prolong the follow-up time to household contacts seeking active disease, and promoting isoniazid preventive therapy in guidelines for children age <5 years in Colombia. |
Introduction
Tuberculosis in children is considered a public health emergency that affects mainly low-income countries 1. In these countries, it is estimated to represent 15% of reported tuberculosis cases 2-4. The pathogenesis and epidemiology of childhood tuberculosis are still poorly understood, possibly because most of the actions in tuberculosis prevention, diagnostic, and therapeutic options are focused mainly on adults 1. Further, there are surveillance gaps. However, since 2011 WHO estimates tuberculosis incidence for patients <15 years of age, accounting 1.01 million (10%) of the 10 million new tuberculosis cases reported worldwide in 2017, 234,000 of which died 5.
In Colombia, 16,000 new tuberculosis cases were estimated in 2017, 11.3% of which would be children 6. However, only 12,439 cases were reported, 598 of which were <15 years old, and represented 4.8% of total reported cases 7.
Tracing contacts of patients with microbiologically confirmed tuberculosis is one of the main strategies to control this disease. It aims early identification of infected individuals in order to initiate preventive therapy, especially in those most at risk of developing active disease such as children <5 years old 4,8. This strategy has proved to be cost-effective for the detection of new cases 9 when combined with the required access to diagnosis and treatment 10.
Children household contacts of adult tuberculosis patients who become infected but do not develop active childhood tuberculosis (primary tuberculosis), represent the main reservoir for tuberculosis transmission once reactivation occurs in adulthood (post-primary tuberculosis) 1. In a given community, a decrease in the number of children with active tuberculosis is an indicator of transmission decrease because they usually get infected within the nuclear family 11.
Previous studies showed that among children (<15 years of age) in close contact with patients with pulmonary tuberculosis, the proportion of active tuberculosis cases can range from 6.0% to 8.0%, being higher in children <5 years. Also, the proportion of children with tuberculosis infection is estimated at 40.4% (95% CI: 38.7-42.2%) being lower in children <5 years 12.
This study, the first of its kind in Colombia, aimed to assess the risk of tuberculosis infection and disease in children <15 years old household contacts of adult patients with tuberculosis disease through a cohort study in three major Colombian cities.
Materials and Methods
Study design and setting
A cohort study of children household contacts of patients with pulmonary tuberculosis in three Colombian cities (Medellín’s metropolitan area, Cali, and Popayán -including five small towns nearby-) was performed between 2005 and 2009. In this study were included the children household contacts of 382 adult index cases with smear-positive pulmonary tuberculosis recruited (275 from Medellín, 77 from Cali and 30 from Popayán).
Between 2005-2009 Medellín reported a tuberculosis incidence of 43.2-50.4 cases per 100,000 13, in Cali was 46-43 cases per 100,000 14 and in Popayán 24.5-12.4 15,16 cases per 100,000. During 2005, characteristics of these cities included population size of 2,219,861 in Medellín, 2,075,380 in Cali, and 258,653 in Popayán; and an Unsatisfied Basic Necessities indicator of 12.42 in Medellín, 11.01 in Cali and 18.07 in Popayán 17. The sample size was calculated assuming an estimated infection prevalence of 50%, an active tuberculosis incidence rate of 5%, confidence of 95%, power of 80% and over sampling of 25% to compensate for loss to follow-up. This resulted in a sample size of 2,816 household contacts of all ages.
Participants
A child household contact was defined as an individual who had spent time regularly every week with an index case in the same household, for at least one month before the confirmation of the tuberculosis case. An index case was defined as the first tuberculosis case identified in each household, diagnosed with active pulmonary, smear-positive tuberculosis. (Adapted from CDC) (18.
Recruitment conditions for index cases and children household contacts were the same for all cities, as previously published about Medellin 19. Briefly, once an adult case was notified to the local tuberculosis control program, the patient and children household contacts were invited to participate in the study, followed by the signature of informed consent.
Follow-up
After index cases were identified and they approved a first (baseline) visit, the research team went to the household to explain the study and obtain written informed consent from parents or responsible adult guardians, to collect socio-demographic and exposure information, and to perform the tuberculin skin test. Follow-up occurred throughout visits every six months and telephone calls every three months. The total follow-up time was at least 24 months. Also, local epidemiological surveillance databases were checked for active tuberculosis cases reported.
Mycobacterium tuberculosis was confirmed by culture in liquid (BD Bactec MGIT960) and/or solid media (Lowenstein-Jensen) from sputum (in adults) and gastric aspirate (in children) for all index and incident cases. Tuberculin skin test was done by intradermal injection of 2 IU of RT23 tuberculin (Staten Serum Institute, Copenhagen, Denmark) in the inner surface of the forearm. Tuberculin skin test reading was done 48 to 72 hours after injection and was considered positive when measured induration was ≥10 mm, according to the Colombian National Tuberculosis Program. Following the recommendations of the American Thoracic Society 20, an analysis of tuberculin skin test positivity using a cutoff of 5 mm of induration was also included. The annual risk of infection was determined through the tuberculin skin test conversion of a child household contact with a negative tuberculin skin test at baseline. The conversion was defined as an increase of at least 6 mm of induration a year after the initial test 21.
