Remark
| 1) Why was this study conducted? |
| This paper presents the applications of damage control in the management of patients with lung trauma. |
| 2) What were the most relevant results of the study? |
| Identification of specific patterns and the recognition of hypothermia, acidosis or coagulopathy, constitute the indication for a damage control thoracotomy. In these cases, the surgeon executes an abbreviated procedure with packing of the bleeding surfaces, primary management with packing of some selected peripheral or transfixing lung injuries, and the postponement of lung resection, clamping of the pulmonary hilum in the most selective way possible. The abbreviation of the thoracotomy closure is achieved by suturing the skin over the wound packed, or by installing a vacuum system. |
| 3) What do these results contribute? |
| The present article proposes an algorithm for the management of lung trauma, in which the decision point lays in characteristics of the injury, like the localization, whether it is peripheric, central or multiple, and in its hemodynamic status. Providing a practical approach regarding surgical management and when to decide to perform damage control surgery. |
Introduction
Surgical damage control techniques seek to minimize time and surgical magnitude in order to restore the initial physiological derangement, for a following definite surgery to complete the final repair. These techniques were initially described for the management of abdominal injuries 1,2). Damage control surgery has been extended beyond the abdomen and to situations other than trauma, after it has demonstrated to increase probability of survival in physiologically exhausted patients with shock 3.
This paper presents the applications of damage control in the management of patients with lung trauma. This article is a consensus that synthesizes the experience acquired during the last 30 years in trauma management, general surgery and critical care of the trauma and emergency surgery group (cirugía de Trauma y Emergencias-CTE) in Cali, Colombia, conformed by experts from the Hospital Universitario Fundación Valle del Lili and the Hospital Universitario del Valle “Evaristo García”, with the Universidad de Valle and Universidad Icesi, in collaboration with the Colombian Association of Surgery and the Pan-American Trauma Society, joined by international specialist from United States.
Epidemiology
Frequency of thoracic trauma is highly variable, depending on the reported setting (pre-hospital, hospital or post-mortem), trauma mechanism and reporting of all thoracic injuries or only the ones with severity criteria. The reported prevalence of lung trauma fluctuates between 5-78%, with a median of 22% 4-8. The prevalence of thoracic injuries was higher in patients with penetrating thoracic trauma, compared to patients with blunt thoracic trauma and those patients who died 4,5.
Most thoracic trauma cases are solved with minor procedures, and only a small proportion requires thoracotomy. Historically, the proportion of patients requiring a thoracotomy fluctuates between 15-25%, being higher in penetrating trauma 9-12.
Damage control surgery for the management of lung trauma has been reported by a limited number of authors, although in recent years the number of publications on the subject has increased. Vargo and Battistella 13 from Sacramento-United States, and García et al. from Cali-Colombia 14, reported experiences prior to 2015 with a rate of less than 6% of patients with thoracotomy who have undergone damage control surgery. However, reports of the last five years showed a rate of 22% and 21%, respectively 12,15. Chica et al., reported that 42% of patients who returned to spontaneous circulation after an emergency thoracotomy were managed with damage control surgery 16.
The lung is the most frequently compromised organ in patients requiring damage control thoracotomy. A sub-analysis of patients with thoracic trauma who underwent emergency thoracotomy in Cali-Colombia reported that 72% of patients managed by damage control thoracotomy presented lung trauma, compared to those who did not required damage control thoracotomy, only 56% presented with lung trauma 16. Similar to the case series from Baltimore-United states, were 86% of patients who require damage control thoracotomy presented with lung injuries 15.
The reported experience of managing lung damage control with anatomical lung resection is limited. Some case series have reported a pneumonectomy or lobectomy rate of 32% O’Connor and 26% Deane for Baltimore-United States 13,15, 20% García and 15% Chica for Cali-Colombia 14,16. This last group has reported a rate greater than 3% of patients with lung resections without the need damage control thoracotomy 16.
Indications
Exsanguination that leads the patient to depletion of the physiological reserve is the primary condition in a patient with thoracic trauma who will benefit from the management damage control principles. There are no clearly defined criteria as indications for thoracic damage control. Traditionally, it has been established that the identification of the triad of death, or the recently proposed lethal diamond (hypothermia, acidosis, coagulopathy and hypocalcemia), allows to recognize the individual who requires a damage control procedure 17-19.
Research aimed to identify variables associated with the risk of damage control thoracotomy have found that alterations in arterial pH, lactate levels, base deficit, preoperative hypothermia 15, preoperative neurological status with alteration of consciousness, magnitude of hemothorax, need of aortic occlusion, central lung injury, mayor vascular injury, tracheobronchial injury or multiple bleeding sites are associated with the decision of a damage control surgical strategy 16. (Table 1)
Table 1 Indications for damage control thoracotomy
| Indications for damage control thoracotomy | |
|---|---|
| Physiological parameters | Glasgow <9 |
| Temperature < 35 C at the beginning of surgery | |
| Arterial pH <7.2 | |
| Base deficit >15 mmol/L | |
| Lactate >5 mmol/L | |
| Clinical parameters | Need for resuscitative thoracotomy |
| Need for aortic occlusion | |
| Massive hemothorax | |
| Central lung injury | |
| Mayor vascular injury | |
| Tracheobronchial injury | |
| Multiple bleeding sources | |
| Clinical coagulopathy | |
It should be borne in mind that that methods that seek for laboratory test alteration have the risk of delaying decision-making while waiting for results, in a context where every minute counts. Therefore, it is advised that in addition to the components of the triad of death, the identification of certain clinical patterns previously mentioned can aid in early recognition of the indication for damage control 16-18.
Initial approach and diagnosis
Initial management should be directed towards hemodynamic stabilization according to the ATLS (Advanced Trauma Life Support) guidelines and the principles of damage control resuscitation. Upon arrival of a patient with a penetrating thoracic injury, it must be determined whether it warrants immediate surgical exploration or additional imaging studies, depending on its hemodynamic status. If the hemodynamic condition is stable, imaging methods such as FAST ultrasound, chest X-ray or chest CT are adequate to assess periclavicular, transmediastinal o thoracoabdominal injuries 11.
Lung trauma is commonly classified according to the American Association for the Surgery of Trauma (AAST) classification. Nevertheless, this classification has a limited value in defining surgical treatment (Table 2).
