Remark
| 1) Why was this study conducted? |
| There are few prospective studies on the frequency of early cardiac toxicity in children receiving chemotherapy and possible predictors of this toxicity in children receiving acute leukemia treatment. |
| 2) What were the most relevant results of the study? |
| Up to 75% of children have some abnormal cardiovascular finding during treatment. The usefulness of advanced echocardiography tools in predicting the risk of cardiac compromise with significant OR. We established GLS and GLS delta’s role as possible early markers of cardiac toxicity in children who received treatment for acute leukemias. Biomarkers were not predictors of cardiotoxicity in this study. The relationship between cardiac disease and cumulative anthracycline doses was demonstrated. |
| 3) What do these results contribute? |
| Motivating the identification of cardiovascular compromise as an adverse effect of chemotherapy in children during cancer treatment and promoting measures to mitigate it, such as the use of cardiac protection strategies. It is necessary to conduct long-term follow-up of leukemia survivors to identify late chronic cardiovascular effects. |
Introduction
Hematolymphoid neoplasms are the leading cause of childhood cancer in the world 1. Advances in pediatric cancer therapeutics have led patients to higher overall survival and increased adverse effects from their therapy (2. The percentage of children with cancer with symptomatic cardiovascular toxicity ranges from 7% to10% (3. The cellular damage produced by antineoplastics in the heart muscle is well known (4. Survivors of childhood cancer have an eight times higher risk of developing cardiovascular diseases throughout their lives (5. High cumulative doses of anthracyclines and chest radiotherapy exposure are the main risk factors for heart failure. However, other triggers have been described: high doses of alkylating agents, female gender, age less than four years, having Down syndrome, and the Afro-descendant race (6. We classified cardiac toxicity derived from cancer therapy according to the time of onset in the following categories: acute, when it occurs in the first week after exposure to the neoplastic agent; early-onset, which occurs between one week and up to a year after the end of antineoplastic treatment; and late-onset, that which occurs after one year of having finished treatment (7,8. Despite the existence of guidelines for detecting and monitoring cardiovascular diseases in the adult population (9, few evidence-based consensus or reviews provide recommendations for the diagnosis and follow-up of cardiovascular complications (10 for patients with childhood cancer. Echocardiography is the primary diagnostic technique to evaluate the left ventricle’s systolic performance by estimating its ejection fraction. Other cardiovascular evaluation tools are serum biomarkers such as troponin T, troponin I, Brain Natriuretic Peptide, and the electrocardiogram. (7.
In a survival study of a cohort of children with acute lymphoblastic leukemia at a reference center for childhood cancer in Bogotá, a grade 3-4 cardiac toxicity frequency of 26.9% was found (11. There are very few data on the frequency of cardiovascular complications derived from antineoplastic therapy in Colombian children.
The study’s main objective was to describe the frequency of early cardiac involvement in children with acute leukemias who received antineoplastic treatment at a referral center for childhood cancer. Secondary objectives were to describe the echocardiographic and electrocardiographic findings and describing the serum biomarkers’ behavior in the study group.
Materials and Methods
This is a prospective descriptive study, nested in a cohort of patients with a confirmed diagnosis of acute leukemias: acute lymphoid leukemia and acute myeloid leukemia, older than one year and younger than 18 years, in the period between October 1, 2017, and March 31, 2019. Participants were selected by non-probabilistic sequential convenience sampling of patients with a new diagnosis of acute lymphoblastic leukemia and acute myeloid leukemia.
Inclusion criteria
Patients older than 12 months and younger than 18 years with a new diagnosis of acute lymphoblastic leukemia and acute myeloid leukemia performed at HOMI Fundación Hospital de la Misericordia.
Exclusion criteria
Patients who did not start treatment at HOMI Fundación Hospital de la Misericordia.
Patients with a diagnosis of relapse of acute lymphoblastic leukemia or acute myeloid leukemia. Patient with a diagnosis of heart disease before the start of cancer treatment. Patients who did not complete at least two evaluations. Patients whose parents did not authorize their inclusion in the study.