For detecting infection, the tuberculin skin test was available to perform in 458 (44%) of the household contacts <15 years old from whom were willing to attended from Cali and Popayán, and an incidental sample from Medellín. From protocol design, participants from Medellín were assessed with an in-house interferon-gamma, data related to the immune response to M. tuberculosis, and incident tuberculosis in household contacts (adults and children) was published previously for the Medellín cohort 19.
Nutritional status was evaluated according to Z score for weight and height as follows: between -1 and 1, normal; less than -1, underweight; and score >1, overweight 22. Socioeconomic strata were recorded according to classifications used by local public services provider and categorized as low socioeconomic strata yes or not. Proximity to the index case was classified as follows: children household contacts slept in other households, children household contacts slept in the same household, children household contacts slept in the same room. Crowding was considered when three or more persons shared the same bedroom 19. Having other relatives with tuberculosis was defined as an additional contact with a tuberculosis case besides the index case.
Incident active tuberculosis cases were diagnosed following the Stop tuberculosis Partnership Childhood tuberculosis subgroup’s guidelines 23, considering contact history, clinical, immunological, microbiological, and radiographic criteria. Evaluation of children in Cali was performed through a diagnostic algorithm 24. This tool was not applied for Medellín and Popayán´s cohorts.
Statistical analysis
Social, demographic and clinical characteristics, as well as the degree of proximity to the index case, were compared using the distribution of absolute and relative frequencies. Also, the chi-square test was calculated to establish significance (p <0.05).
The tuberculin skin test positivity and its association with children’s characteristics were analyzed using a prevalence ratio (PR). Adjusted PR was estimated using binomial regression. The annual risk of infection and its association with the index case’s characteristics were calculated using relative risk (RR) adjusted in a binomial regression. Incidence estimated for active TB was calculated for each city and four age groups (<1-year-old, 1-4 years old, 5-9 and 10-14), as well as for other household associate characteristics by using the hazard ratio (HR). HR was adjusted by a Cox regression. Age groups were selected based on described children’s tuberculosis risk and immune responses25. Finally, two multivariate models were built using BCG vaccination status (evidenced by the presence of a vaccine’s scar), given that an additive interaction was found when using a stratified Mantel-Haenzel method. The Kaplan-Meir method was used to estimate time to the disease for each age group. Every association measure and multivariate model was adjusted by cluster, given that outcomes (positive tuberculin skin test and active tuberculosis) could be influenced by natural clusters such as family and city, 95% confidence interval (95% CI) was estimated. p values <0.05 were considered significant. Analyses were done using Stata 12.0, SPSS 22.0, and Epidat 3.1.
Ethical aspects
This study, including the informed consent used, was approved by the Ethical Committee of Facultad de Medicina, Universidad de Antioquia, and it applied for each city. The study was also supported by the National Tuberculosis Program and the regional public health authorities. At the time of data collection, national health policies only included the recommendation of isoniazid preventive therapy for children <5 years old with tuberculin skin test >10 mm without BCG vaccination. Most children involved in the study did not receive isoniazid preventive therapy because the majority were vaccinated. Active tuberculosis cases received tuberculosis treatment according to National Tuberculosis Program.
Results
Of the total household contacts, 1,040 were children <15 years’ old, included in this analysis. 742 (71.3%) from Medellín, 216 (20.8%) from Cali and, 82 (7.9%) from Popayán. Children represented 35% of household contacts in the overall cohort study. The demographic and epidemiological characteristics of the 1,040 children included in this study are shown in Table 1. Most children belonged to low socioeconomic strata (79.2%), had a BCG vaccination scar (80.6%), and were household contacts of an index case with a high bacillary load. From the whole cohort, 1.8% of Medellín, 4.8% from Cali, and 1.7% from Popayán were voluntarily withdrawn; and 1.0% from Medellín, 5.0% from Cali and 1.3% from Popayán were lost of follow-up.
Table 1 Characteristics of children (<15 years old) household contacts of adult patients with pulmonary tuberculosis (TB) by city, in three Colombian cities, 2005-2009.
