Remark
| 1)Why was this study conducted? |
| From discovery to actual immunization to protect populations against COVID-19, the process might take several months, or even years. Then, we propose exploring alternatives to organize preventive measures to adapt our lives, behaviour and structures to this new reality. |
| 2) What were the most relevant results of the study? |
| We find out that using Combination HIV Prevention Programmes as a model to organize COVID-19 prevention in absence of a vaccine is a suitable option. So far, we don’t have an effective HIV vaccine. Nonetheless, we have learned a lot on how to prevent HIV and reached major achievements. Proposing Combination COVID-19 Prevention Programmes is an alternative to face this pandemic situation until we obtain a safe and effective vaccine for the population. |
| 3) What do these results contribute? |
| We expect that health authorities can use this approach and set up Combination COVID-19 Prevention Programmes for local epidemiological settings. In an editorial of Science, published on the same day or our paper, the authors advocate for this approach. Our manuscript goes one step forward by providing examples on how this approach can be translated from HIV to COVID-19. |
Introduction
The emergence of COVID-19 pandemic has posed an unprecedent burden for healthcare systems. Exponential growth of infection rate and demand of intensive care in short periods of time demanded more aggressive strategies to decrease the transmission. Measures to decrease the reproductive number (R) of SARS-CoV-2 infection distribute the number of cases requiring healthcare in a longer period. Such effect, called “flattening the curve”, can be reached by implementation of rigorous control of social interactions with a heavy impact in individual and collective behaviors as well in economic activities 1.
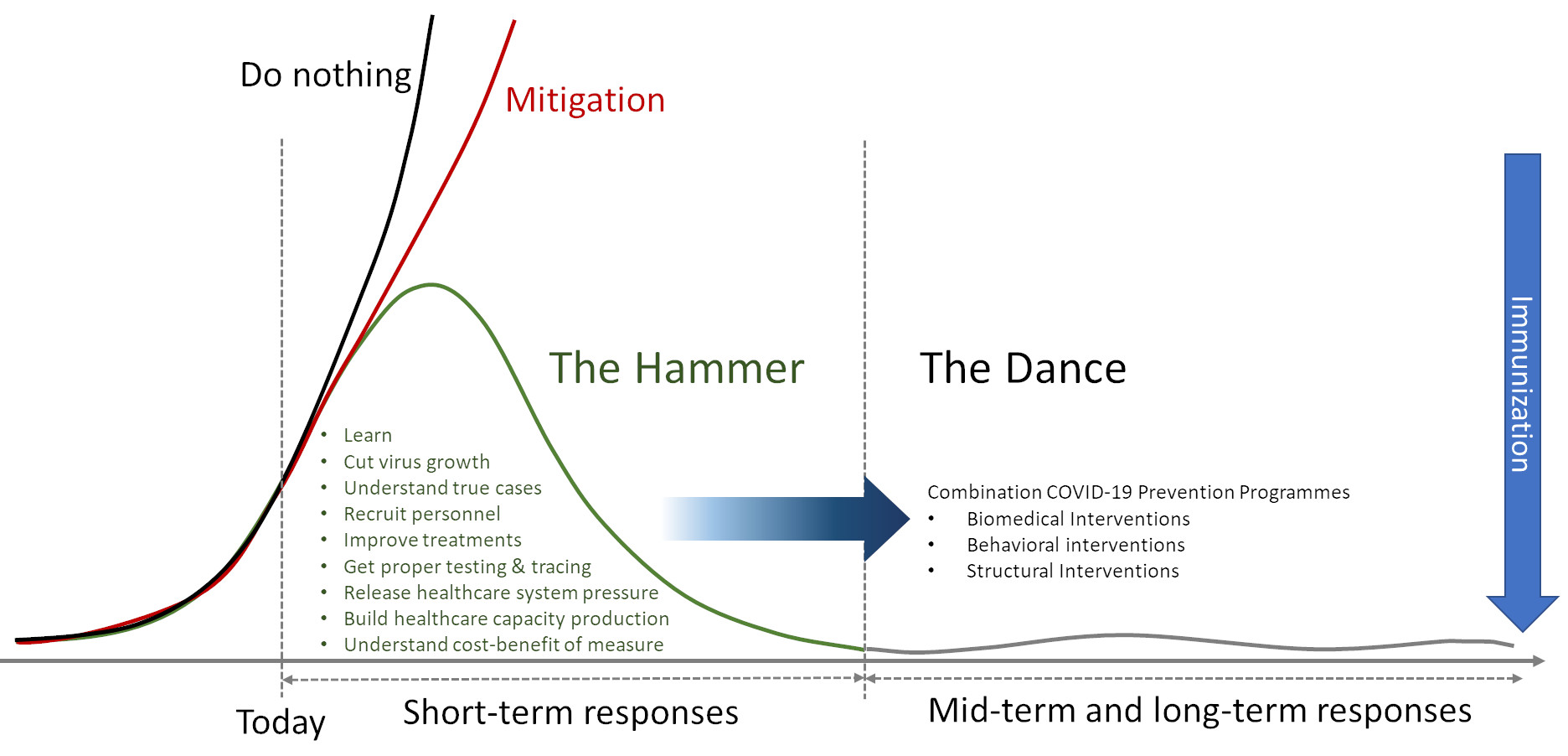
In an enlightening essay entitled “Coronavirus: The Hammer and The Dance”, Tomás Pueyo concludes that suppression strategies, “The Hammer” to hit the R, are critical to buy time to face the COVID-19 pandemic 2. This time is valuable to boost healthcare capacity, refining therapeutic approaches, and testing treatments and vaccines. When severe restrictions control the outbreak, “The Dance” with the R starts. In that phase, measures aim to keep the R low enough to have a manageable amount of cases (Fig. 1). “The Dance” will keep buying additional time to obtain better solutions like immunization.