Definitions
The patients with acute lymphoid leukemia were classified according to their risk as standard, intermediate, and high. The risk classification for acute lymphoid leukemia is based on the ALLCI protocol of the BFM group (Appendix 1) (12.
Cardiac dysfunction related to cancer therapy: the decrease in the left ventricle’s systolic performance by estimating its ejection fraction below 53%, by estimating with the biplane Simpson’s method (13,14.
Global longitudinal myocardial deformation: Considering the manufacturer of the echocardiography equipment used and the available literature, a deformation greater than -20.1% was considered a normal absolute value for all age groups 13,14.
A decrease in the percentage of deformation greater than 15% compared to the baseline value at follow-up was considered significant (global longitudinal myocardial deformation Delta) (15.
Other associated cardiovascular disorders: coronary artery disease, arrhythmias, pericardial effusion, valve insufficiency (other than mild tricuspid or mitral regurgitation and considered physiological), thromboembolic disease, systemic arterial hypertension (defined as systolic or diastolic blood pressure higher than the 95th percentile for age, gender, and height), and pulmonary hypertension 8 (defined as an echocardiographic estimate of a pulmonary systolic pressure greater than or equal to 40 mmHg from the tricuspid regurgitation velocity and an estimate of pressure at the level of the right atrium in relation to the percentage of inferior vena cava collapse).
Acute toxicity: cardiovascular manifestations that appear in the first week of antineoplastic treatment (7,8.
Early-onset toxicity: the appearance of cardiovascular manifestations after the first week and up to the first year after the end of treatment (7,8.
Alterations in serum biomarkers: significant elevation of brain natriuretic peptide above 100 pg/mL of TnI with a value greater than 0.05 in ng/L and TnT value above 40 ng/L (16.
Alterations in the electrocardiogram (ECG): identifying alterations in impulse generation, AV conduction (atrioventricular), IV conduction (interventricular), and repolarization disorders (8.
Echocardiography evaluation: The taking and analysis of the echocardiographic studies were carried out by two pediatric cardiologists with more than five years of experience in evaluating patients with childhood cancer, performing ECG synchronized acquisitions (5 beats) for moving images. The three methods used to evaluate the systolic performance of the left ventricle were the ejection fraction (LVEF) estimated by the Simpson biplane method (LVEF (S)), the LVEF estimated by the Teicholz method (LVEF (T)), and the shortening fraction. An analysis of the global longitudinal strain was performed using the particle follow-up technique (Speckle tracking).
For quantifying the ejection fraction using the biplane Simpson method and the global longitudinal myocardial deformation analysis, the frames were obtained from the apical window in 4-chamber, 2-chamber, and three-chamber axes. The acquisition analysis was performed with the Phillips Affiniti 70C ultrasound machine software, version 1.7.1. For all cases, the operators located the basal points and the apical point to be analyzed in the three defined views, allowing automatic recognition of the endocardium in the first instance and manually correcting its location if necessary, using the editing function.
For the quantification of the ejection fractions by the Teicholz method and the shortening fraction, we used a static image acquired from the short axis of the ventricles.
All patients underwent 12-lead ECG evaluations and two-dimensional (2D) echocardiography with the Phillips Affiniti 70C, 5-1, and 8-3 MHz transducers.
Assessments were performed with serum biomarkers measured in peripheral or central venous blood processed by rapid test immunochromatography. The serum biomarkers evaluated were brain natriuretic peptide, troponin I (TnI), and troponin T (TnT). The reference values correspond to the technique used.
All the previously mentioned evaluations (echocardiogram, electrocardiogram, and biomarkers) were performed before starting treatment and at specific points of the acute lymphoid leukemia and acute myeloid leukemia treatment (Figure 1). For patients with high-risk of the acute lymphoid leukemia, the end of treatment was considered the last evaluation at the end of reinduction or the last evaluation before hematopoietic stem cell transplantation. For patients with acute myeloid leukemia, the end of treatment was considered the last evaluation before TPH or the evaluation after three cycles of consolidation.