Table 2 Lung injury classification from the AAST
| Grade* | Injury type | Description |
|---|---|---|
| I | Contusion | Unilateral, <1 lobe |
| II | Contusion | Unilateral, single lobe |
| Laceration | Simple pneumothorax | |
| III | Contusion | Unilateral, >1 lobe |
| Laceration | Persistent (>72 h) air leak from distal airway | |
| Hematoma | Nonexpanding intraparenchymal | |
| IV | Laceration | Major (segmental or lobar) air leak |
| Hematoma | Expanding intraparenchymal | |
| Vascular | Primary branch intrapulmonary vessel disruption | |
| V | Vascular | Hilar vessel disruption |
| VI | Vascular | Total uncontained transection of pulmonary hilum |
*Advance one grade for bilateral injuries up to grade III. Hemothorax is scored under thoracic vascular injury scale
The present article proposes an algorithm for the management of lung trauma, in which the decision point lays in characteristics of the injury, like the localization, whether it is peripheric, central or multiple, and in its hemodynamic status. Providing a practical approach regarding surgical management and when to decide to perform damage control surgery (Figure 1).

Figure 1 Algorithm for Surgical Management of Lung Trauma. Anotations: 1. Aortic occlusion can be made during the preoperative phrase by an endovascular balloon occlusion (REBOA). If the device or the protocols are not available, a left thoracotomy for aortic-cross clamping is indicated. 2. If a resuscitative thoracotomy is required, a left anterolateral incision must be performed. A sternotomy is indicated in central wounds. 3. Consider autotransfusion. 4. Packing around a peripheral injury or packing of a through- and-through wound should only be considered in patients with other more complex injuries requiring surgical team’s attention. 5. Packing lung wound should be only deemed the definitive method only in complicated cases with exhausted physiologic reserve and hemorrhage control. 6. Anatomic resection in the index operation is recommended only if hemorrhage control is immediate and physiologic is almost completely restored. 7. Temporary chest closing can be achieved by packing the wound and suturing the skin over the pads or by a vacuum system.
Surgical approach
Step 0: Profound preoperative hypotension (systolic arterial blood pressure <70 mmHg) constitutes an indication for therapeutic aortic occlusion to provide hemodynamic support 20. This can be achieved through an emergency thoracotomy to perform an aortic cross-clamping or with the placement of a REBOA (Resuscitative endovascular balloon occlusion of the aorta) in zone I 21, this last one, especially when the aorta is not easily accessible, for example when performing a right thoracotomy 22.
The placement of a REBOA, ideally, should be managed by the articulation of two surgical teams, while one is performing the endovascular procedure the other one is performing the main surgical approach. Additionally, the massive transfusion protocol must be activated, along with the administration of tranexamic acid. However, none of these actions should delay the immediate surgical intervention.
Step 1: Access to the thoracic cavity depends on the surgeon’s experience and the thoracic area where the injury is suspected. The topographic localization of the injury and the identification of massive hemothorax detected through imaging studies or drainage through a chest-tube guides the decision. The most common reported surgical approaches are the left or right anterolateral thoracotomy. An incision is made in the fourth or fifth intercostal space up to the midaxillary line, and if further surgical exposure is needed, an extension of the surgical incision can be made to the contralateral side of the chest, in a Clamshell incision 12-14.
Median sternotomy is a surgical approach that allows for a better cardiac and great vessel visualization, and if further surgical exposure is needed, an extension of the surgical incision can be made with a cervicotomy or supraclavicular incision 12-14. However, its execution usually requires an extended surgical time, therefore it must be performed in specific situations, by trained and experienced surgeons counting with the accessibility to surgical instruments for its rapid execution.
Upon entering the thoracic cavity, the surgeon must:
Confirm correct intubation: verify lung expansion
Evacuate of the hemothorax: introducing suction into the cavity
Identify the possible source of massive bleeding: packing of the posterior and anterior region must be performed using the minimum number of lap sponges to have a better visualization of the surgical field when controlling the bleeding.
After these initial maneuvers, the surgeon must reevaluate the patient’s hemodynamic status, if hypotension persists, descending aortic occlusion is required. Digital occlusion can be performed if hypotension is present and the sources of bleeding are easily controlled. However, if profound hypotension or bradycardia are present, or the need to perform advance maneuvers for bleeding control are required in different organs, aortic cross-clamping is indicated 23.
If an associated cardiac injury is suspected, a diagnostic pericardial window should be performed. In case of cardiac arrest or ineffective cardiac activity, cardiac massage should be performed 23.
Step 2: transient bleeding control. Injury to most thoracic structures can cause considerable blood loss in a short time. The surgeon must be able to execute rapid and effective maneuvers for bleeding control, causing the least possible additional trauma, simultaneously to the damage control resuscitation.
Transient bleeding control of lung hemorrhage depends on the localization of the lesion, which can be central, peripheral, or multiple injuries.
Peripheral injuries: bleeding control can be achieved by manual compression of the tissue or by the placing a Duval forceps 14,24 (Figure 2). Transient bleeding control of small injuries can be achieved by the placement of one or two lap sponges between the thoracic wall and the lung parenchyma 18,24-26. Transient bleeding control of a transfixing lung injury can be achieved by manual compression of the tissue, by the placement of Duval forceps or by the insertion of a lap sponge through the wound (Figure 3) 24. These procedures should be performed in hemodynamically unstable patients, with multiple bleeding sites and possibly damage control candidates. When performing thoracic packing, attention must be taken to avoid compressing the heart or mediastinal shifting, which can affect diastolic filling with a decrease in cardiac output 27.