| Characteristic | Medellín | Cali | Popayán | Total |
|---|---|---|---|---|
| n= 742 (%) | n= 216 (%) | n= 82 (%) | n= 1040 (%) | |
| Age in years | ||||
| <1 | 30 (4.0) | 9 (4.2) | 7 (8.5) | 46 (4.4) |
| 1-4 | 207 (27.9) | 59 (27.3) | 31 (37.8) | 297 (28.6) |
| 5-9 | 241 (32.5) | 72 (33.3) | 28 (34.1) | 341 (32.8) |
| 10-14 | 264 (35.6) | 76 (35.2) | 16 (19.5) | 356 (34.2) |
| Sex | ||||
| Female | 365 (49.2) | 97 (44.9) | 39 (47.6) | 501 (48.2) |
| Low socioeconomic stratum | ||||
| Yes | 551 (74.3) | 184 (85.2) | 80 (97.6) | 815 (78.4) |
| No information | 8 | 3 | 0 | 11 |
| Nutritional condition | ||||
| Normal | 427 (57.5) | 119 (55.1) | 46 (56.1) | 592 (56.9) |
| Underweight | 178 (24.0) | 57 (26.4) | 17 (20.7) | 252 (24.2) |
| Overeight | 129 (17.4) | 37 (17.1) | 19 (23.2) | 185 (17.8) |
| No information | 8 | 3 | 0 | 11 |
| BCG scar | ||||
| Yes | 626 (84.4) | 138 (63.9) | 69 (84.1) | 833 (80.1) |
| No information | 6 | 1 | 0 | 7 |
| History of another relative with TB* | ||||
| Yes | 256 (34.5) | 85 (39.4) | 37 (45.1) | 378 (36.3) |
| Proximity to the index case | ||||
| HHC slept in other household | 171 (23.0) | 36 (16.7) | 15 (18.3) | 222 (21.3) |
| HHC slept in same household | 340 (45.8) | 111 (51.4) | 38 (46.3) | 489 (47.0) |
| HHC slept in same room | 231 (31.1) | 69 (31.9) | 29 (35.4) | 329 (31.6) |
| Persons per room | ||||
| < 3 | 493 (66.8) | 137 (64.3) | 49 (59.8) | 679 (65.7) |
| ≥ 3 | 245 (33.2) | 76 (35.7) | 33 (40.2) | 354 (34.3) |
| Sputum smear load | ||||
| + | 287 (38.7) | 54 (25.1) | 18 (22.0) | 359 (34.6) |
| ++ | 192 (25.9) | 78 (36.3) | 33 (40.2) | 303 (29.2) |
| +++ | 263 (35.4) | 83 (38.6) | 31 (37.8) | 377 (36.3) |
| Exposure time | ||||
| ≤ 3 months | 487 (65.8) | 166 (76.9) | 49 (59.8) | 702 (67.6) |
| > 3 months | 253 (34.2) | 50 (23.1) | 33 (40.2) | 336 (32.4) |
HHC: household contact
* History of another relative with TB: had had any other relative with tuberculosis besides the identified index case in the cohort.
From the 458 tuberculin skin test performed, 205 were performed in Cali, 81 in Popayán, and 172 in Medellín. The tuberculin skin test at baseline was positive (≥10 mm) in 43.7% (95% CI: 39.2-48.2) of tested children. Regardless of cutoff, factors associated with having a positive tuberculin skin test were age 10-14 years and the presence of a BCG scar (p <0.05). Underweight, being a household contact for more than 3 months, and closer proximity to the index case were associated with a higher prevalence of a positive tuberculin skin test (≥10 mm). The same factors showed a similar trend, although not significant when a cutoff point of 5 mm was applied (Table 2). An association trend was found between both tuberculin skin test cutoff points and proximity to the index case (chi-square test for trend p <0.01).
Table 2 Associated characteristics to positive tuberculin skin test prevalence in children (<15 years old) household contacts of adult patients with pulmonary tuberculosis (TB), in three Colombian cities, 2005-2009.
| Characteristic | N | Positive TST ( ≥5mm) | Positive TST ( ≥10 mm) | ||||
|---|---|---|---|---|---|---|---|
| n (%) | PR | (95%CI)* | n (%) | PR | (95%CI)* | ||
| Age in years n(%) | |||||||
| <1 | 19 | 7 (36.8) | 0.81 | (0.4 - 1.6) | 2 (10.5) | 0.37 | (0.1 - 1.4) |
| 1-4 | 137 | 66 (48.2) | 1 | (0.8 - 1.3) | 55 (40.1) | 1.03 | (0.8 - 1.3) |
| 5-9 | 153 | 74 (48.4) | 1 | 59 (38.6) | 1 | ||
| 10-14 | 149 | 96 (64.4) | 1.3 | (1.1 - 1.6)† | 84 (56.4) | 1.43 | (1.1 - 1.9)‡ |
| Sex n (%) | |||||||
| Male | 244 | 125 (51.2) | 1 | 105 (43.0) | 1 | ||
| Female | 214 | 118 (55.1) | 1.17 | (1.0 - 1.4) | 95 (44.4) | 1.17 | (1.0 - 1.4) |
| Low socioeconomic stratum | |||||||
| No | 54 | 24 (44.4) | 1 | 23 (42.6) | 1 | ||
| Yes | 401 | 217 (54.1) | 1.04 | (0.7 - 1.7) | 175 (43.6) | 0.84 | (0.5 - 1.4) |
| Nutritional condition | |||||||
| Normal | 255 | 139 (54.5) | 1 | 111 (43.5) | 1 | ||
| Underweight | 125 | 68 (54.4) | 1.07 | (0.9 - 1.3) | 61 (48.8) | 1.27 | (1.0 - 1.6)† |
| Overeight | 75 | 35 (46.7) | 0.96 | (0.7 - 1.3) | 28 (37.3) | 0.97 | (0.7 - 1.4) |
| BCG scar | |||||||
| No | 109 | 48 (44.0) | 1 | 35 (32.1) | 1 | ||
| Yes | 346 | 193 (55.8) | 1.34 | (1.0 - 1.7)† | 163 (47.1) | 1.52 | (1.1 - 2.1)† |
| History of another relative with TB** | |||||||