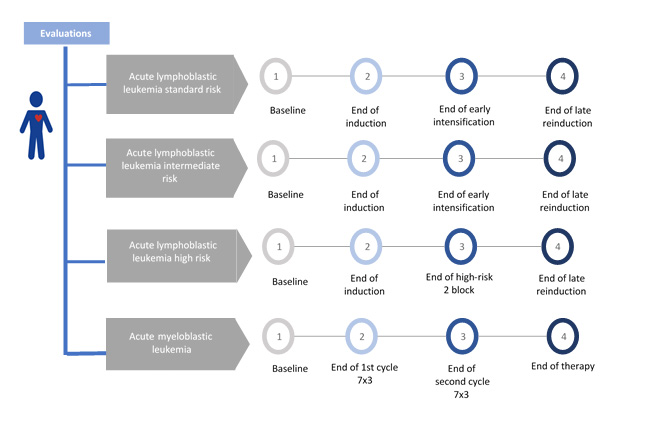
Figure 1 Study schema: time of evaluations with echocardiographic measurements, 12-lead electrocardiogram, and with biomarkers, according to type of leukemia.
The cumulative doses of anthracyclines by pathology were converted to equivalent doses of doxorubicin: 1 mg of daunorubicin is equivalent to 0.833 mg of doxorubicin (17.
Statistical analysis
According to the distribution after analysis with normality tests, the quantitative variables’ statistical analyses were carried out with central tendency and dispersion measures, means, standard deviation, or medians and ranges, after analysis with normality tests (Kolmogorov-Smirnov or Shapiro Wilk) to establish the behavior of the data as parametric or nonparametric. Qualitative variables were analyzed with Pearson’s Ji 2 test and Fisher’s exact test. The echocardiography data were analyzed according to the accumulated dose of anthracyclines. In each treatment group, the Wilcoxon test for nonparametric paired samples was used. For the analysis of biochemical markers such as brain natriuretic peptide, patients were divided into two groups according to the presence or absence of cardiotoxicity and were analyzed with the Mann-Whitney U test for independent nonparametric samples. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) for Windows, version 25.0. A value of p <0.05 was considered significant.
Ethical aspects
This study was classified as a “Minimal Risk” project. Informed consent was obtained from the parents of each patient. Assent was obtained from each of the patients or the patient’s legal representative if that were the case. This research protocol was approved by the institutional ethics committee, act 4848 CEI-11-17.
Results
One hundred thirteen patients were admitted: 94 with acute lymphoid leukemia and 19 with acute myeloid leukemia. One patient with acute myeloid leukemia was excluded from the study because he did not have data from the second evaluation; the results correspond to 112 patients. We analyzed 38 patients (33.9%) with high-risk acute lymphoblastic leukemia, 42 (37.5%) with intermediate-risk, 14 (12.5%) standard risk, and 18 patients with acute myeloid leukemia (16.0%). Thirteen patients died during follow-up; data was collected until pre-death assessment. Table 1 describes the demographic characteristics of the study population.
Table 1 Demographic characteristics of the study population.
| Variables | LLA RE | LLA RI | LLA RA | LMA | Total |
|---|---|---|---|---|---|
| Number of patients (%) | 14 (12.5%) | 42 (27.5%) | 38 (33.9%) | 18 (16%) | 112 (100%) |
| Median age in years (range) | 3.4 (1-6) | 6.6 (1.7-17.2) | 9.4 (1.5-17.7) | 9.0 (1.5-17.0) | 6.35 (1.0- 17.7) |
| Female | 8 (57.1%) | 17 (40.5%) | 13 (34.3%) | 7 (38.9%) | 45 (40.2%) |
| Median weight in kilograms (range) | 12.8 (10.9-25.0) | 20 (10.4-74.0) | 19 (10-73) | 31.5 (10-69) | 20 (10-74) |
| Median height in cms (range) | 97 (86-118) | 118.5 (75-180) | 122.5 (83-181) | 129 (70-167) | 114 (70-181) |
| Accumulated dose of anthracycline mg / m2 | 170 | 220 | 270 | 298 | NA |
ALL: acute lymphoblastic leukemia, ER: standard risk, IR: intermediate risk, AR: high risk.
Early-onset of cardiac dysfunction related to cancer therapy developed in 20 patients (17.9%); no patient had acute heart disease.