In the latest four decades, the world learned how to make “The Dance” with HIV. Despite the search for a vaccine has not succeed yet, time and resources have produced a broad spectrum of biomedical technologies 3,4, as well as understanding of behavioral components 5, in the framework of an enabling structure that represent the current combination HIV prevention programmes 6. This article aims to assess the components of this strategy in order to assess whether they can be adapted to the current COVID-19 pandemic.
Definition of a Combination Prevention Programme
The Joint United Nations Programme on HIV/AIDS (UNAIDS) Prevention Reference Group stated in 2009 the following definition:
Combination prevention programme are “rights-based, evidence-informed, and community-owned programmes that use a mix of biomedical, behavioural, and structural interventions, prioritized to meet the current HIV prevention needs of particular individuals and communities, so as to have the greatest sustained impact on reducing new infections. Well-designed combination prevention programmes are carefully tailored to national and local needs and conditions; focus resources on the mix of programmatic and policy actions required to address both immediate risks and underlying vulnerability; and they are thoughtfully planned and managed to operate synergistically and consistently on multiple levels (e.g. individual, relationship, community, society) and over an adequate period of time. They mobilize community, private sector, government and global resources in a collective undertaking; require and benefit from enhanced partnership and coordination; and they incorporate mechanisms for learning, capacity building and flexibility to permit continual improvement and adaptation to the changing environment.”.7
Placing COVID-19 instead of HIV in the above-mentioned definition can provide a proper multilevel programme to face the unprecedent magnitude of this new pandemic. Furthermore, this definition acknowledges human rights, science and community as the basis for a successful programme.
There is not “one-size-fits-all” design for combination prevention programmes. Availability and offer of several preventive methods make possible that individuals mix biomedical and behavioral strategies in the frame of structural interventions sensitive to specificities of individuals and communities 8. The success of the programme will not depend only on isolated actions of individuals, communities or governments but to combined actions at every level. Every combination prevention programme covers three main areas: biomedical, behavioral and structural areas. Examples of strategies in these areas are presented in Table 1 and will be detailed below.