| No | 289 | 152 (52.6) | 1 | 122 (42.2) | 1 | ||
| Yes | 169 | 91 (53.8) | 1.05 | (0.8 - 1.3) | 78 (46.2) | 1.1 | (0.8 - 1.4) |
| Proximity to the index case | |||||||
| HHC slept in other household | 99 | 42 (42.4) | 1 | 27 (27.3) | 1 | ||
| HHC slept in same household | 224 | 119 (53.1) | 1.25 | (0.9 - 1.7) | 100 (44.6) | 1.61 | (1.1 - 2.4)† |
| HHC slept in same room | 135 | 82 (60.7) | 1.34 | (1.0 - 1.8) | 73 (54.1) | 1.79 | (1.1 - 2.8)† |
| Persons per room | |||||||
| <3 | 289 | 137 (47.4) | 1 | 108 (37.4) | 1 | ||
| ≥3 | 166 | 104 (62.6) | 1.27 | (0.9 - 1.8) | 90 (54.2) | 1.44 | (0.9 - 2.2) |
| Sputum smear load | |||||||
| + or ++ | 296 | 161 (54.4) | 1 | 129 (43.6) | 1 | ||
| +++ | 161 | 82 (50.9) | 1.07 | (0.8 - 1.4) | 71 (44.1) | 1.23 | (0.9 - 1.7) |
| Exposure time | |||||||
| ≤3 months | 303 | 146 (48.2) | 1 | 115 (38.0) | 1 | ||
| >3 months | 155 | 97 (62.6) | 1.24 | (1.0 - 1.6) | 85 (54.8) | 1.37 | (1.0 - 1.8)† |
TST: Tuberculin Skin Test. HHC: household contact.
* PR: Prevalence Ratio adjusted by all the other variables in the table and adjusted by family and city intraclass correlation.
** History of another relative with TB: had had any other relative with tuberculosis besides the identified index case in the cohort.
† p <0.05
‡ p <0.01
For those children with a baseline tuberculin skin test <5 mm (173 children), the M. tuberculosis annual risk of infection was 12.1% (95% CI: 7.2-17.1), whereas in the group of children with initial tuberculin skin test <10 mm (206 children) annual risk of infection was 17% (95% CI: 11.8-22.2). A significant correlation was found between M. tuberculosis annual risk of infection and a higher bacillary load of the index case for those with an initial tuberculin skin test <5 mm (RR= 2.61, 95% CI: 1.2-5.9, p= 0.020 adjusted by age, low socioeconomic stratum, history of another relative with tuberculosis and exposure time) as well as for those with an initial tuberculin skin test <10 mm (RR= 2.12, 95% CI: 1.0-4.3, p= 0.037 adjusted by age and nutritional condition) (Table 3).
Table 3 Associated characteristics to Annual Risk of Infection in children household contacts of adult patients with pulmonary tuberculosis (TB), in three Colombian cities, 2005-2009.
| Characteristic | ARI (baseline TST <5 mm)* | ARI (baseline TST <10 mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| N=173 | n (%) | RR | (95%CI)** | N=206 | n (%) | RR | (95%CI)** | |
| Age in years | ||||||||
| <5 | 68 | 5 (7.4) | 1 | 80 | 9(11.3) | 1 | ||
| 5-14 | 105 | 16 (15.2) | 2.07 | (0.8-5.3) | 126 | 26(20.6) | 1.83 | (0.9-3.6) |
| Sex | ||||||||
| Male | 97 | 10 (10.3) | 1 | 111 | 20(18.0) | 1 | ||
| Female | 76 | 11 (14.5) | 1.4 | (0.7-3.0) | 95 | 15(15.8) | 0.88 | (0.5-1.5) |
| Low socioeconomic stratum | ||||||||
| No | 25 | 5 (20.0) | 1 | 26 | 5(19.2) | 1 | ||
| Yes | 147 | 15 (10.2) | 0.51 | (0.2-1.4) | 179 | 29(16.2) | 0.84 | (0.3-2.3) |
| Nutritional condition | ||||||||
| Normal | 89 | 11 (12.4) | 1 | 111 | 22(19.8) | 1 | ||
| Underweight | 51 | 7 (13.7) | 1.11 | (0.5-2.5) | 55 | 8(14.5) | 0.73 | (0.4-1.5) |
| Overweight | 31 | 2 (6.5) | 0.52 | (0.1-1.9) | 37 | 4(10.8) | 0.55 | (0.2-1.4) |
| BCG scar | ||||||||
| No | 54 | 7 (13.0) | 1 | 62 | 11(17.7) | 1 | ||
| Yes | 118 | 14 (11.9) | 0.92 | (0.4-2.1) | 143 | 24(16.8) | 0.95 | (0.5-1.9) |
| History of another relative with TB† | ||||||||
| No | 114 | 17 (14.9) | 1 | 136 | 25(18.4) | 1 | ||
| Yes | 59 | 4 (6.8) | 0.46 | (0.2-1.4) | 70 | 10(14.3) | 0.78 | (0.3-1.8) |
| Proximity to the index case | ||||||||
| HHC slept in other household | 47 | 6 (12.8) | 1 | 59 | 11(18.6) | 1 | ||
| HHC slept in same household | 126 | 15 (11.9) | 0.93 | (0.4-2.5) | 147 | 24(16.3) | 0.88 | (0.4-1.8) |
| Persons per room | ||||||||
| <3 | 115 | 15 (13.0) | 1 | 136 | 26(19.1) | 1 | ||
| ≥3 | 57 | 6 (10.5) | 0.81 | (0.3-2.4) | 69 | 9(13.0) | 0.68 | (0.3-1.6) |
| Sputum smear load | ||||||||
| + or ++ | 109 | 9 (8.3) | 1 | 132 | 17(12.9) | 1 | ||
| +++ | 63 | 12 (19.0) | 2.31 | (0.9-5.7) | 73 | 18(24.7) | 1.91 | (0.9-3.9) |
| Exposure time | ||||||||
| ≤3 months | 131 | 17 (13.0) | 1 | 155 | 24(15.5) | 1 | ||
| >3 months | 42 | 4 (9.5) | 0.73 | (0.2-2.6) | 51 | 11(21.6) | 1.39 | (0.6-3.0) |
TST: Tuberculin skin Test. ARI: Annual Risk of Infection (increase ≥6 mm). HHC: household contact.