There was early-onset toxicity in patients with acute lymphoid leukemia high-risk with a median of 30 days in 4 patients and 120 mg/m2 accumulated anthracyclines. By the third evaluation (end of intensification), eight patients presented early cardiotoxicity, with cumulative anthracyclines doses of 150 mg/m2. At the last evaluation (end of reinduction), a cumulative 11 patients had cardiac involvement with cumulative anthracycline doses of 270 mg/m2.
Cardiac dysfunction related to cancer therapy was found in two intermediate-risk patients with cumulative doses of 220 mg/m2 of doxorubicin at the end of reinduction. In the standard-risk group, no patients had cardiac dysfunction related to cancer therapy.
In the children with acute myeloid leukemia group, seven patients had early cardiotoxicity, one of them after 180 mg/m2 accumulated anthracyclines, and four patients after 298 mg/m2 of anthracyclines. By the end of treatment, two additional patients had early heart disease. The decrease in LVEF (S) during follow-up, greater than ten percentage points compared to the previous LVEF (S), was a predictor for cardiotoxicity in patients with acute myeloid leukemia with an OR of 42.42 (95% CI: 4.6- 391.0).
Abnormal findings on the echocardiogram were presented in 65 patients (Tables 2 and 3).
Table 2 Early-onset cardiovascular findings according to the type of leukemia.
| Description of the heart condition | Acute lymphoblastic leukemia | Acute myeloid leukemia | TOTAL | ||
|---|---|---|---|---|---|
| Standard risk n: 14 | Intermediate risk n: 42 | High risk n: 38 | n: 18 | n: 112 | |
| CDRCT (FEVI <53%) | 0 (0%) | 2(1.8%) | 11(9.8%) | 7(6.3%) | 20(17.9%) |
| ECG abnormalities | 1(0.9%) | 16(14.3%) | 9(8.0%) | * 5(4.4%) | 31(27.6%) |
| Pericardial effusion | 2 (1.8%) | 0 | 7(6.3%) | * 5(4.5%) | 14(12.5%) |
| Valvular insufficiency | 0 | 0 | 2(1.8%) | * 5(4.5%) | 7 (6.2%) |
| Arterial hypertension | 2 (1.8%) | 5(4.5%) | 7(6.3%) | 0 | 14 (12.5%) |
| Pulmonary hypertension | 1 (1.8%) | 5(4.5%) | 9 (8.0%) | 2(1.8%) | 17 (15.1%) |
| No abnormal cardiovascular findings | 8 (57.1%) | 9 (21.4%) | 5 (13.1%) | 5(27.7%) | 27 (24.1%) |
* Patients may have combined electrocardiographic and echocardiographic abnormalities or electrocardiographic abnormalities on more than one evaluation. CDRCT: Cardiac dysfunction related to cancer therapy
Table 3 Description of echocardiography values by type of leukemia and time of evaluation.
| High-risk acute lymphoblastic leukemia | ||||
|---|---|---|---|---|
| At diagnosis (n= 38) | At the end of induction (n = 38) | At the end of consolidation (n = 30) | At the end of reinduction (n = 17) | |
| LVEF (T) median (range) | 66.0 (48- 77) | 65.5 (37- 73) | 70.5 (65- 76) | 64.5 (50- 75) |