Table 1 Examples of strategies for a combined prevention programme in HIV and in COVID-19
| Area | Objective | Strategy in HIV | Strategy in COVID-19 |
|---|---|---|---|
| Biomedical Interventions with clinical and medical methods | Physical barriers to prevent infection | Condoms and lubricants | Use of masks and personal protective equipment |
| Identifying infected asymptomatic individuals able to transmit the infection | Mass testing (Serology) | Mass testing (RT-PCR) | |
| Prophylactic measures in case of potential exposure | PREP and PEP | Washing hands and cleaning of objects with potential exposure | |
| Identifying individuals that are unlikely to transmit infections | Undetectable = Untrasmittable (HIV viral load in infected individuals) | Serology testing | |
| Treating underlying medical conditions associated to increased risk | STI diagnosis and treatment | Treatment of diabetes mellitus, chronic lung disease, and cardiovascular disease and other comorbidities | |
| Behavioral Interventions that promote healthy behaviors | Reduction of risk in potential interactions between susceptible and infected individuals | Serosorting and sexual harm reduction practices | Isolation, quarantine, social distancing and community containment measures |
| Providing basis for behavioral changes and social marketing campaigns | Comprehensive sex education and campaigns to promote use of prevention methods | Comprehensive education in scientific basis to support COVID-19 prevention measures and campaigns to promote use of prevention methods | |
| Structural Interventions that promote an enabling environment | Identification and actions on key and vulnerable populations | Avoiding marginalization and targeted policies for vulnerable populations | Measures to improve living conditions of senior citizens and minorities and social welfare policies to reduce inequality |
| General protection for populations | Universal access to prevention, diagnosis and treatment of HIV | Financial assistance to affected households and firms |
Biomedical Area
Biomedical interventions are those based on clinical and medical methods. They can be pharmacological or non-pharmacological and relies on use of hard technology. This kind of interventions requires to warrant access to supplies as well as individual and community adherence to the technology.
Physical barriers to prevent infection
Individuals with asymptomatic or oligosymptomatic infections can spread viruses in contact with susceptible individuals. As far as those infections are not apparent, susceptible individuals cannot distinguish between infected and non-infected individuals in interactions. Then, as a rule, all the individuals should be considered as potential source of infections and physical barriers to avoid infections should be requested to all individuals.
Universal use of condom is a cornerstone of HIV prevention since the earlier prevention strategies. Use of condom is widely promoted and make it available is a key activity in HIV prevention. Individuals are encouraged to use or request the use of condom and learn its proper use, including when to use also lubricant help to reduce additional risks 9-11.
Transmission from asymptomatic and pre symptomatic individuals has been identified in clusters of COVID-19 12. About four fifths of SARS-Cov-2 sources of infections are asymptomatic individuals and can spread infections to susceptible individuals at large scale 13. But, empirical efficacy of face masks in prevention of SARS-CoV-2 spreading is still limited 14. Potential benefit weighted by the United Stated Centers for Disease Control and Prevention (CDC) lead to a recommendation for use of masks, regardless the presence of symptoms, to create a physical barrier in areas with significant community-based transmission since April 3,2020 15. Protection to acquire infection requires specific personal protective equipment. Then, use of non-professional masks is mainly intended as altruistic measure to protect others if an asymptomatic/ presymptomatic infected individual is unaware of that condition, rather than protecting the person wearing the mask. This message should be conveyed to the community to emphasize that people more likely to have asymptomatic infection adhere to this measure and avoid discredit of the measure if someone using the mask get infected.
Identifying infected asymptomatic individuals able to transmit the infection
Asymptomatic individuals usually do not actively procure healthcare services for diagnosis. Nonetheless, if those individuals are identified, additional measures to avoid spreading and monitor early complications due to the infection can decrease transmission and morbidity.
Active HIV rapid test offering allow earlier treatment leading to decrease transmission and avoid progression to AIDS 16. Current technologies have made over-the-counter tests available and peer-based community testing campaigns 17,18. Germany and South Korea implemented a mass testing policy for SARS-CoV-2, in contrast to other countries that restrict testing only to symptomatic or severe cases 19. This strategy allows further measures in those identified as infected including quarantine and health monitoring. Mass testing and identification of asymptomatic and pre symptomatic individuals account for a considerable part of the success of those countries in facing COVID-19.