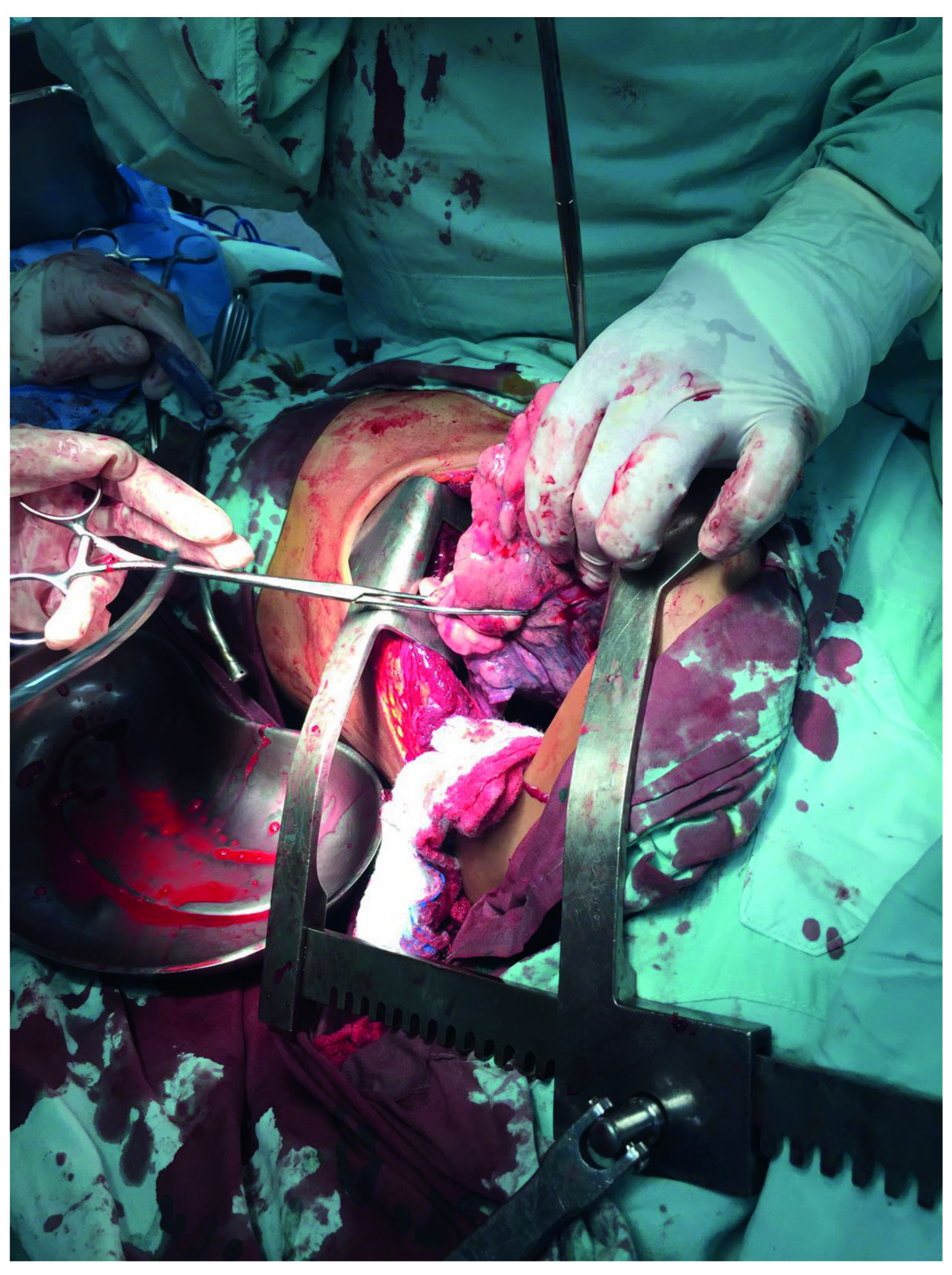
Figure 2 Transient hemorrhage control for peripheral injuries. Direct pressure with the surgeon's hand or a Duvall clamp frequently stops the hemorrhage transiently in a peripheral parenchymal wound.
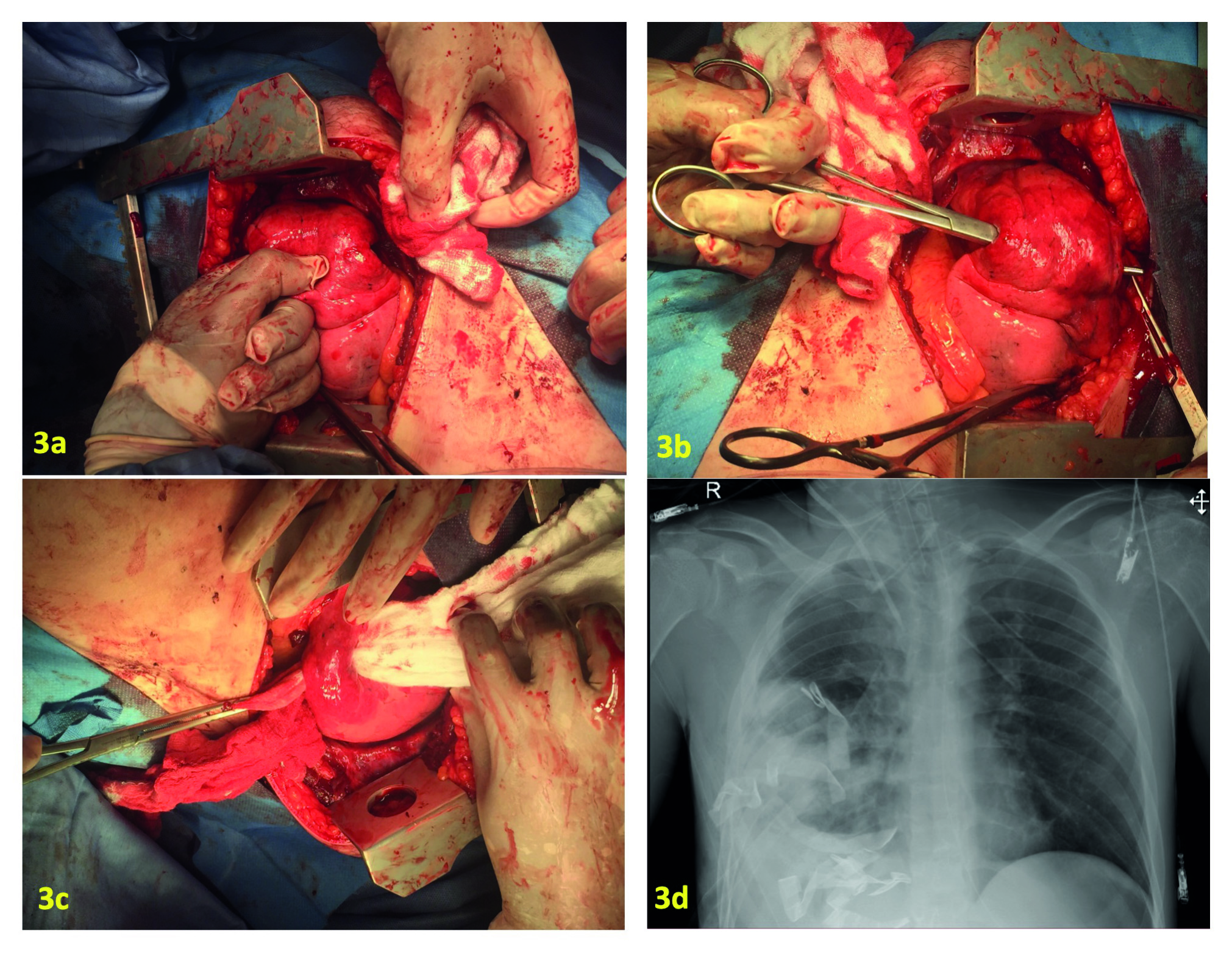
Figure 3 Packing of the tract in a through-and- through peripheral lung wound. a) The surgeon introduces a finger gently in the tract, exploring it, and preparing to guide a hemostatic clamp. B) The hemostatic clamp has been introduced in the wound guided by the surgeon’s finger. C)The surgeon exerts traction with a laparotomy pad driving it into the wound, and gently adapting it to the wound shape. D) A Postoperative Chest X-Ray obtained immediately post procedure depicts, the intraparenchymal packing filling the lower part of the thorax, permitting the lung expansion without mediastinal deviation.