| LVEF (S) median (range) | 63.5 (46- 70) | 54.0 (42- 73) | 62.5 (60- 65) | 64.7 (53- 67) |
| FS median (range) | 36.0 (23- 46) | 35.0 (17- 41) | 39.0 (34- 44) | 34.5 (25- 43) |
| TAPSE median (range) | 23 (14- 31) | 19 (12- 25) | 20 (18- 22) | 21 (13- 25) |
| GLS median (range) | -22.0 (-30-18) | -16.6 (-22-15) | -22.5 (-23-22) | -24.2 (-26.3, -18.2) |
| Intermediate-risk acute lymphoblastic leukemia | ||||
| n= 42 | n = 41 | n = 40 | n = 36 | |
| LVEF (T) median (range) | 68.5 (54- 78) | 68.5 (48- 79) | 66.0 (56- 74) | 64.5 (46- 74) |
| LVEF (S) median (range) | 62.9 (53.8- 74.0) | 62.1 (54- 71) | 63.0 (54.8- 76.0) | 62.1 (48- 75) |
| FS median (range) | 37.0 (27- 46) | 37.3 (24- 46) | 36.0 (29- 43) | 34.1 (22- 42) |
| TAPSE median (range) | 22.0 (14- 33) | 20.0 (13.5- 26.0) | 22.0 (15- 32) | 21.5 (14- 27) |
| GLS median (range) | -24.4 (-30-18) | -22.5 (-27.5, -17.2) | -23.3 (-31.0-19.6) | -22.7 (-28.0-17.4) |
| Standard-risk acute lymphoblastic leukemia | ||||
| n= 14 | n= 14 | n= 14 | n= 14 | |
| LVEF (T) median (range) | 65.5 (40- 74) | 70.0 (57- 77) | 65.0 (58- 72) | 63.0 (60- 72) |
| LVEF (S) median (range) | 60.2 (38- 65) | 58.6 (55- 63.3) | 60.0 (54.7- 67.7) | 59.0 (55.7- 67.2) |
| FS median (range) | 34.5 (19- 43) | 38.5 (29- 44) | 35.0 (30- 40) | 33.0 (31- 40) |
| TAPSE median (range) | 19.5 (16- 23) | 19.0 (16- 25) | 20.0 (16- 27) | 18.0 (12- 23) |
| GLS median (range) | -23.4 (-26.6, -16.8) | -21.3 (-25.9, -16.3) | -24.1 (-26.7, -20.0) | -23.2 (-31, -19) |
| Acute Myeloid Leukemia | ||||
| n = 18 | At the end of 7+3 (1st cycle), n = 18 | At the end of 7+3 (2nd cycle), n = 18 | At the end of treatment, n = 16 | |
| LVEF (T) median (range) | 68.0 (64- 77) | 68.0 (54- 76) | 64.0 (38- 70) | 63.0 (43- 73) |
| LVEF (S) median (range) | 62.2 (55.5- 74.3) | 63.0 (52.2- 69.8) | 58.8 (32- 71) | 59.9 (41.0- 64.4) |
| FS median (range) | 37.0 (34- 48) | 38.0 (27- 42) | 33.0 (18- 38) | 34.0 (21- 42) |
| TAPSE median (range) | 22.0 (13- 32) | 21.0 (13- 31) | 22.0 (14- 31) | 21.0 (12- 30) |
| GLS median (range) | -21 (-30-15) | -21.2 (-26.8, -17.4) | -21.5 (-27.0, -15.8) | -22.6 (-26.1, -16.1) |
LVEF(T): left ventricular ejection fraction Teicholz method, LVEF (S): left ventricular ejection fraction Simpson method, FS: fraction shortening, TAPSE: tricuspid annular, plane systolic excursion, GLS: global longitudinal strain.
The most common ECG abnormalities were impulse generation disturbances and repolarization disorders (Appendix 2). There were no events of thromboembolism, peripheral vascular disease, coronary disease, or stroke.
It can be seen in Figure 2 how the LVEF evaluated with the Simpson method decreases from the start of treatment as the accumulated doses of anthracyclines are given. It is significant with values of accumulate anthracycline dose over 150 mg/m2 (Figure 2).