Prophylactic measures in case of potential exposure
Events with potential exposure to a pathogen cannot always avoided. But exposure does not lead to infection in all the cases. Determinants of infection include the dose of the pathogen during exposure and the contact of the pathogen with cells susceptible to the infection. Prophylactic measures to change these determinants can be used either before or after exposure. In this item, the focus is on use of chemical substances used as pharmacological or non-pharmacological prophylactic measures.
The use of prophylaxis related to exposure in HIV had to milestones: occupational accidents in healthcare workers and prevention of mother-to-child HIV transmission. In both cases, the use of antiretroviral drugs was successful to decrease early infection events after potential exposure. Based on those findings, use of antiretroviral was extended to potential after potential sexual exposure to HIV, initially as post-exposure prophylaxis (PEP) 20-22 and later as pre-exposure prophylaxis (PrEP) 3,23. Prophylactic use of antiretroviral drugs is widely incorporated to HIV prevention programs worldwide.
Until now, no medication has been reported to have proven potent antiviral effect in clinical trials against SARS-CoV-2. But repurposing of drugs approved for other indications can provide a faster way for a treatment and, eventually, becoming suitable for prophylaxis. In the meantime, other prophylactic measure can be used to destroy viral particles before they can get in contact with susceptible cells. SARS-Cov-2, like other respiratory viruses can remain viable in fomites for several hours, and even days 24, and can get contact with mucosae through the hand of an individual in contact with infected fomites. Use of chemical substances, like soap and alcohol-based sanitizers, to clean hands can avoid an effective infection in a person with exposure to a contaminated area. At a larger extent, surfaces in contact with susceptible individuals should be disinfected to avoid exposure 25.
Identifying individuals that are unlikely to transmit infections
Those individuals who either for control or recovering of infection can block the transmission chain might not be requested to take other preventive measures, i.e. physical barriers. Then, identification of an individual as unable to transmit can offer significant secondary gains.
People living with HIV have suffered discrimination because other people feared to acquire the infection during interaction. Then, when studies demonstrated that sexual partners of people living with HIV on effective antiretroviral therapy with viral load reduction below the detection levels had no infection after potential exposure, the motto “Undetectable = Untransmittable” was coined 26-28. This scientific evidence relieved in part the discrimination and suffering of many people living with HIV.
In the case of COVID-19, some researchers have proposed the use of serological testing to determine who already had infection and acquired immunity against SARS-CoV-2. “Immunity certificates” of those who cannot get or transmit the infection might safely return to work 29. Such type of proposals is still limited by the restricted knowledge on mid-term and long-term immunity after SARS-CoV-2 infection. In human coronavirus 229E, experimental infection occurred only on volunteers with low nasal IgA and serum IgG levels 30, meaning that high antibody levels are required to obtain protection. Decay of antibodies is well documented after SARS-CoV-1 infection in a 3-years follow-up period 31 and in patients after 34 months after MERS-CoV infection 32. In an animal model, MERS-CoV reinfection was only possible in the absence of neutralizing antibodies 33. Those results are warning signs to be cautious in establish parameters to declare an individual as immune to a new SARS-CoV-2 infection and to establish policies on this foundation.
Treating underlying medical conditions associated to increased risk
Host conditions can affect natural history of infection, either by increasing risk of infectivity or progression to severe disease. Then, controlling or eliminating those underlying diseases can decrease infection or morbidity risks.
Genital inflammation in sexually transmitted infections might increase risk of HIV transmission, then identification and treatment of those infections can offer an additional benefit to those patients 34,35.
SARS-CoV-2 has not association to underlying conditions in terms of infection, but in case severity. Beside older age, diabetes mellitus, chronic lung disease, and cardiovascular disease and other comorbidities are more frequent among those patients with severe COVID-19 36. Unbalance in the ACE2 receptor downregulation associated to those conditions can be a mechanistic way to explain such severity 37. On the other hand, patients with poor functional reserve or with unstable baseline conditions are in worse position to resist the infection-related injuries. Then, improving underlying conditions might decrease risk of severe COVID-19 in case of infection 38.
