Remark
| 1) Why was this study conducted? |
| To analyze the local control and survival of patients with BMs from BC treated via volumetric modulated arc radiosurgery, as well as some of the main factors that influence them (e.g. molecular subtypes of BC, baseline situation of patients and the location of BMs). |
| 2) What were the most relevant results of the study? |
| On the one hand, our results suggest that the survival of patients with brain metastases from breast cancer with VMAT-RS, is comparable to that of other radiosurgery techniques. On the other hand, we showed that the cerebellum was the predominant site of breast cancer BMs, and also suggested that HER2+ BMs had a predilection for some structures of the posterior circulation, such as the cerebellum, brainstem and occipital lobes. |
| 3) What do these results contribute? |
| Our findings suggest that the spatial distribution of the BMs in the central nervous system could differ according to the genetic composition (molecular subtype) of the primary breast cancer, which could be of importance for the planning of different treatment strategies in the future. |
Introduction
Currently, brain metastases (BMs) are the most frequent intracranial tumours, occurring in about 20-40% of cancer patients 1,2. BMs in patients lead to a significant increase in morbidity and mortality 3. Its incidence has been increasing in recent years due to the improvement in the quality of neuroimaging diagnostic techniques (e.g. magnetic resonance imaging -MRI-) and improved effectiveness of treatment regimens 4-7. Breast cancer (BC) represents the second most common cause of BMs in adult patients (15-25%) 1,8. To date, several molecular subtypes of BC with prognostic and therapeutic differences have been described. Thus, we know that patients with human epidermal growth factor receptor (HER)2 positive breast cancer and triple negative breast cancer have an increased risk of developing BMs compared to luminal BC subtypes 6,9-11. According to Witzel et al. (6 , the frequency of brain metastases among patients with metastatic breast cancer would be: HER2-positive (11-20%), triple negative breast cancer (25-27%), luminal B (11%) and luminal A (8-15%).
Whole-brain radiation therapy and stereotactic radiosurgery (SRS) are two treatment modalities commonly utilised to treat BMs. Traditionally, radiotherapeutic treatment has been performed using whole-brain radiation therapy, however recent evidence has revealed the potential development of cognitive impairment and its impact on the quality of life of some treated patients 12-14. In this context, SRS has been of increasing importance, since it allows the administration of high doses of radiation on the tumour lesion with a significant dose gradient, minimising adverse effects on healthy brain tissue 15. Currently, the use of SRS in patients with 1-4 BMs is widely accepted as a standard treatment 3, and even some authors extend it as an alternative treatment in patients with 5-10 BMs 16. Recent studies have not found a decrease in overall survival of patients treated with SRS alone versus those treated with SRS plus whole-brain radiation therapy (11,13,14,17. Among the radiosurgical modalities, Gamma knife (GK-RS) has been the most commonly used 18; however currently there are other treatment modalities that offer procedural advantages, such as, radiosurgery using volumetric modulated arc therapy (VMAT-RS).
In this study, we analyzed on the one hand, the response to VMAT radiosurgery treatment in terms of overall survival and local control in patients with BMs from BC. On the other hand, we analyzed the distribution of brain metastases in the central nervous system and the possible influence of molecular biotype.
Materials and Methods
Study cohort
This is an observational, descriptive and retrospective study of case series that includes all patients with BM from BC who were treated with VMAT-RS from October 2012 to July 2018. Patients were treated in the Department of Radiation Oncology of the Santa Lucia University Hospital (Cartagena, Spain). The patients treated with SRS in single fractions (single-fraction SRS), as well as, those treated by fractionated stereotactic radiotherapy (fSRS) 11,19 were included in the study. The experimental protocol was approved by the Ethical Committee of Clinical Research of the Murcian Health Service of the Autonomous Community of the Region of Murcia (Spain).
Data regarding age, sex, number of BMs, presence of metastases outside the central nervous system (CNS), previous and/or subsequent local therapy of BMs, systematic treatments after diagnosis of brain metastasis and primary tumor characteristic as histologic subtype, hormone receptor (HR) and HER2 status were collected for each patient. HR status referring to the presence or absence of either estrogen or progesterone receptors positivity. The Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG score) were collected and modified Breast-Graded Prognostic Assessment scores 20 were calculated for each patient (Table 1), except for a patient with breast sarcoma, where DS-GPA for breast was applied 21. The survival time of each patient was calculated from the date of radiosurgery to the date of death or the last clinical follow up.
Table 1 Modified Breast-GPA Score.
| Factor | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
|---|---|---|---|---|---|
| KPS | ≤50 | 60 | 70-80 | 90-100 | - |
| BC subtype | TNBC | HR+/HER2- | HR-/HER+ | HR+/HER+ | - |
| Age, years | >50 | ≤50 | - | - | - |
| Nº of BM | >3 | 1 to 3 | - | - | - |
BC: breast cancer, BM: brain metastases, GPA: Graded prognostic assessment, HER2: human epidermal growth factor receptor 2, HR: hormone receptor, KPS: Karnofsky Performance Status, TNBC: triple-negative breast cancer. Prognostic categories: 0-1.0; 1.5-2.0; 2.5-3.0; 3.5-4.0 20,22.
Patients were classified according to immunohistochemistry and fluorescence in situ hybridization tests performed on the primary tumor, in four BC subtypes 22: a) HR-/HER2- (triple negative breast cancer), b) HR+/HER2-, c) HR+/HER2+ and d) HR-/HER2+. The summary of clinical characteristics, as well as, the molecular subtype of the primary tumour of each patient is shown in Tables 2 and 3.