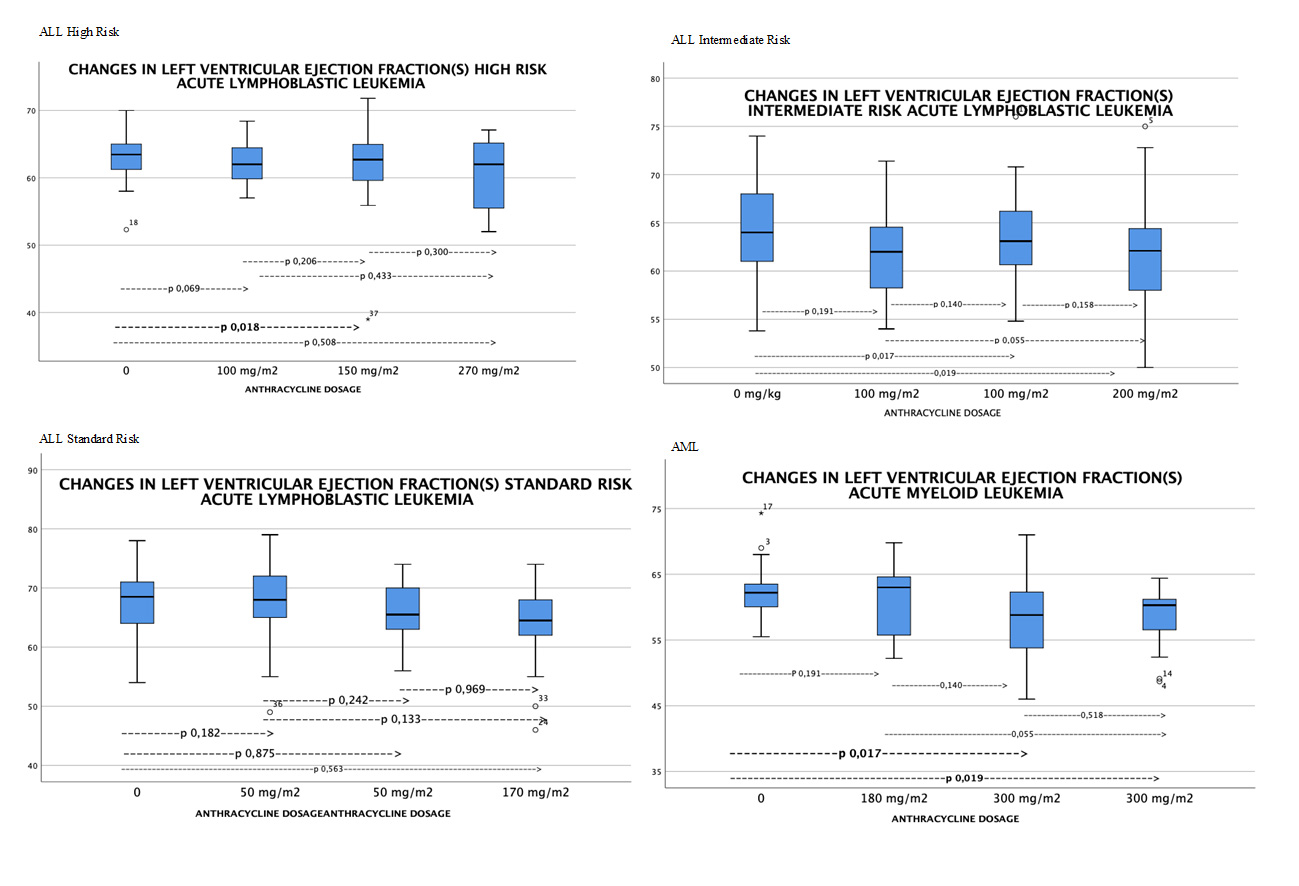
Figure 2 Changes in left ventricular ejection fraction by Simpson method by type of leukemia (evaluated with Wilcoxon test, statistically significant differences p <0.05).
In patients with high-risk leukemia, the factors related to cardiotoxicity were the presence of systemic arterial hypertension at any time of treatment with an OR: 8.57 (IC 95%: 1.35-54.35) p: 0.022, pulmonary hypertension with an OR: 4.11 (IC 95%: 1.12-15.05) p: 0.038, the global longitudinal myocardial deformation delta (global longitudinal myocardial deformation difference greater than 15%) with an OR: 19.5 (CI 95%: 4.57-83.09) p <0.000 and the decrease in LVEF (S) by more than ten percentage points with respect to the baseline evaluation by echocardiogram, with an OR: 56 (CI 95%: 6.31- 496) p< 0.000.
When performing the Wilcoxon test analysis for paired samples, a statistically significant difference was observed for LVEF (S) p: 0.018 and global longitudinal myocardial deformation p: 0.004 between the baseline echocardiogram evaluation and the evaluation after receiving 150 mg / m2 of doxorubicin.
