Remark
| 1) Why was this study conducted? |
| This article summarizes the recommendations of the Trauma and Emergency Surgery Group (CTE) Cali-Colombia about the specific situations concerning re-interventions of patients undergoing damage control surgery. |
| 2) What were the most relevant results of the study? |
| We suggest packing as the preferred bleeding control strategy, followed by unpacking within the following 48-72 hours. In addition, a deferred anastomosis is recommended for management of intestinal injuries, and patients managed with vascular shunts should be re-intervened within the first 24 hours for definitive repair. Furthermore, abdominal and/or thoracic wall closure should be attempted within the first 8 days upon admission. |
| 3) What do these results contribute? |
| These strategies aim to decrease the overall complication, morbidity, and mortality of these patients. |
Introduction
Rotondo and Schwab first described damage control surgery in 1993 and has become one of the most important advances in trauma surgery. Damage Control Surgery has impacted the morbidity and mortality of patients with major physiological impairment 1. This strategy is based on an abbreviated laparotomy with abdominal packing, which allows deferring definitive surgical management until the stabilization of physiological and hemodynamic parameters 2. Damage control surgery comprises a diverse spectrum of procedures such as intestinal discontinuity, solid organ packing, retroperitoneal packing, vascular shunts, and the open abdomen. All of these are temporary measures performed in response to a severe injury and metabolic debt, which requires an aggressive hemostatic resuscitation, deferred definitive management until an optimal physiological status is achieved 3.
When damage control measures have been completed; the next step is to establish the time and strategy for performing the next interventions 4. This decision is led by the evolution of physiological status and the severity of the injuries. However, it is accepted that to reduce morbidity and mortality rates, all definitive procedures such as intestinal/vascular reconstruction or abdominal/thoracic wall closure should be performed only when the diamond is lethal (metabolic acidosis, hypothermia, hypocalcemia, and coagulopathy) is corrected 5,6. This article summarizes key recommendations concerning the most relevant controversies in surgical re-intervention during damage control surgery such as unpacking, time-lapse for intestinal anastomosis, ostomy requirement, shunt removal time, vascular reconstruction, and strategy for definitive closure of the abdomen.