* Does not include 33 children with a baseline TST ≥10 mm
** RR: Risk Ratio adjusted by family and city intraclass correlation.
† History of another relative with TB: had had any other relative with tuberculosis besides the identified index case in the cohort.
‡ p<0.05
Nineteen children developed active tuberculosis during follow-up (7.2%, 95% CI: 3.9-10.5), among those with an initial tuberculin skin test ≥5 mm (18/243) or an increase of 6 mm of induration per year (1/21). Active tuberculosis incidence was higher in children without BCG scar (8/55, 14.5%, 95% CI: 7.3-26.4) than in those with a BCG scar (11/207, 5.3% 95% CI: 2.9-9.4) (p= 0.033). Among children without documented tuberculosis infection (initial tuberculin skin test <5 mm and less than 6 mm induration increase per year), incidence of active tuberculosis was 0.7% (1/152, 95% CI: 0.02-3.6). Figure 1 shows the number of children who developed active tuberculosis according to the result of the initial tuberculin skin test and the increase of tuberculin skin test induration per year.
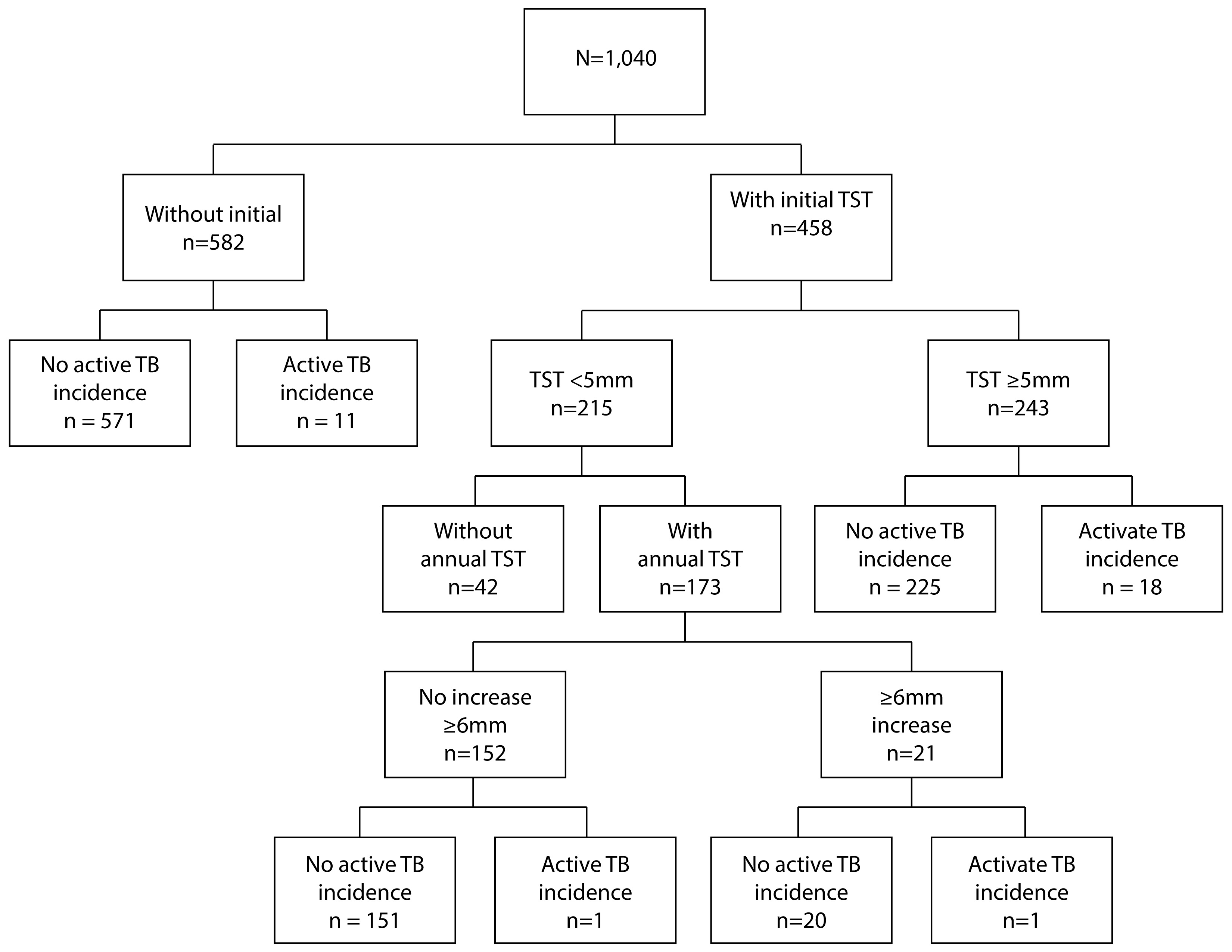
Figure 1 Flow chart of active tuberculosis cases by initial tuberculin skin test result (cutoff point 5 mm) and one year later (infection incidence: increase of 6 mm or more compare to initial tuberculin skin test). TST: Tuberculin Skin Test. TB: tuberculosis.