Serum biomarker analysis
No relationship was observed between the presence of cardiac involvement and alteration in TnI or TnT (Appendix 3).
Acute lymphoid leukemia standard risk: no patient presented elevation in serum biomarkers throughout all evaluations.
Acute lymphoid leukemia intermediate risk: 69% of patients with acute lymphoid leukemia intermediate-risk had no abnormality in serum biomarkers. There was an elevation of brain natriuretic peptide in 12 patients (29.3%), 11 after intensification, and one patient in the end-of-treatment evaluation.
In two patients with altered biomarkers, an associated repolarization disorder was found, without echocardiographic manifestations. Elevation of TnT was found in one patient; in the last evaluation, this patient presented LVEF <53% asymptomatic.
Acute lymphoid leukemia high risk: There was no alteration in TnT or TnI. In 8 patients (24.2%), brain natriuretic peptide elevation> 100 pg/mL was found during the study. One patient with elevated brain natriuretic peptide at the end of induction had a severe infection associated with it. At the time of the third evaluation (at the end of the second high-risk block 2), three patients presented elevation of brain natriuretic peptide secondary to cardiomyopathy in the clinical context of infection; in 2 patients, an elevation not related to infection was found. At the last evaluation, two patients had elevated brain natriuretic peptide, none related to infection or sepsis.
Acute myeloid leukemia: No patient presented alterations in troponin T or troponin I. Regarding brain natriuretic peptide, one patient presented elevation at diagnosis with recovery to normal values during follow-up, two presented elevation in the third evaluation (after the second 7+3 cycle), and three at the end of the treatment, one of them persisted with the abnormal value from the previous measurement.
Discussion
Cardiovascular compromise is the most frequent adverse effect, with a deterioration in the quality and life expectancy of children cured of acute leukemias 18,19. The prospective methodology of this study allowed the real-time evaluation of the identification of early-onset cardiovascular compromise. Few prospective studies evaluate this complication; a critical aspect for identifying and reporting early toxicity is the difference in times used by the different authors 19,20.
Early-onset toxicity was found in 13.5% of patients with acute lymphoid leukemia high-risk 21, very similar to that found by Lipschultz et al. (19, who found 10% within the first year of exposure to anthracyclines. In this study, acute lymphoid leukemia high-risk patients presented cardiotoxicity with 150 mg/m2 anthracycline cumulative doses at a median of 99 days. This lower frequency is similar to that reported by Militaru et al.’s prospective study, which found an LVEF drop below 50% in 28.5% of children with acute lymphoid leukemia at three months of treatment (22. It is not clear why the difference in the incidence of this condition with a similar evaluation period. There is an association between the accumulated dose of anthracycline and cardiomyopathy. A greater probability is reported with an accumulated dose> 250 mg/m2, however, there is evidence of toxicity at doses starting at 45 mg/m2 (23,24. During this cohort’s follow-up, no patient presented toxicity with doses lower than 50 mg/m2. Therefore, echocardiographic surveillance of patients with higher doses of anthracyclines must be carried out throughout treatment.
In the follow-up of childhood cancer survivors exposed to anthracyclines, estimating myocardial deformation and its changes over time exposed to chemotherapy can predict the appearance of cardiac dysfunction related to cancer therapy 14,25,26. Despite the heterogeneity in the data regarding the age of the patient, the type of cancer, and the evaluation techniques used, a meta-analysis found that changes in myocardial deformation precede changes in LVEF in exposed patients to anthracyclines, even at doses lower than those historically thought to be cardiotoxic (27. Oikonomou et al. (28 defined an abnormal absolute value of the global longitudinal myocardial deformation between -18 and -19% (with General Electric platforms), a cut-off point associated with cardiac dysfunction related to cancer therapy with an OR: 12.27 (CI 95%: 7.73-19.47); this is similar to what was found in the cohort of our study. The evidence of alterations in global longitudinal myocardial deformation is strong in favor of predicting the subsequent decrease in LVEF at diagnostic values of cardiac dysfunction related to cancer therapy (less than 53%), and that is why international guidelines have adopted the concept of subclinical left ventricular dysfunction in those patients with an LVEF greater than 53% but with a decrease in myocardial deformation (global longitudinal myocardial deformation delta) greater than 15% with respect to the baseline evaluation (8.14). Our study found that patients with acute lymphoid leukemia high-risk with global longitudinal myocardial deformation delta more than 15% had cardiac dysfunction related to cancer therapy.