Central or multiple injuries: clamping of the pulmonary hilum must be performed using a vascular clamp (Figure 4) 14,24,28,29. Clamping is achieved by advancing the surgeons' left hand between the heart and the lung, sliding the palm over the lung surface until reaching and embracing the hilum with the index and middle fingers for a posterior occlusion of the hilum with a vascular clamp. Another reported technique is the pulmonary hilum twist in an emergency room thoracotomy 30.
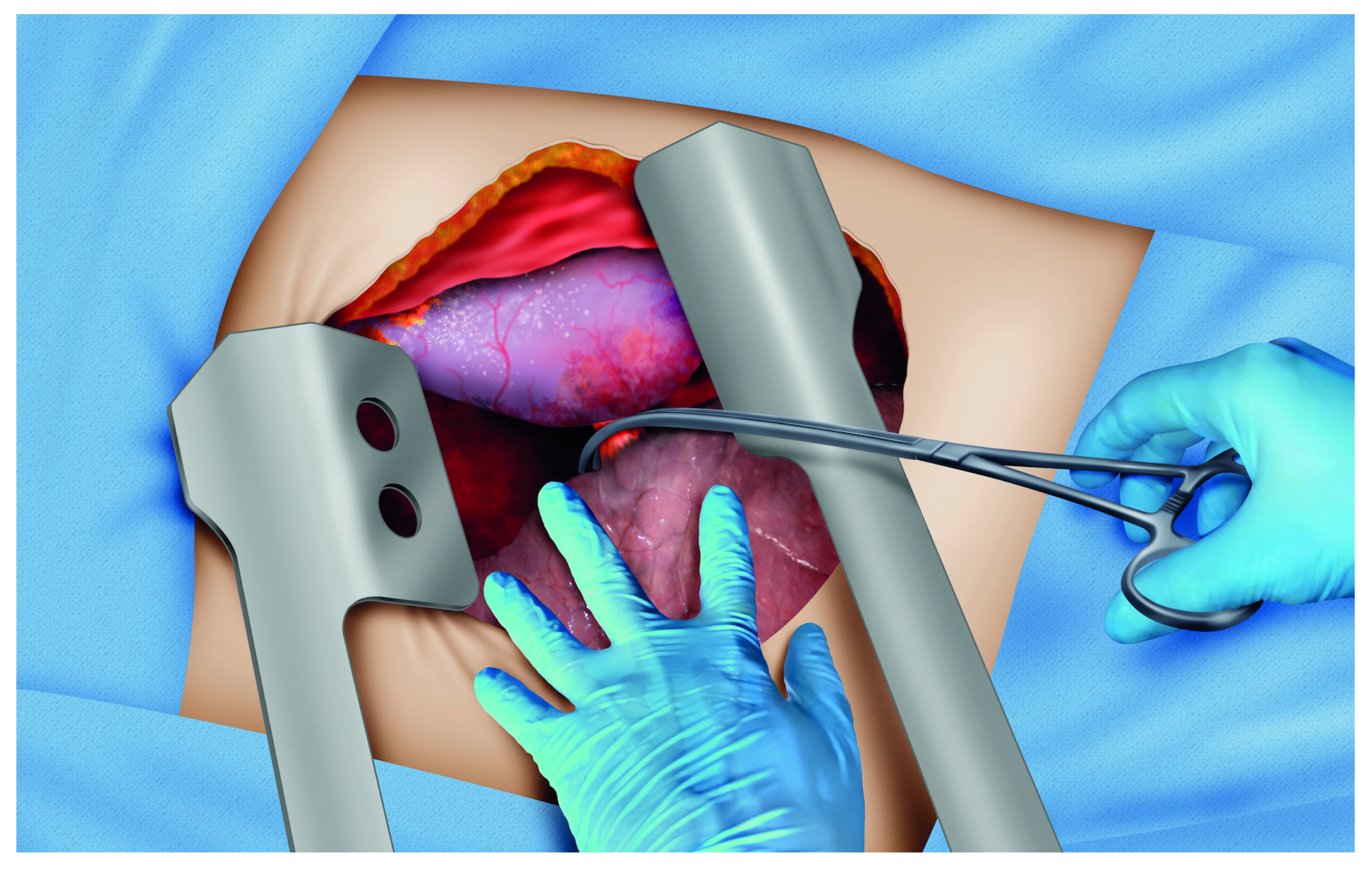
Figure 4 Cross clamping of the lung hilum. This patient has multiple exsanguinating lung wounds. The surgeon's left hand is introduced between the lung and the heart, making room for the clamp application. The index and middle fingers embrace the hilum to make facilitate the placement of the atraumatic clamp.
Step 3: Wound control. After bleeding control is achieved, management of the lung injury should be performed using conventional or damage control techniques. Localization of the wound must be determined if it is peripheral or central, single or multiple.