The general incidence rate for active tuberculosis was 12.4 per 1,000 persons-year. As shown in Figure 2, the rate at which active tuberculosis develops differs according to age (Log-rank=10.10, p= 0.006). In particular, early development of active tuberculosis was found more frequently in children without BCG scar HR= 6.00 (95% CI: 1.3-28.3 adjusted by the history of another relative with tuberculosis, proximity to the index case, and persons per room). Also, a positive association was found between crowding and the early development of active tuberculosis, but it was not significant when the BCG scar was considered. Having an initial tuberculin skin test ≥5 mm had a significant positive association with the earlier development of active tuberculosis (tuberculin skin test 5-9 mm HR= 8.55, 95% CI: 2.5-29.2; tuberculin skin test ≥10 mm HR= 8.16, 95% CI: 2.0-32.9) (Table 4).
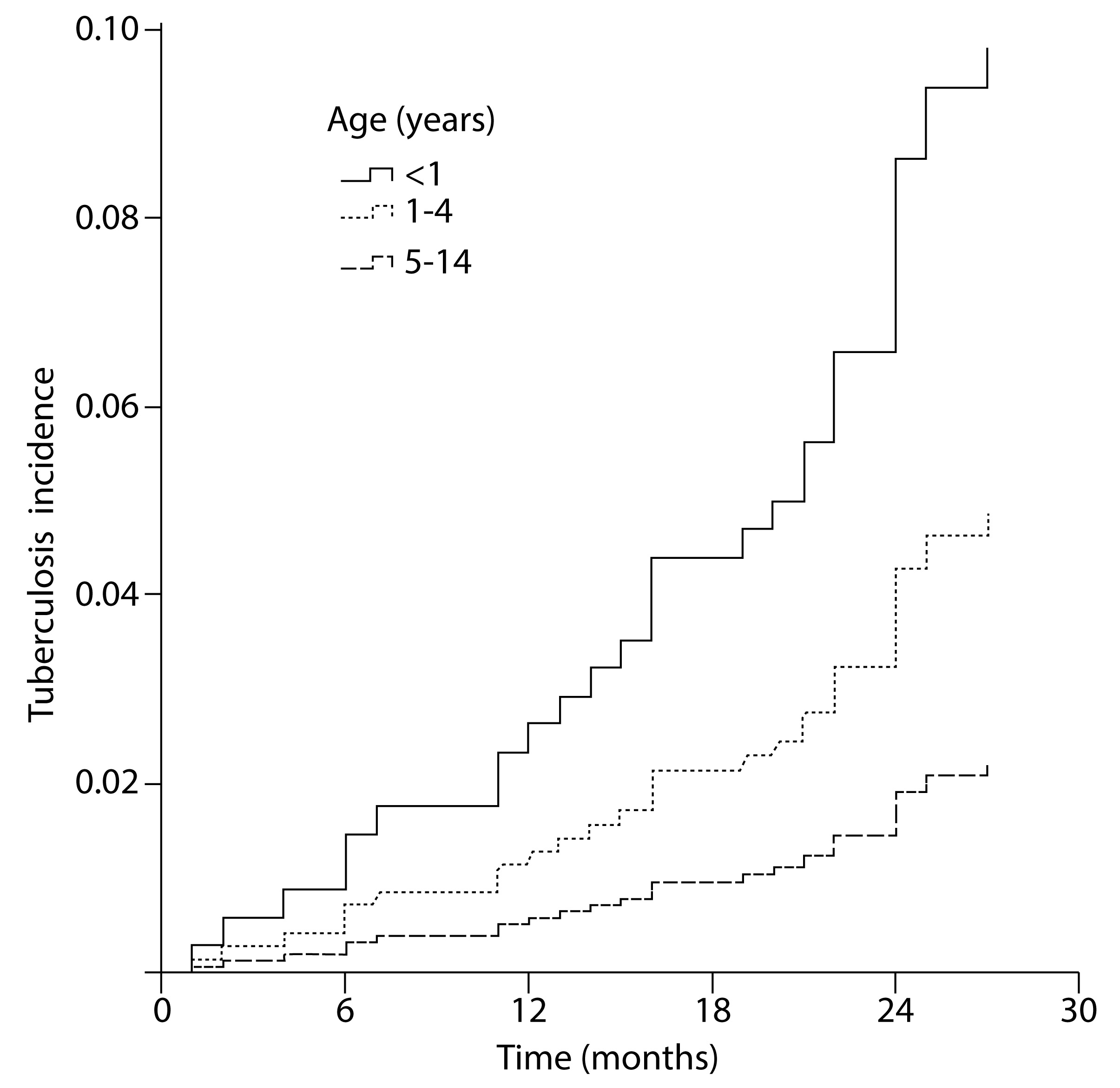
Figure 2 Risk to develop active tuberculosis by age group in children household contacts of adult patients with pulmonary tuberculosis, in three Colombian cities, 2005-2009. Log-rank= 10.10, p= 0.006. Age groups 5-9 and 10-14 years were grouped in one because they had a similar trend.