Behavioral Area
Promoting healthy behaviors is a soft technology with focus in behaviors and individual choices. However, such choices are not only depending on the free willing of the individual, but on several external constrains outside of the control of the individual. Then, many of these behavioral interventions have companion measures to modify external factors and incentivize the desired choice, either by creating restrictions or breaking barriers.
Reduction of risk in potential interactions between susceptible and infected individuals
In the absence of acquired immunity, one of the most effective ways to decrease the reproductive number of an infection is curtailing opportunities of transmission. Segregation between infected and susceptible individuals is challenging due to limitation to assess asymptomatic and presymptomatic infected individuals in timely manner. Then, behavioral changes should affect all interactions between individual related to ways of transmission.
Serosorting is one of the behavioral strategies based on segregation documented in HIV. That is restrict the choices of sexual partner only to those with the same serostatus, i.e. a person living with HIV will limit sexual relationships to other people living with HIV. Other strategy to reduce risk of HIV transmission is changing behavior during sexual intercourse, i.e. non-penetrative sexual practices 39-42.
Control of viral respiratory infections is more difficult because transmission is possible with a limited interaction. The most extreme measures are required for contention of those cases. That was the case in the SARS outbreak in 2003. The centuries-old quarantine, meaning complete isolation of infected individuals and their contacts, was settled in place by health authorities to contain the SARS outbreak 43. This precedent was useful to enable the same kind of measures when COVID-19 emerged. A scale-up of measures from isolation to quarantine, then social distancing, and finally community containment (also known as lockdown) became essential to face COVID-19 outbreak 44. Application of this set of methods to restrict interaction between susceptible and infected individuals represent not only an increasing chance to interrupt transmission, but degrees of disruption of social and economic life. Resistance to adhere to these set of measures is directly proportional to the magnitude of such disruption. Chinese authorities implemented this kind of measures to an unprecedent level in history. This proof-of-concept was a remarkable success able to deplete susceptible individuals to curb the reproductive number 45,46. SARS-CoV-2 basic reproductive number (R0) was calculated in 5.7 (95% CI of 3.8-8.9) 47, meaning that one infected individual can spread the infection to near 6 other individuals, beside many of them are asymptomatic. Such high R 0 explains the need for decreasing social interaction to lower the transmission rate. One of the biggest challenges in COVID-19 prevention for the upcoming months is finding tools for fine tuning this kind of measures. Societal and mental health consequences as well as deepening of inequalities and injustices are advisable as a result of disruptions in social and economic activities 29. Repertoire of restrictions and changes in daily life and labor is extensive and their impact is subject of intense mathematical modeling to guide authorities 1. Most models are founded on the classical SIR (Susceptible/Infected/Removed) dynamics, but if the immunity to SARS-CoV-2 wanes as occurred after infections with other coronaviruses 30-32, those models can fail at mid-term to predict the effect of relaxing restrictions to social interactions. Further data on monitoring the effect of measures and natural history of SARS-CoV-2 infection are urgently needed to feed mathematical models.
Providing basis for behavioral changes and social marketing campaigns
In the same way that healthcare providers need to educate a lay person with a new diagnosis to promote healthy behaviors, societies require learning on the reasons to change behaviors. Leadership usually relays in health authorities with support of multilateral agencies, like World Health Organization, and scientific societies and academic institutions. Communities should easily identify trustable sources and receive culturally appropriated messages to warrant success.
There is a long history on educative actions in HIV/AIDS, including natural history of disease, modes of transmission, diagnosis, treatment, and prevention methods. Strategies included use of mass media, targeted actions in key populations and schools, and activities organized by community centers, among others 42-48.
Reports on community educational activities in COVID-19 are still limited, despite mass campaigns and media coverage has been intense since the beginning of the outbreaks49-50. Monitoring risk perception through qualitative research can guide authorities to modulate the message51-52. Building trust in authorities by transparent and reliable information is also an important domain to deliver inputs to the public to support behavioral changes 53. Social media is getting a critical role as mean of communication in the latest years. Engagement at the beginning of the COVID-19 in China was related to government measures as well as to distrust related to scandals 54. Proactive communication and monitoring of this new environment are challenges to follow in this pandemic.