Tractotomy has being reported between 9-44% in patients with lung trauma and damage control surgery 13,14. This technique allows control of peripheral and transfixing wounds in an expeditious manner while preserving lung tissue. This technique must be carried out by opening the wound tract, taking the lung tissue with atraumatic forceps (intestinal or aortic Crawford clamps) and making a manual cutting of the tissue with surgical scissors (31), or by mechanical linear stapler GIA 60-80 32 (Figure 5). Once the tract has been opened, the surgeon must identify bleeding sites and air leaks, then ligate them with a cross-stitch (in X) of polypropylene 3-0 or 4-0 31,32. If the wound tract has been open by cutting the lung tissue between atraumatic clamps, a continuous horizontal mattress hemostatic suture should be performed, with stitches below the clamps along the cross section 31 (Figure 5). Frequently, a second line of running suture is necessary to achieve complete hemostasis. However, it is recommended to open the wound tract with one or two shots of linear stapler, which saves time and minimizes additional lung trauma. In coagulopathic patients, one or two lap sponges should be placed on the lung surface, since superficial bleeding may be present 14.
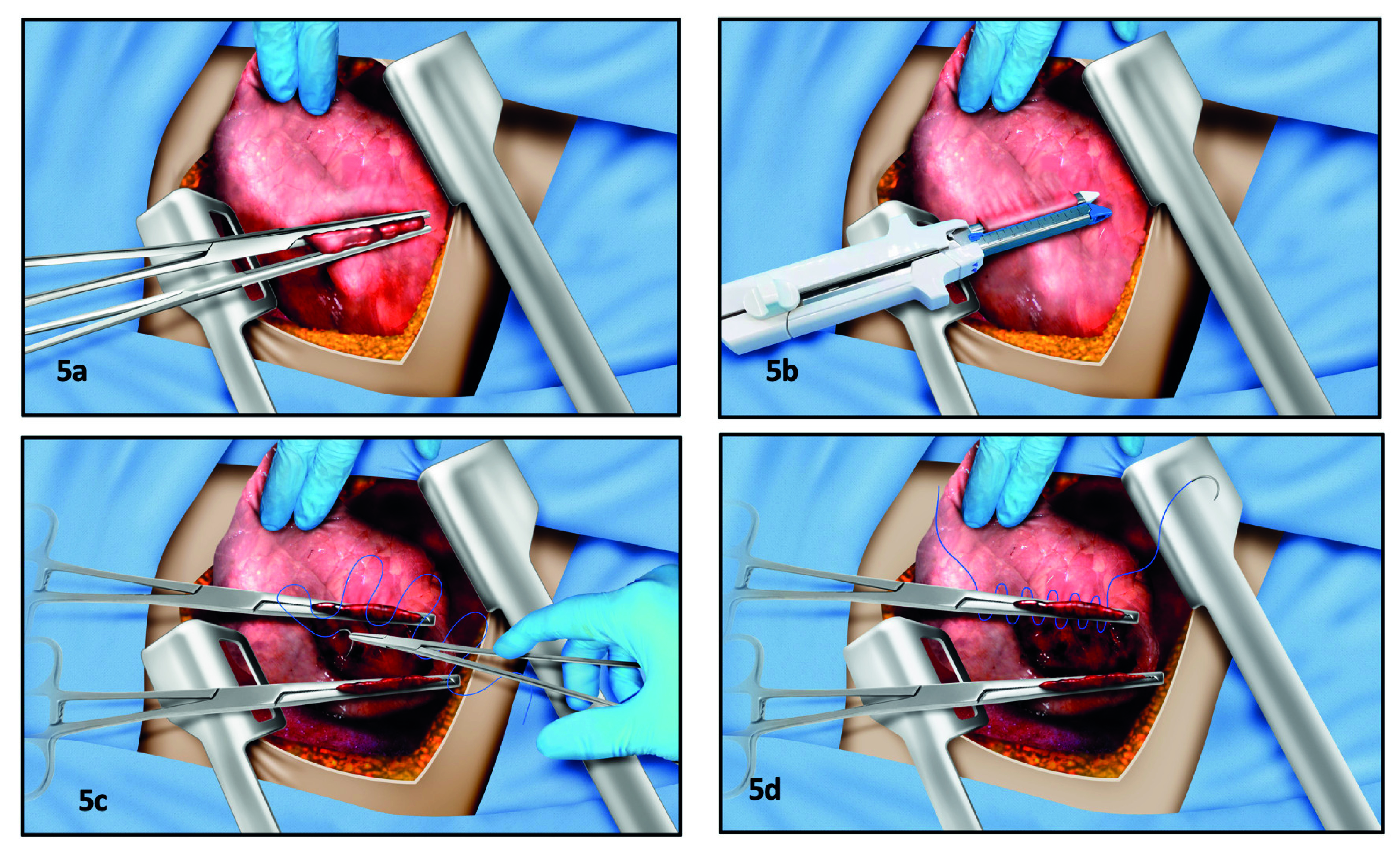
Figure 5 Pulmonary tractotomy. A) Two atraumatic clamps take the bridge of tissue across the tract created by the penetrating injury. The tissue is cut with a scalpel or a scissor, exposing the interior of the wound. B) The surgeon can drive the linear staple along opening of the tract, thus reducing surgical trauma, saving time, and avoiding blood loss. C) After exposing the inner aspect of the wound, the bleeding vessels and the air leaks are selectively ligated with 3/0 monofilament in figure of “8” stitches. D) If the tract was opened with clamps and scissors or scalpel, the tissue beneath the clamps must be oversewn with a monofilament 3-0 suture. Occasionally a second suture line must be performed to achieve the hemostasis, as is shown in the figure.
Performing a tractotomy is no synonym of damage control surgery. The decision to determine if the patient can be managed definitely in the first surgery or if an abbreviated approach is required, is determined by the analysis of the physiological state, concomitant injuries and the need to perform additional procedures of greater technical skill.
The management of patients requiring emergency thoracotomy performed in Cali-Colombia were reported by Chica et al 16. The characteristics and clinical results are described in Table 3.