Table 4 Associated characteristics to active tuberculosis (TB) incidence in children household contacts of adult patients with pulmonary tuberculosis, in three Colombian cities, 2005-2009.
| Characteristics | TB incidence | Total | HR | (95%CI)* § |
|---|---|---|---|---|
| n (%) | N | |||
| Age in years n(%) | ||||
| <5 | 17 (5.0) | 343 | 2.55 | (1.3-4.9)‡ |
| 5-14 | 14 (2.0) | 697 | 1 | |
| Sex n (%) | ||||
| Male | 16 (3.0) | 539 | 1 | |
| Female | 15 (3.0) | 501 | 0.99 | (0.5-1.9) |
| Low socioeconomic stratum | ||||
| No | 4 (1.9) | 214 | 1 | |
| Yes | 26 (3.2) | 815 | 1.69 | (0.5-6.1) |
| Nutritional condition | ||||
| Normal | 18 (3.0) | 592 | 1 | |
| Underweight | 6 (2.4) | 252 | 0.8 | (0.3-2.0) |
| Overeight | 6 (3.2) | 185 | 1.04 | (0.3-3.3) |
| BCG scar | ||||
| No | 12 (6.0) | 200 | 1 | |
| Yes | 19 (2.3) | 833 | 0.37 | (0.2-0.7)‡ |
| History of another relative with TB** | ||||
| No | 24 (3.6) | 662 | 1 | |
| yes | 7 (1.9) | 378 | 0.5 | (0.2-1.4) |
| Proximity to the index case | ||||
| HHC slept in other household | 3 (1.4) | 222 | 1 | |
| HHC slept in same household | 28 (3.4) | 818 | 2.56 | (0.9-7.5) |
| Persons per room | ||||
| <3 | 12 (1.8) | 679 | 1 | |
| ≥3 | 18 (5.1) | 354 | 2.95 | (1.2-7.3)† |
| Sputum smear load | ||||
| + or ++ | 21 (3.2) | 662 | 1 | |
| +++ | 10 (2.7) | 377 | 0.84 | (0.3-2.1) |
| Exposure time (months) | ||||
| ≤3 | 23 (3.3) | 702 | 1 | |
| >3 | 8 (2.4) | 336 | 0.75 | (0.3-2.0) |
| Initial tuberculin skin test (mm) | ||||
| 0-4 | 2 (0.9) | 215 | 1 | |
| 5-9 | 3 (7.0) | 43 | 8.55 | (2.5-29.2)‡ |
| ≥10 | 15 (7.5) | 200 | 8.16 | (2.0-32.9)‡ |
* HR: Hazard Ratio adjusted by family and city intraclass correlation.
** History of another relative with TB: had had any other relative with tuberculosis besides the identified index case in the cohort.
† p <0.05
‡ p <0.01
§Age in years HR stratified by BCG scar and adjusted by the history of another relative with TB, proximity to the index case, and persons per room: without scar HR= 6.00 (95% CI: 1.3-28.3, p: 0.024), with scar HR= 1.33 (95% CI: 0.5-3.4, p: 0.559).
Discussion
This study showed a high prevalence of tuberculosis infection in children (age <15 years) household contacts of adult patients with pulmonary tuberculosis, associated with having a BCG scar and age between 10-14 years. Closer proximity and time spent with the index case (exposure) greater than three months had a positive association with tuberculin skin test ≥10 mm. A systematic review and meta-analysis of the evidence on the yield of household contact investigations in countries with similar epidemiological characteristics than Colombia, found a prevalence of tuberculosis infection of 51.4% (95% CI 50.6-52.2), in children ranging from 18.5% to 69.2%, and being greater in children 5-14 years of age than in children <5 years 12, similar to our findings. In Brazilian children exposed to TB (evaluated by tuberculin skin test and interferon-gamma release assay, significant factors associated with infection were being a contact of an adult with active disease (0-60 days OR= 6.9; >60 days OR= 27.0) and sleeping in the same room with the index case (OR= 5.2) 26, as it was found in the present study.