This article is a consensus that synthesizes the experience earned during the past 30 years in trauma critical care management of the severely injured patient from the Trauma and Emergency Surgery Group (CTE) of Cali, Colombia which is made up of experts from the University Hospital Fundación del Valle "Evaristo García", the University Hospital Valle del Lili, the Universidad del Valle and Universidad Icesi, the Asociación Colombiana de Cirugia, the Pan-American Trauma Society and the collaboration of national and international specialists of the United States of America.
Packing in damage control surgery
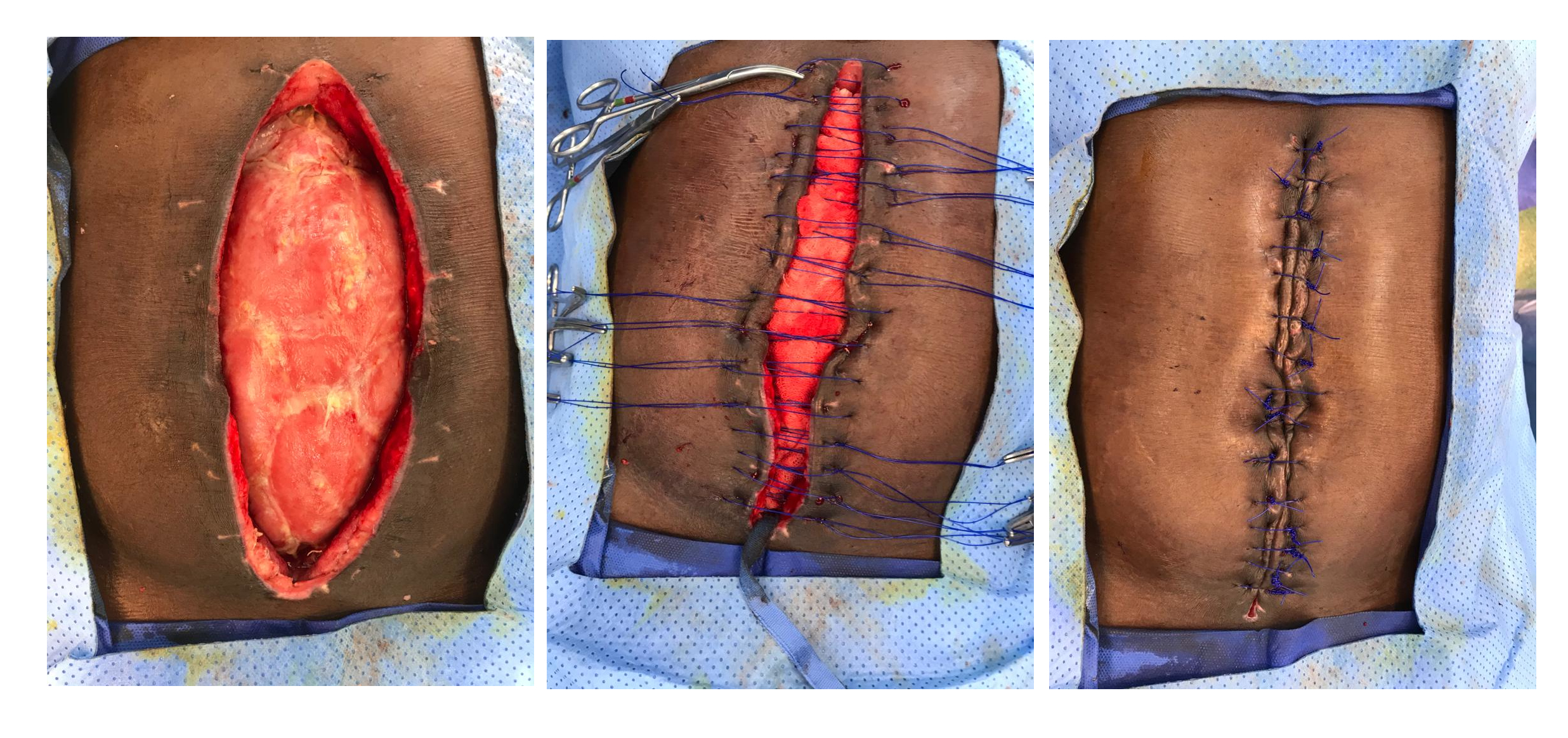
Cavity or organ packing is a widely used strategy to rapidly achieve hemorrhage control during the initial phase of damage control surgery. It is usually performed when massive bleeding is found secondary to severe organ destruction, where time is limited. Packing aims to temporarily or permanently stop the bleeding by applying sustained pressure with either gauze or compresses, which results in more than 90% efficacy 7. While indications for abdominal packing seem to be clearly defined, the optimal time to remove all packed material (gauze, compresses, or hemostatic agents) remains uncertain; most articles have reported a time range between 24-72 hours 8-11 (Figure 1).
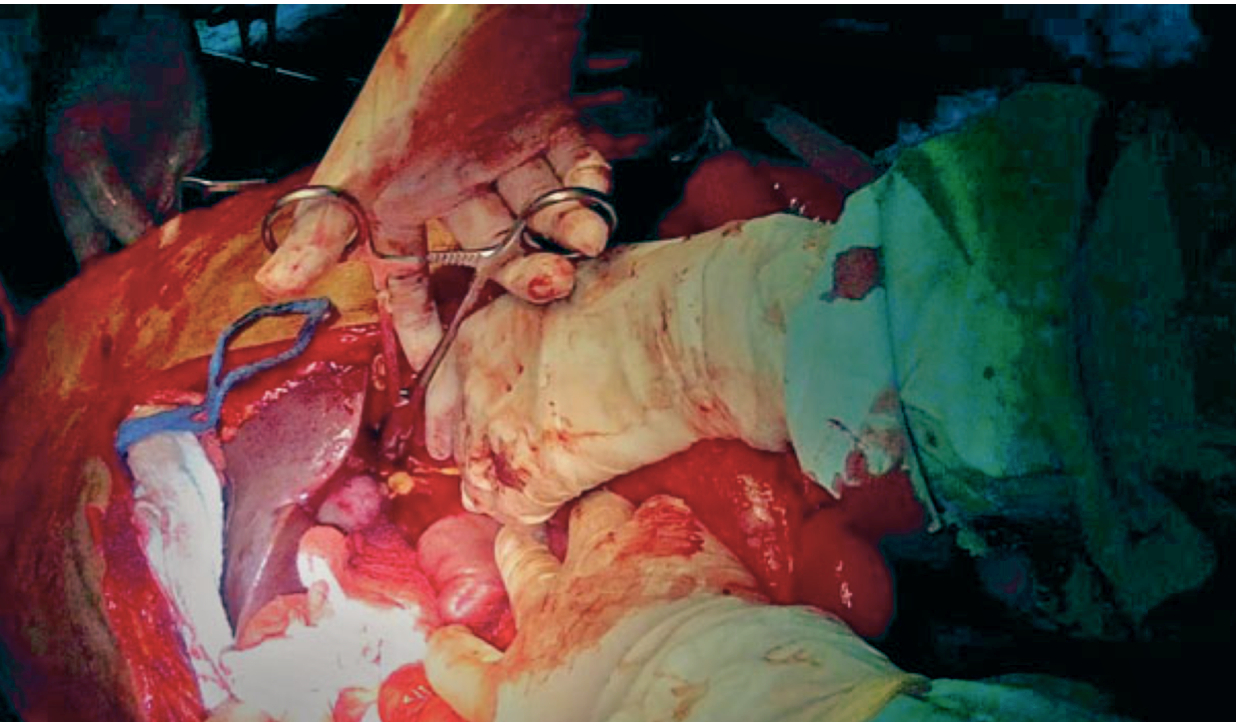
Figure 1 Abdominal Packing. Patient with a severe liver trauma that had been controlled via perihepatic packing to control of hemorrhage.
Aydin et al. described the use of abdominal packing in patients with severe liver trauma. Patients who were re-operated within the first 24 hours of trauma had ineffective packing (misplacement, excess or insufficient compresses) and required a longer stay in the intensive care unit and a higher transfusion of blood products 12. Nicol et al. obtained similar results when they compared patients who were intervened within the first 24 hours vs. those who intervened in 48 hours. They described a higher re-bleeding rate in the first group but no differences in the infectious complications between groups 13. Ordóñez et al., describe that premature packing removal increases the risk of re-bleeding while prolonging packing for more than 72 hours increases infectious complications 7. Kang et al. compared patients with high-grade liver trauma who underwent unpacking before and after 48 hours. They found significantly lower ventilator-associated pneumonia rates and shorter mechanical ventilation time when unpacking was performed in the first 48 hours. However, this group had a higher hemocomponent transfusion and non-invasive procedures such as arteriography/embolization 14.