Table 3 Management characteristics and clinical results of patients with emergent thoracotomy with and without damage control surgery.
| Variable | Damage control surgery (n = 53) | Initial definitive surgical management (n = 63) |
|---|---|---|
| Resuscitative thoracotomy, n (%) | 14(26.4) | 4(6.4) |
| Aortic clamping, n (%) | 18 (34.0) | 5 (7.9) |
| Lung trauma, n (%) | 38 (71.7) | 35 (55.6) |
| Pneumorrhaphy, n (%) | 12 (22.6) | 9 (14.3) |
| Tractotomy, n (%) | 16 (30.2) | 16 (25.4) |
| Lung resection, n (%) | 8 (15.1) | 2 (3.2) |
| Lung packing | ||
| Primary method, n (%) | 9 (17.0) | -- |
| Complementary method, n (%) | 14 (26.4) | -- |
| Clamping of the pulmonary hilum, (n%) | 10 (18.9) | 4 (6.3) |
| Vascular trauma, n (%) | 25 (47.2) | 18 (28.6) |
| Intercostals or internal thoracic minor vessels, n (%) | 17 (32.1) | 16 (25.4) |
| Mayor vascular injury | 8 (15.1) | 1 (1.6) |
| Cardiac trauma, n (%) | 9 (17.0) | 26 (41.3) |
| Thoracic wall packing, n (%) | 23 (43.3) | -- |
| Extrathoracic damage control, n (%) | 24 (45.8) | 9 (14.3%) |
| In-hospital mortality | 13 (24.5) | 6 (9.5) |
Pneumorrhaphy or wedge lung resection with a linear stapler after clamping injured lung tissue with a Satinsky forceps is rarely used during a damage control thoracotomy 14,15. Nevertheless, they can be suitable for definite management of small peripheral injuries in patients with multiple bleeding sites.
In central or multiple injuries patients are candidates for an anatomical lung resection (pneumonectomy or lobectomy). These procedures are associated with a high mortality rate, that has not changed in the last 45 years 33. It is higher in patients with blunt trauma, and it increases as the extent of resection is greater 32,34,35. Due to the exsanguinating nature of central lesions, these patients are candidates for a damage control procedure. Authors have proposed transient clamping of the lung hilum to allow postponement of lung resection, once coagulopathy, hypothermia, and hypoperfusion have been reversed 14.
For this purpose, once bleeding control is achieved by clamping the lung hilum, the surgeon must evaluate the magnitude of the injury, and decide if it is a peripheric injury than can be managed by a wedge lung resection or a tractotomy. If it is a central injury that warrants resection 18, it must be evaluated if it is possible to selectively occlude the circulation of the injured lobe or if the hilum needs to stay clamped 14. Unless the patient has stabilized and a definitive hemodynamic stability is assured, it will be preferable to conclude the surgery with abbreviated maneuvers to allow resuscitation, and to perform the lobectomy or pneumonectomy in a semi-elective manner in a subsequent procedure, within the following 24 hours, in the company of a thoracic surgeon (Figure 6).
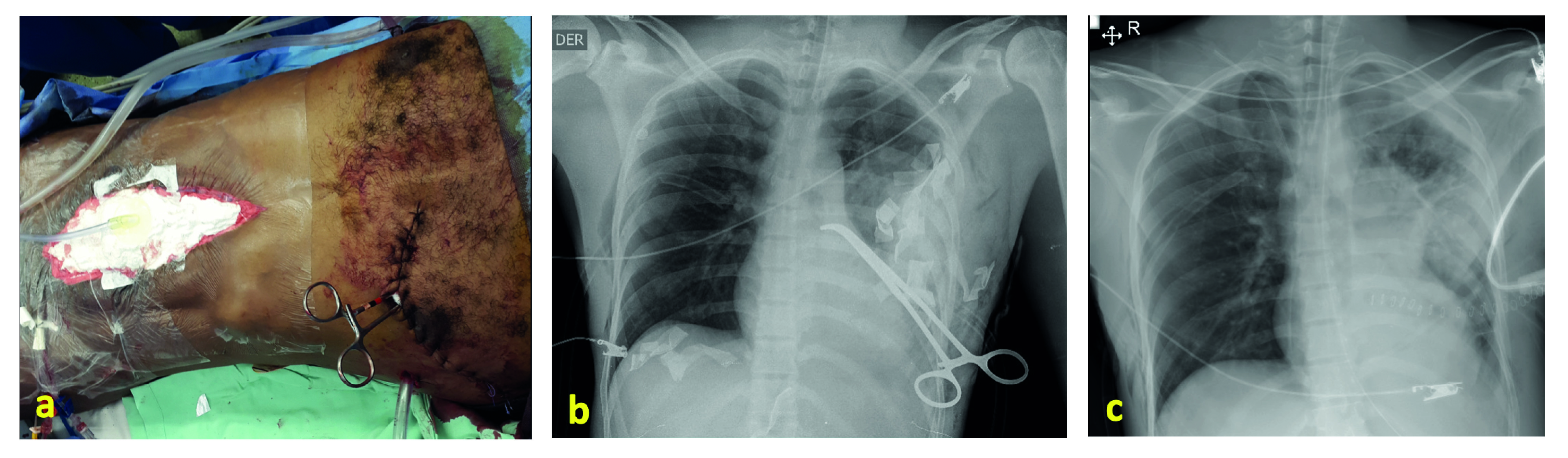
Figure 6 Clamping of the pulmonary hilum for a deferred anatomic resection. A) This patient was submitted to a resuscitative thoracotomy because of exsanguinating injuries in the lower left lobe, near the hilum, and the left hepatic lobe. He received open cardiac massage, aortic cross-clamping, and defibrillation (#3). Hemorrhage was arrested by clamping the vascular structures of the inferior lobe of the left lung and by liver packing. The chest was closed by suturing the skin over pads and the abdomen with a vacuum system. B) Postoperative chest x-ray showing the left superior lobe completely expanded. The clamp occludes the inferior left lobe's vascular structures, and there are pads packed in the lower part of the chest and upper abdomen. C) Chest X-rays after the lobectomy, which was performed two days after the index surgery.
Step 4: After completing the damage control procedure, the surgeon should check the bleeding sites and confirm absence of active bleeding. If necessary, lap sponges must be replaced, ensuring hemostasis with the minimum number of them. It must be checked that the packing is not compressing the vena cava or the heart. Abbreviated closure of a thoracotomy or a sternotomy is achieved by installing a negative pressure system or by packing the muscular layer and closing the skin 13,36,37. This last method is recommended by the authors, since it is a quick procedure to execute with a successful rate of bleeding control of the surgical wound. This technique must be performed by placing the lap sponges in the wound angles, especially in the area where blood is leaking through the thoracic wall, keeping them in place while the skin is being closed by a continuous suture, adjusting the lap sponge as the suture advances (Figure 7). The placement of a chest-tube is recommended to watch over thoracic bleeding.