Structural Area
Implementation of biomedical and behavioral interventions requires an enabling environment to make them feasible. Such environment is only possible through structural changes agreed by social forces, including government, legislative bodies, justice administration, private sector, and civil society.
Identification and actions on key and vulnerable populations
Key populations are those at higher risk regardless the presentation of the epidemic in a territory or the local context. Vulnerable populations are the ones with higher risk in specific context of an epidemic setting that are not affected equally in other settings. Identification of both, key and vulnerable populations, is relevant to determine approaches at different levels.
In HIV, five key populations are identified: 1) men who have sex with men, 2) people who inject drugs, 3) people in prisons and other closed settings, 4) sex workers and 5) transgender people 55. Those populations are more prone to marginalization and discrimination. Policies to obtain effective recognition of human rights take them out of the margin and allows efficient approach from the healthcare and social protection systems 56,57. In country-specific setting, vulnerable populations are identified, i.e. female teenagers in some African countries. The HPTN 068 study found that low school attendance in female teenagers was associated to increased risk of HIV acquisition in South Africa 58. Therefore, school attendance might be a tool to decrease HIV incidence on those settings.
In COVID-19, key populations were evident since first reports. Elderly people and those with underlying medical conditions have higher mortality in different epidemiological settings 36,59. As a consequence. concerns on mode of living of senior citizens raised awareness for specific actions to address this key population 60,61. Vulnerable populations for COVID-19 in countries like United States include minorities with a considerable disadvantage to understand the new disease and how to get prepared 62. Analogous situation can also occur in other countries with limited social protection and important inequality 63. A higher impact of disease is expected in socially disadvantaged populations. Targeted policies to improve social welfare can ease such impact.
General protection for populations
Some major preventive measures are only possible in the framework of warrants beyond key and vulnerable populations. Such protections are usually established by governments and implies in mobilization of large amount of resources.
Widespread unrestricted access to all population to HIV diagnosis and treatment, regardless health insurance coverage for other medical conditions, is the basis of the 90-90-90 strategy launched by UNAIDS in 2014. The proposed goal of this strategy is reaching by 2020 90% of HIV-infected individuals diagnosed, 90% of them on antiretroviral treatment and 90% of them with undetectable viral load 64. The access to diagnosis allows identification of asymptomatic individuals and the access to treatment switch their status to be unlikely to transmit the infection. This ambitious strategy enables successful implementation of biomedical interventions.
In COVID-19, restriction to social interaction stopped several economic activities and lead to recession. Therefore, governments are challenged to flatten two simultaneous curves: the pandemic and the recession curves 65. Furthermore, only governments can hold the pressure to take measures to protect lives through social distancing and to provide financial assistance to affected households and firms during the disruption of some economical activities 66. Mid-term and long-term measures might affect several sectors of the economy depending on gathering activities. Those sectors might face reformulation of business model and adaptation to survive. Without proper financial and legal support from the government, social distancing is not sustainable.
Conclusion
The search for a HIV vaccine started in the 1980’s and no product was licensed so far 67. In the meantime, preventive measures have reached remarkable success currently consolidated in Combination HIV Prevention Programmes. Vaccines against SARS-CoV-2 are the most hopeful way to have a game-changing measure to control current pandemic. Many initiatives are underway 68, nonetheless, those candidates have risks to overpass and are months away from licensure. Eventual licensed vaccines to protect against COVID-19 might need additional time to be accessible due to manufacturing constraints 69. Then, it is time to learn how to perform a long “Dance” with the SARS-CoV-2 reproductive number. There are many other interventions in addition to those abovementioned as examples. Each epidemiological setting should build, monitor, and review the best mix of interventions. But in all cases, a strong commitment of individuals, communities, civil society, and authorities is required to control these ongoing pandemics, HIV/AIDS and COVID-19.











 text in
text in