Thoracic cavity packing has been initially described as a bleeding control strategy in cardiac surgery, pulmonary resection, or pleurectomy 15-18. The chest cavity packing is one of the most commonly used techniques for controlling bleeding from injured surfaces, repaired tissue area, or associated with coagulopathy. 19. Moriwaki et al. showed that packing had been successfully used in severe bleeding from the chest wall, paravertebral space, lung parenchyma, and between the chest wall and diaphragm. In this cohort, only half of the patients developed packing-related reversible effects on ventilation or oxygenation. Unpacking was performed between the second and third day, with no procedure-related infectious complications 20. In 2019, Garcia et al. described the wound tract packing in transfixing injuries of the lung parenchyma as a damage control technique, which rapidly and safely controlled the hemorrhage in all patients (n = 4). They also recommended packing removal within the next 24-48 hours 21.
Pelvic packing is one of the first-line strategies for hemorrhage control in patients with pelvic fractures and hemodynamic instability. In these patients, unpacking is recommended in the next 24-48 hours 22,23.
In conclusion, cavity or organ solid packing should not be removed before 48 hours or after 72 hours. However, in severe liver trauma, the evidence supports unpacking before 48 hours as long as it is performed along with arteriography/embolization techniques and optimal blood products transfusion 14.
Postoperative care and deferred intestinal anastomosis
If fecal contamination of the peritoneum was identified during the surgical exploration, a therapeutic antibiotic scheme covering both aerobic and anaerobic microorganisms should be indicated. In contrast, if the lesion shows no signs of contamination, only a preoperative prophylactic dose of antibiotics is required.
If intestinal surgery has been performed, it is recommended to place a nasogastric tube, which has a decompressive effect on the bowel and can be used for enteral nutrition in the postoperative period, if needed.