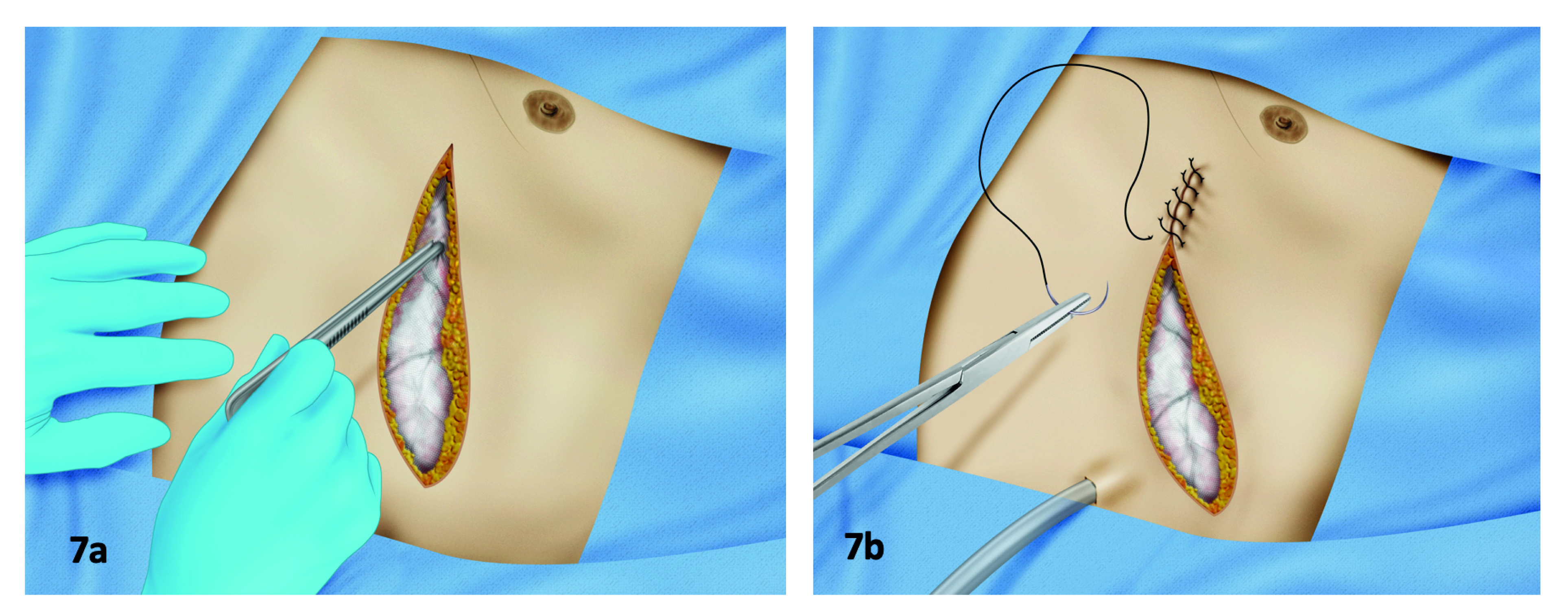
Figure 7 Packing and abbreviated closure of the thoracic wall. A) This technique expedites the closure of the thoracic incision. After checking for mechanical bleeding, the surgeon places two or three laparotomy pads in the wound, between the muscular layers and the subcutaneous fat, packing the edges and by doing this, oozing around the edges of the wound is controlled. B) The skin is sutured over the pads, completing the packing effect.
Prognosis
Patients treated with damage control thoracotomy are at high risk of complications. The need for an emergency reintervention due to persistent bleeding occurs in 14-24% of cases 13,14,26. Infectious complications such as pneumonia have been reported between 20-32%, while empyema has a prevalence between 4-23%. Organ dysfunction can occur in up to 88% of patients. Respiratory dysfunction has been reported between 25-84%, cardiovascular between 41-76% and renal between 20-43%. The requirement of renal-replacement therapy is present between 4-30% in patient with damage control thoracotomy (Table 4).
Table 4 Complications and intrahospital mortality before damage control thoracotomy
| Complication | O’Connor (n = 44) | Mackowski (n = 25) | García (n = 25) |
|---|---|---|---|
| Pneumonia (%) | 27 | 32 | 20 |
| Empyema (%) | 23 | 4 | 16 |
| Respiratory Dysfunction (%) | 25* | 36** | 84*** |
| Cardiovascular Dysfunction (%) | 41§ | -- | 76¶ |
| Renal Failure (%) | 43 | -- | 20 |
| Thoracic Compartment Syndrome (%) | 2 | 0 | 4 |
| Reintervention by bleeding (%) | 14 | 0 | 24 |
| Intrahospital Mortality (%) | 23 | 40 | 24 |
* Respiratory Distress Syndrome
**Prolonged mechanical ventilation
*** More than 2 days of ventilatory support.
§ Vasopressors support to discharge of surgery
¶ Vasoactive or inotropic drugs therapy
The in-hospital mortality associated with damage control surgery for lung trauma has been estimated at 28% according to a systematic review that grouped results from various case series 18. It has been identified that the severity of the trauma (measured by the Injury Severity Score - ISS), the magnitude of the required lung resection, renal dysfunction, requirement of renal-replacement therapy, extracorporeal membrane oxygenation support (ECMO) and penetrating trauma are associated with higher mortality 13,26. However, the experienced reported by Garcia et al. showed a higher survival rate in patients who required mayor lung resection (pneumonectomy or lobectomy), when the procedure was carried out in a subsequent surgery by leaving the lung hilum clamped in the first intervention, contrasting the experience reported by the case series from north America 14.
Intuitively, the reduction of bleeding due to the early decision to intervene the patient, the prompt bleeding control, the early application of the principles of damage control, and the decision of performing an abbreviated procedure will improve the survival probability of patients with severe thoracic trauma with depletion of the physiological reserve.