Additionally, the incidence rate of infection after one year of follow-up was high, suggesting that a significant risk for developing active tuberculosis persists after one year. tuberculosis annual risk of infection gives information about the degree of transmission in a given community, used to focus vaccination programs, and ultimate control of the disease. Usually, it is calculated from the observed infection prevalence in scholar age children, given that even in places where a high transmission occurs, measurement of infection by using tuberculin skin test sequentially will require significant sample sizes, and the results may be influenced by booster effects 27-29.
Performing annual tuberculin skin test, our study showed that annual risk of infection in children household contacts of adults with pulmonary tuberculosis is higher than the one reported in general population in Russia with a moderate disease incidence (annual risk of infection in 1991: 0.2%, in 2000: 1.6%) 30. In contrast, in two countries with high tuberculosis incidence as Zambia and South Africa, the reported annual risk of infection in children between 6-11 years old was 2.0% and 4.2%, respectively (tuberculin skin test cutoff 10 mm) in 2009 31. Another study in South Africa in 2016 described an annual risk of infection in children <15 years old of 3.1% 32. These studies differ from our study in the way the annual risk of infection was calculated (estimated from data obtained with prevalence surveys) and in the cutoff point for considering a positive tuberculin skin test; even though, our data support that the infection risk for tuberculosis remains greater in children household contacts of patients with active tuberculosis (annual risk of infection 12.1%), compared to general population even one year after being identified as a TB contact.
In the same way, a higher incidence of active tuberculosis was demonstrated during the two years of follow-up with an earlier presentation in children <5 years old without a BCG scar. Results from several studies showed an increased risk for developing active tuberculosis in children <5 years old, having HIV, or sharing the bed or the household with an adult with active tuberculosis, proximity to the index case, crowding and being a passive smoker as well as severe undernourished and not having a previous BCG vaccination 33-36, similar to present study findings regarding age less than 5 years and BCG vaccination. The risk of developing active tuberculosis decreases with age, and the protective effect of BCG vaccination for infection, active tuberculosis, and severe disease have been reported 25,33,37 (using interferon-gamma as an infection marker) 38.
Our findings support the protective effect described for the BCG vaccine in children <5 years old in conditions of a moderate prevalence of tuberculosis 38. However, these effects vary in different geographical regions, which could be related to the genetic dependent immune response that affects even tuberculin skin test results 39.
Although tuberculin skin test is widely used to determine infection by M. tuberculosis, the test has several pitfalls, which makes it acceptable but not perfect. Its specificity is poor in BCG vaccinated populations, it has cross-reactivity with non-tuberculous mycobacteria and its sensitivity decrease in immunocompromised patients 25,40,41. These tuberculin skin test characteristics may have a confusing effect, especially in the association found between the presence of BCG vaccination scar and the prevalence of tuberculosis infection.
The present study is the first in our country with these characteristics, which includes the follow-up of a cohort of children exposed to tuberculosis. Currently, the proportion of children with tuberculosis disease reported in Colombia 7 is under the 10% global estimation of new tuberculosis cases. That suggests a weakness in the country activities for identification of children with disease 42. Although BCG vaccination shows protection against the disease, children <5 years old are yet the most vulnerable population to develop active tuberculosis disease.
Our findings allowed to modify some policies in Colombia, including a prolongation of follow-up time to household contacts seeking active disease, and promoting isoniazid preventive therapy in guidelines for children age <5 years.
Study limitations
Children included in the study were part of a cohort of household contacts of patients with pulmonary tuberculosis. Thus the initial sample size was not calculated exclusively for children. An inverse calculation was done for the cohort of children <15 years’ old which consisted in an estimation of the power needed to detect a significant difference (95% confidence value) between the 2% of incidence rate of active tuberculosis in individuals with a BCG scar vaccination (exposed) and the 6% of incidence rate in non-vaccinated individuals. The results showed a power of 81% using sample size software (version 1.1) 43. A similar result was obtained for comparison of active tuberculosis incidence rate according to the presence or not presence of crowding.
On the other hand, the tuberculin skin test was not available for every child in Medellín; only an incidental sample could access to this test.
Besides, the estimated tuberculosis annual risk of infection is not comparable to traditional methods used. Currently tuberculosis contact tracing with tuberculin skin test is complemented with commercial interferon-gamma release assay, even in children, results from in-house interferon-gamma quantification were reported previously 19.
Conclusions
tuberculosis infection in children younger than 15 years household contacts of patients with pulmonary tuberculosis is high, as it is the risk of developing active TB. This finding supports the importance of tracing tuberculosis contacts and the application of measures such as the isoniazid preventive therapy or other regimens, especially in children <5 years of age, given that a large part of these children become infected in their homes. New studies should be focussed to evaluate the implementation of recommended tuberculosis preventive measures in the context of the household contacts of patients with active tuberculosis, especially children <5 years old.











 text in
text in 