Intestinal anastomosis
Deferred intestinal anastomosis to reduce surgical times and leakage risk has belonged to damage control surgery procedures. Left abdomen in discontinuity is to prevent further peritoneal contamination, allowing a delayed reconstruction of the gastrointestinal tract after physiological derangement has been corrected (Figure 2).
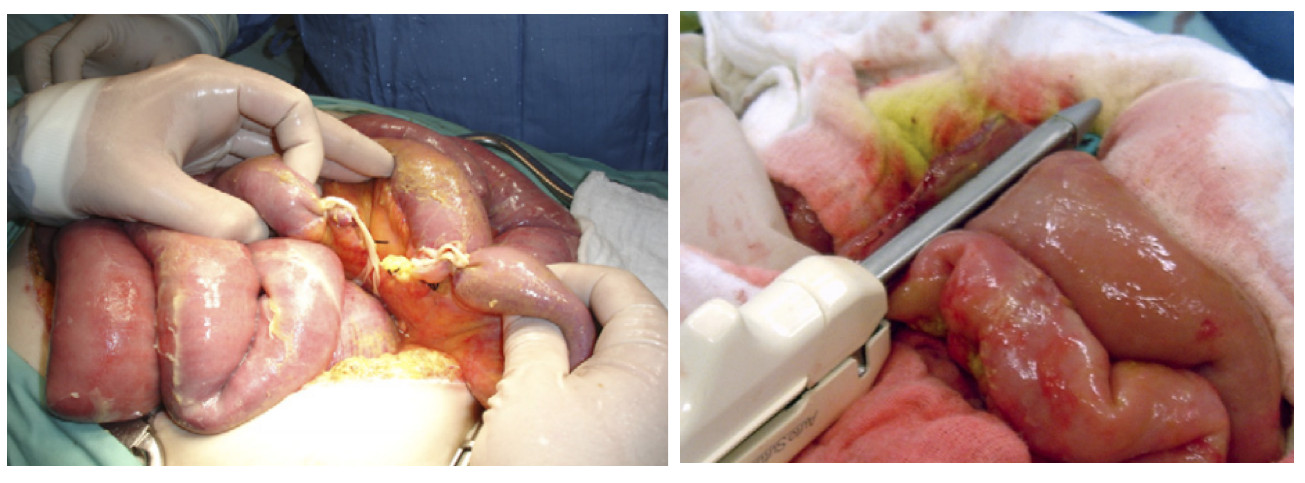
Figure 2 Deferred Intestinal Anastomosis. A. Small bowel injury is left in discontinuity as damage control surgery. B. Intestinal reconstruction via deferred small bowel anastomosis using stapple technique.
The underlying questions are: when is the optimal time to perform bowel repair and/or anastomosis? Moreover, whether or not an ostomy is required? is it necessary or not to perform an ostomy? Dysfunction of the gastrointestinal tract is the potential mechanism leading to bacterial translocation and sepsis. Therefore, it might complicate the prognosis of the patient in the midterm. Weinberg et al. assessed this concern by comparing patients with destructive colonic injuries who underwent definitive management in the first surgery (resection-anastomosis or resection-ostomy) with those who underwent damage control surgery with a deferred anastomosis. They found that a deferred anastomosis was associated with a higher probability of presenting colon-related complications (leakage, abscess formation, and stoma ischemia). This study also described that patients undergoing damage control surgery had a higher trauma severity and that open abdomen was managed with a negative pressure system only in 16% of the cases. Therefore, they recommended management with colostomy in a second surgical time 24.
However, deferred management of destructive or multiple bowel injuries is safe and can be successfully achieved by anastomosis repair, reducing ostomy requirements and the associated morbidity and sequelae 11,25. Furthermore, other studies have reported that complications such as leakage, enterocutaneous fistula, or surgical site infection do not have a frequency higher if the patient underwent definitive or abbreviated management 9,26. In addition, there is not a difference regarding complications rate between patients undergoing deferred anastomosis or deferred diversion 11,27,28.