Discussion
Damage control thoracotomy has evolved from tractotomy, designed to shorten the surgery, to a series of procedures that temporarily control the sources of bleeding, allowing a deferred management of the lung injury. This in order to increase survival of patient with depletion of the physiological reserve, allowing resuscitation and a subsequent definitive management. Indications and execution of damage control procedures are still a matter of debate and controversy. Literature is limited, and few groups worldwide have reported their experience.
Early identification of whether a patient benefits from damage control surgery, or not, enables activation of resuscitation protocols and redirects surgical effort towards a damage control thoracotomy. Two groups have researched the variables that differentiate patients who require thoracic damage control 15,16. They have reported the following conditions that relates to a damage control approach: neurological status upon admission, resuscitative thoracotomy, need of a concomitant laparotomy, severe lung injury, mayor vascular injury, tracheobronchial injury, massive hemothorax, hypothermia, acidosis and coagulopathy.
Refractory hypotension (SBP <70 mm Hg, despite resuscitation) in the preoperatory period or during surgery when there is no access to the aorta, such as a partial sternotomy or a right thoracotomy, are indications for an aortic occlusion by the positioning of a REBOA in zone I, procedure that should be carried out by a surgical team that works simultaneously with those who are performing the surgical approach for the management of the injuries 20,21.
There is concern about increasing bleeding due to increased blood pressure. A recent study that included three porcine models of thoracic trauma (lung injury, thoracic venous injury or subclavian artery injury) revealed that the use of REBOA does not aggravate lung damage or increases exsanguination rate 38.
Authors have provided clinical evidence on the use of REBOA in this group of patients: Ordoñez et al. reported 23 patients with thoracic trauma managed with REBOA. Eleven of them had lung injury and six had involvement of the lung hilum. The most common management was placement of the REBOA in zone I, with sternotomy in 65.2% of patients and thoracotomy in 17.3%. No significant differences were found between observed and expected mortality, nor higher requirement for blood components 19. García et at. analyzed the impact of the REBOA on mortality in a group of patients with penetrating torso injury using a multivariate model. They found that the use of REBOA during surgery was associated with a better chance of survival 21.
The need for a tractotomy does not necessarily implies damage control surgery. The decision to perform, or not, a damage control thoracotomy depends on the physiological derangement presented by the patient. In the series of patients undergoing emergency thoracotomy reported by Chica et al., 50% of the tractotomies were performed outside the context of damage control surgery 16.
Packing is a technique that has been considered in thoracic damage control. Manzano et al. conducted a systematic review of the literature where they identified 14 studies 14. Most of the publications described the use of the technique as a complement to other procedures in coagulopathic individuals. In some reports, packing is described as the primary technique for treating a lung injury. In most cases by placing lap sponges between the surface of the injured lung and the chest wall. The intrapulmonary packing technique 24, was used in selected cases with various bleeding sources, some more complex, which required the attention of the surgical group.
Authors evaluated the impact of thoracic packing on mechanical ventilation. They found a reduction in dynamic compliance leading to a moderate decrease in tidal volume and moderate CO2 retention. Oxygenation was not compromised and in no case did the packaging induce critical difficulties for ventilatory support (Table 5) 39.
Table 5 Comparison between emergency thoracotomy patients treated with or without damage control and thoracic packing.
| Variable | Damage control surgery (n = 53) | Initial definitive surgical management (n = 63) |
|---|---|---|
| Respiratory parameters, first day post-trauma | ||
| FiO2, (median IQR) | 0.5 (0.5 - 0.5) | 0.5 (0.4 - 0.6) |
| PO2/FiO2, (median IQR) | 332.8 (155 - 3909 | 262.9 (173.3 - 413.2) |
| Arterial pH, (median IQR) | 7.27 (7.15 - 7.29) | 7.33 (7.26 - 7.37) |
| PCO2, (median IQR) | 41.6 (36.8 - 44.5) | 36.3 (32.3 - 42.5)* |
| Base deficit mmol/L (median IQR) | 8 (6 - 12.9) | 5.8 (3.7 - 9.0)* |
| Tidal volume ml, (median IQR) | 480 (450 - 500) | 510 (484 - 550)** |
| Respiratory rate, breathings/min (median IQR) | 18 (16 - 22) | 17 (15 - 20) |
| PEEP, H2O cm (median IQR) | 5 (5 - 8) | 5 (5 - 6) |
| Peak inspiratory pressure, H2O cm (median IQR) | 28 (25 - 32) | 25 (22 - 29)** |
| Dynamic compliance, (median IQR) | 21.5 (19.2 - 25) | 25.2 (22.7 - 31.9)** |
| Clinical results | ||
| Days-off of Mechanical Ventilation, (median IQR) | 25 (0 - 27) | 29 (24 - 29)*** |
| Patients with mechanical ventilation complications, n (%) | 9 (33.3) | 7 (18.9) |
| Atelectasis, n (%) | 6 (22.2) | 5 (13.5) |
| Pneumonia, n (%) | 6 (22.2) | 5 (13.5) |
| Pneumothorax, n (%) | 0 (--) | 3 (8.1) |
| Mortality, n (%) | 7 (25.9) | 5 (13.5) |
IQR interquartile range. PEEP Positive end expiratory pressure.
Wilcoxon-Mann-Whitney test * <0.05 ** <0.01 *** <0.001
Patients who require anatomical lung resection have a high risk of death, fluctuating between 50% and 100% in the different published series 31. The Trauma and Emergencies Surgery Group (Cirugía de Trauma y Emergencias - CTE) of Cali, has addressed this situation by occluding of the pulmonary hilum as selectively as possible, allowing resuscitation of the patient and the execution of pneumonectomy or lobectomy in a second stage. Five patients were managed in this way. Three patients underwent pneumonectomy and two lobectomy, only one case had a fatal outcome 14.
The technique of temporary wall closure can be performed by installing a commercial or handcrafted vacuum system, or by packing the thoracic wall and rapid closure with a running suture 13,14,37. There are no comparative studies, and both techniques seem to solve the need for rapid closure with control of thoracic wall bleeding.
Conclusion
Hemodynamic instability and severe lung trauma are indications for damage control surgery. Transient pulmonary bleeding control can be achieved for peripheral wounds via local pressure maneuvers and central or multiples wounds perform a clamping of the pulmonary hilum. The pulmonary resection should be reserved as a deferred procedure after the hemodynamic resuscitation in the intensive care unit.











 text in
text in 