The fall in LVEF (S) by more than ten percentage points, but less than 53%, constituted the leading risk factor for cardiac dysfunction related to cancer therapy in the population analyzed in this study. The vast majority of consensuses consider that a reduction in LVEF between 10% and 20% in asymptomatic patients is not a sufficiently sensitive parameter to define cardiac dysfunction (27-29. Early identification of echocardiographic abnormalities described (defined as predictors of cardiac dysfunction related to cancer therapy) potentially makes it possible to identify patients with a higher risk of developing heart failure over time. The use of medications such as angiotensin-converting enzyme (ACE) inhibitors, beta-blockers (30,31, and the use of cardioprotection (32) could be considered.
In this study, the measurement of the left ventricular ejection fraction by the Teicholz method was compared with the biplane Simpson method, finding that the Teicholz method overestimated baseline LVEF compared to the biplane Simpson method, this is explained by its geometric assumptions and its regional rather than global character in the assessment of LVEF (17,30. Most consensuses recommend using the biplane Simpson method in 2D echocardiography since it has a better correlation with the current method of choice for estimating LVEF: 3D echocardiography. (17. Unfortunately, the use of the Simpson biplane method in pediatric oncology centers in the country is scarce.
No relationship was found between echocardiogram and ECG findings in patients with cardiac dysfunction, similar to that described by Pourier et al. (33, who found no association between these two diagnostic tests in childhood cancer survivors in a 5-year follow-up. Few studies on children define electrocardiographic alterations related to early-onset cardiac dysfunction. Most publications describe the late effects associated with the use of anthracyclines (34. Arrhythmias are more frequent in adults undergoing antineoplastic drugs with pre-existing cardiovascular diseases than in children who have transient rhythm disturbances (35.
In this study, no relationship was found with female gender and age at diagnosis less than four years old, referenced in the literature as risk factors for cardiac toxicity associated with the use of antineoplastic drugs. 36-38.
A higher incidence of cardiac toxicity was recorded in the group of patients with acute lymphoid leukemia for whom systemic arterial hypertension or pulmonary hypertension was documented in the follow-up and in the group of acute myeloid leukemia in whom pulmonary hypertension was found than in patients without these findings. This difference was statistically significant, unlike those reported in the literature on the low association of HTN and PHT with cardiac toxicity in the pediatric population (6.
In this study, no significant difference was documented between alterations in the cardiac biomarkers evaluated and the appearance of cardiac dysfunction related to cancer therapy or as predictors of it. This contrasts with the findings of the systematic review of the literature by Dolci et al. (39, who found that troponin T’s elevation could predict the appearance of left ventricular dysfunction up to three months before the appearance of clinical manifestations of cardiomyopathy.
No association was found between the elevation of brain natriuretic peptide and the cumulative dose of anthracyclines or cardiotoxicity in acute lymphoid leukemia patients. Armenian et al. (40 suggest using brain natriuretic peptide and troponins in symptomatic patients and not as predictors of toxicity.
One limitation of this study is that not all the patients completed the follow-up due to the disease’s circumstances; the variability between the echocardiography equipment and software means that the results found are applied to the patients evaluated with the technology methodology described.
Conclusions
Comprehensive cardiovascular evaluation and monitoring of patients with acute leukemias are essential, taking into account the high incidence of alterations in this system that in the short and long term can affect the quality of life and survival of these patients. In this study, the main risk predictors for cardiac dysfunction related to cancer therapy were a fall in LVEF of 10 percentage points (to values higher than 53%), alteration of global longitudinal myocardial deformation (absolute value and delta of global longitudinal myocardial deformation), and the presence of pulmonary and arterial hypertension. The timely identification of predictors of cardiac dysfunction in the pediatric population undergoing cancer therapy makes it possible to prevent complications and sequelae.











 text in
text in 