The literature suggests that primary anastomosis or deferred anastomosis is safe and significantly reduces the rate of unnecessary ostomies, with no differences in complication rates compared to other procedures. A methodical analysis of the patient's physiological conditions and local variables should be performed when deciding to reconstruct the gastrointestinal tract, with the focus being on colonic anastomoses as high risk and with severe complications. Strategies and recommendations for other intestinal segments (duodenum, jejunum, and ileum) are based on the hemodynamic status and the surgical technique. The duodenum can be managed via primary repair or early duodenal reconstruction. This is consistent with the management of gastroduodenal injuries in our institution. Therefore, we assume that the phrase "less is more" (less surgeries, less reinterventions, fewer procedures) may have full validity in these cases 29,30. On the other hand, nutritional support plays an essential role in the recovery and management of patients undergoing damage control surgery and should be initiated as early as possible 31.
The following are recommendations based on the current evidence:
Definitive anastomosis should be performed as soon as possible, a maximum of 24-48 hours after intestinal ligation to avoid septic complications 24-28.
Duodenal reconstruction should be performed during the first 48 hours after the index surgery. 29,30.
Bowel reconstruction can be deferred until the second or third reoperation if the metabolic acidosis, uncontrolled septic focus, or intestinal edema do not persist. On the contrary, the patient is a candidate to perform a diversion. 9,26.
Total parenteral nutrition is not contraindicated in patients with intestinal ligatures and should be started as early as possible. In cases in which enteral nutrition cannot be established 31.
Enteral nutrition should be initiated once intestinal transit has been re-established and there is no evidence of paralytic ileus 31.
Vascular bypass (Shunt)
In vascular trauma, the early management of injuries aims to restore the blood flow to the compromised organ or limb. When primary repair is not possible, vascular shunts can be artisanal or commercial, but there is no difference between them and increasing the chance of preserving the function of the affected area. They became popular in military warfare with the rising incidence of peripheral vascular trauma and later on extended to civilian trauma centers where they are currently less frequently used, possibly due to the lack of knowledge and training on their use 32 (Figure 3).
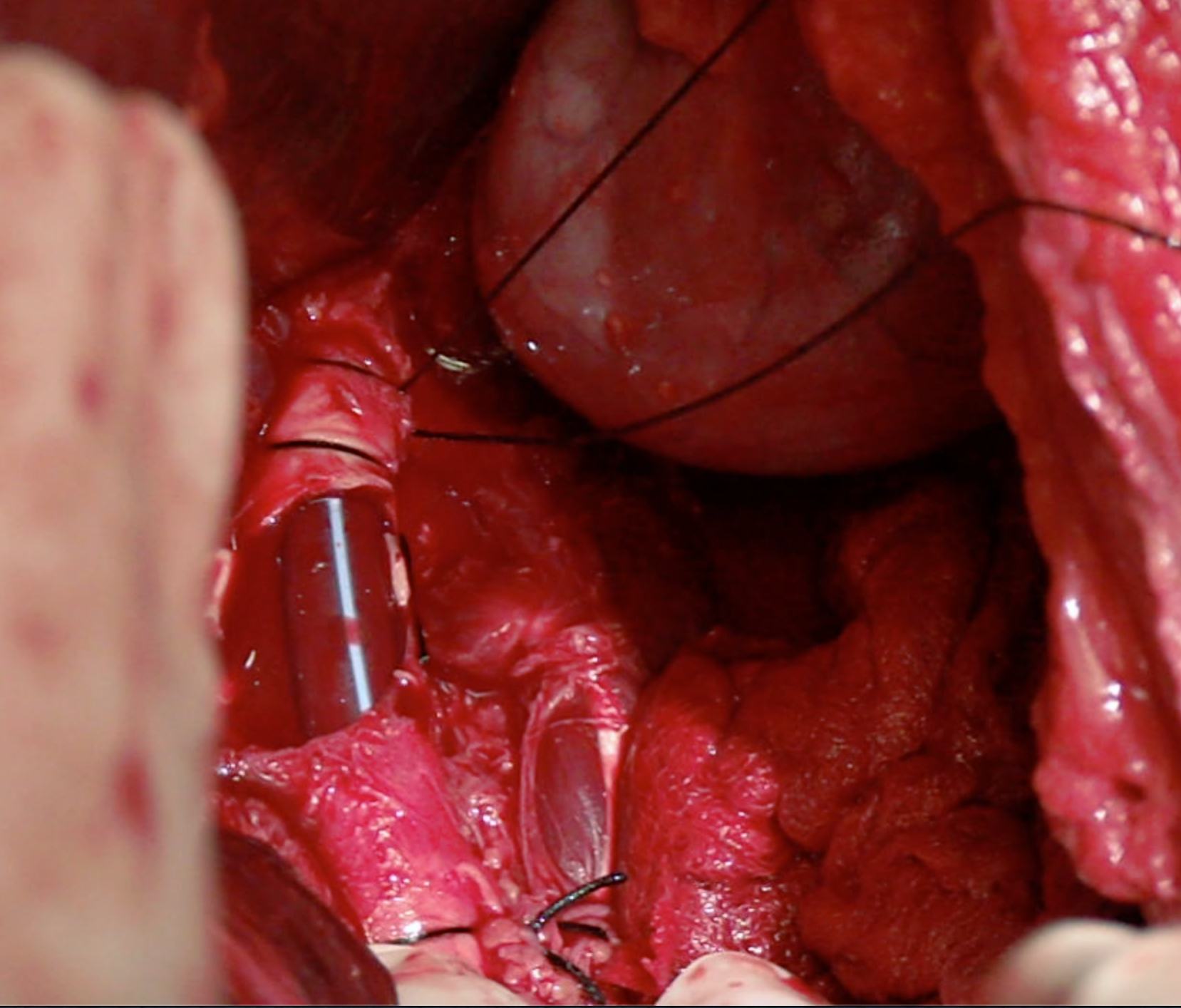
Figure 3 Vascular Shunt on Aorta artery. Infrarenal aorta injury is temporally treated using a vascular shunt
A shunt's patency depends on several variables, including time, placement technique, the type of vessel involved, and the patient's physiological status. Inaba et al. reported shunt thrombosis in 5.6% of the cases and most of them were removed in less than 48 hours without developing any complications 33. Based on animal models, Ding et al. suggested that the ideal time for using a vascular shut is 6 hours to ensure that permeability is preserved 34. However, in several studies, this time ranges from 2 to 52 hours without the need for anticoagulation.
The evidence conclude that vascular shunts are an appropriate clinical practice in the context of damage control surgery. Vascular shunt must only be used during the first 6 hours after the index surgery because the early definitive management of vascular injury should be a priority.
Abdominal wall closure
About 10% to 15% of laparotomies are managed through damage control surgery resulting in the open abdomen and consequently associated abdominal wall defects, fistula formation, malnutrition, and prolonged stay in the intensive care unit. The question would then be: When is the right time for abdominal wall closure? This is a controversial issue as it depends on many factors: time of re-intervention, number of re-interventions, number of fluids administered during resuscitation, and the temporary closure strategy being used (Figure 3). While it is sometimes impossible to close the abdominal cavity due to bowel loops edema or the patient's hemodynamic status, temporary closure techniques must be employed to avoid bowel dehydration, water and electrolyte disorders, tissue inflammation, and the most feared loss of abdominal wall dominance due to myofascial retraction and tension of abdominal components, all of which will technically hinder future reconstruction 35. Therefore, closure should be performed as soon as possible once the patient is fully resuscitated, with no further procedures required and no risk of abdominal compartment syndrome. Some authors have considered that the optimal time for abdominal wall closure is during the first nine days 36. Miller et al. reported 344 damage control laparotomies, with a primary fascial closure rate of 65%, showing that a fascial closure before 8 days was associated with better clinical outcomes 24,37. When comparing the outcomes of abdominal wall closure at the first re-intervention versus subsequent re-interventions, some studies have shown a lower rate of intra-abdominal abscesses, ventilatory failure, renal failure, and sepsis in early closures. When these variables are adjusted for age, gender, and some trauma scores, it is observed that infectious and non-infectious complications are lower with an early wall closure.
Keeping in mind an early closure of the abdominal wall, we must consider that evisceration is a serious complication, and the use of prophylactic meshes could be a suitable strategy in these patients, balancing the risks and benefits related to their use (surgical site infection, seromas, and prosthesis rejection) 38. Some consensus recommendations include the use of strategies such as negative pressure therapies or dynamic fascial traction to prevent tissue retraction. In cases of large defects, techniques such as separation of abdominal wall components should not be ruled out (Figure 4). However, excessive efforts to close the abdominal wall could have unfortunate consequences, and planned eventration is an acceptable strategy in this group, which is ideally recommended after 6 months 39.
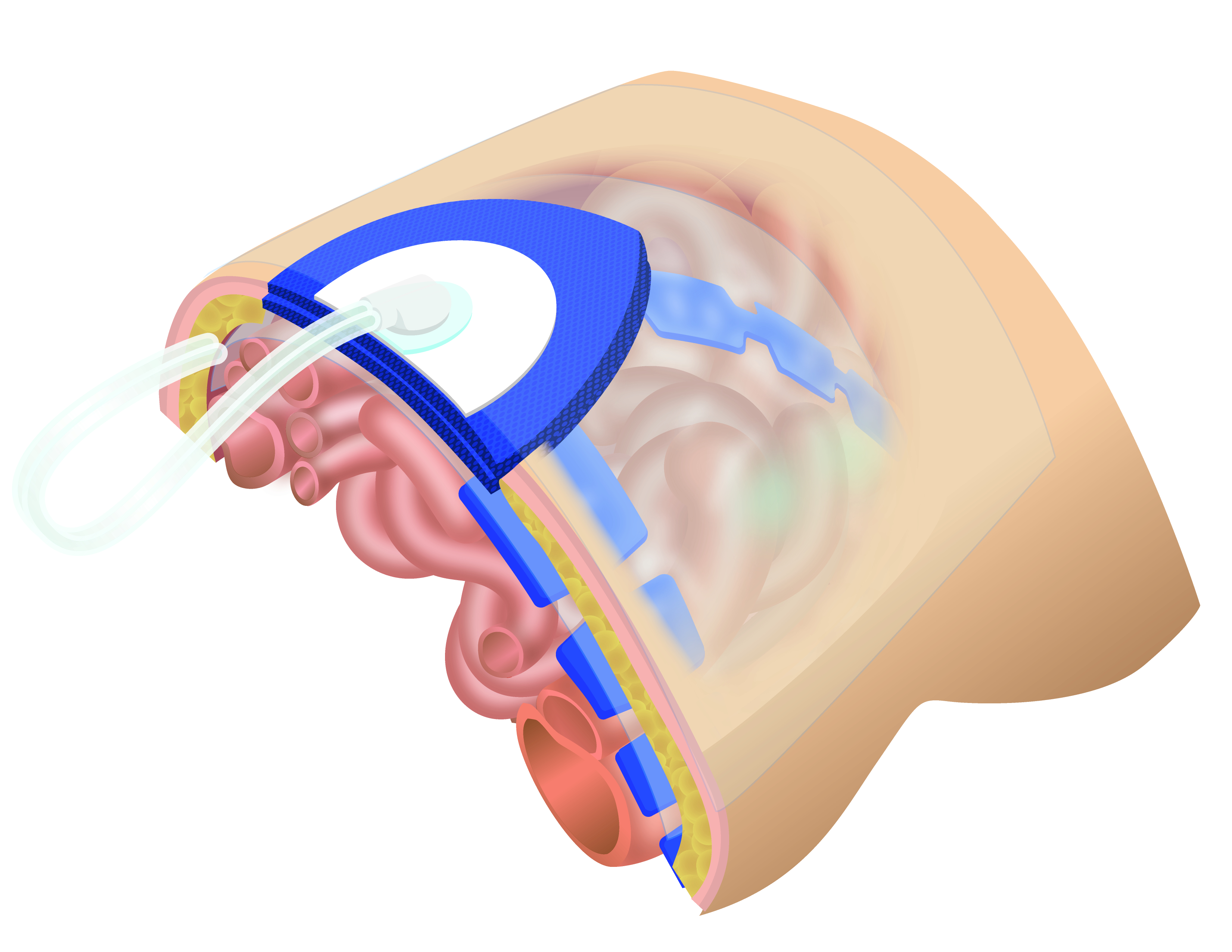
Figure 4 Negative Pressure Wound Therapy to deferred closure of the abdomen. A fenestrated plastic separates intra-abdominal organs, while foam sponges or dressings are placed over it and secured beneath a double layer of adhesive sheets. The suction device is installed over the adhesive film by cutting out a 3 to 3.5 cm diameter circle
Our recommendation is to perform definitive closure of the abdomen in the second or third reoperation with a maximum time of five or seven days after the index surgery. If the definitive closure of the abdomen does not include fascial or skin, only the skin should be closed to avoid abdominal wall complications (Figure 5).











 text in
text in