Table 2 Clinical and therapeutic characteristics of patients.
| Characteristics | N. of patients | % | |
|---|---|---|---|
| Sex | Female | 18 | 100 |
| Age at Radiosurgery | ≤50 years | 6 | 33.3 |
| >50 years | 12 | 66.7 | |
| Tumour histology | Ductal carcinom | 14 | 77.8 |
| Lobular carcinom | 3 | 16.7 | |
| Sarcoma | 1 | 5.5 | |
| Molecular subtype | TNBC | 3 | 17.7 |
| HR+/HER2- | 3 | 17.7 | |
| HR-/HER2+ | 4 | 23.5 | |
| HR+/HER2+ | 7 | 41.1 | |
| Number of BM | 1 | 8 | 44.4 |
| 2 | 6 | 33.3 | |
| 3 | 3 | 16.7 | |
| >3 | 1 | 5.6 | |
| Modified Breast-GPA | 3.5-4 | 4 | 22.2 |
| 2.5-3 | 7 | 38.9 | |
| 1.5-2 | 5 | 27.8 | |
| 0-1 | 2 | 11.1 | |
| KPS | 90-100 | 3 | 16.7 |
| 70-80 | 11 | 61.1 | |
| 60-50 | 4 | 22.2 | |
| Extracranial Metastases | Yes | 14 | 77.8 |
| No | 4 | 22.2 | |
| Previous Treatment | None | 9 | 50.0 |
| Surgery | 1 | 5.6 | |
| WBRT | 7 | 38.9 | |
| Both | 1 | 5.6 | |
| Posterior Treatment | None | 11 | 61.1 |
| Radiosurgery | 3 | 16.7 | |
| WBRT | 1 | 5.6 | |
| Both | 3 | 16.7 | |
| Systemic therapy after BM | Yes | 17 | 94.4 |
| No | 1 | 5.6 | |
| Kind of systemic therapy | Chemotherapy | 16 | 88.9 |
| Hormonal therapy | 6 | 33.3 | |
| HER2 targeted therapy | 10 | 55.6 |
TNBC: triple negative breast cancer
WBRT: Whole-brain radiation therapy
Table 3 Clinical and therapeutic characteristics of brain metastases.
| Variable | Parameter | n | % |
|---|---|---|---|
| Fractionation treatment | Single-fraction SRS | 33 | 80.5 |
| Fractioned SRS (fSRS) | 8 | 19.5 | |
| Variable | Parameter | cc | Cc |
| PTV | Median (IQR) | 2.45 | (4.6) |
| Mean (SD) | 4.31 | (4.99) | |
| GTV | Median (IQR) | 0.6 | (2.3) |
| (BMs from all subtypes of BC) | Mean (SD) | 1.51 | (2.0) |
| Cumulative tumor volume | Median (IQR) | 3.15 | (4.1) |
| (∑GTV of BMs from breast cancer) | |||
| ∑GTVHER2+ | Median (IQR) | 4.2 | (3.9) |
| ∑GTVHER2- | Median (IQR) | 2.8 | (4.4) |
| Variable | Parameter | mm | mm |
| Diameter (Φ) of the largest BM | Median (IQR) | 16 | (7.7) |
| ΦHER2+ | Median (IQR) | 20 | (10) |
| ΦHER2- | Median (IQR) | 16 | (12) |
| Variable | Parameter | Gy | Gy |
| Dose | Single-fraction SRS Median (IQR) | 18 | (4) |
| Fractioned SRS Median (IQR) | 30 | (0) | |
| Variable | Parameter | min | min |
| Procedural time | Global Median (IQR) | 26 | (14) |
| Single isocenter Median (IQR) | 24.5 | (7) | |
| Two isocenters Median (range) | 48 | (13) | |
GTV: gross tumour volume, PTV: planning target volume.
SRS: stereotactic radiosurgery
Clinical and radiotherapeutic characteristics of BM
For each metastasis, anatomical localisations in CNS, pre-treatment diameter, post-treatment diameter (both in the first and in the second neuroimaging control), gross tumour volume (GTV), planning target volume (PTV), planned dose, dose fractionation scheme, time to neuroimage-check and response to treatment were analyzed. The doses of the prescribed treatments were 12-20 Gy for BMs treated by SRS; 30-35 Gy for those treated by fSRS and administered with 5-6 fraction schemes. The prescribed treatment dose for whole-brain radiation therapy was 30 Gy in 10 fractions. As a general rule, for patients who previously received whole-brain radiation therapy, the prescribed dose was from 12 to 15 Gy, while for the remainder, the prescribed dose ranged from 16 to 20 Gy (Table 2). Nevertheless, fSRS was performed on localized metastases close to the brain stem or optical nerve, or those greater than 35-40 mm in diameter.
Tumour response assessment was performed on contrast-enhanced MRI (T1 sequences) according to mRECIST 23,24. The slice thickness of images was 1 mm. Patients were classified according to the following four possibilities: complete response (CR), partial response (PR), progressive disease (PD) and stable disease (SD). In patients with more than one BM, local control was evaluated individually.
Characteristics of the treatment technique
All patients were treated using a VMAT technique consisting of 5 non-coplanar arcs and 6 MV X-rays, using optimized rotational delivery (gantry and couch rotations) in order to avoid collision between gantry and patient while simultaneously minimising treatment time according to a previous study 15 (Figure 1). Patients treated with fractional treatment schemes (fSRS) were also included. Thus, while the planning aimed for an isodose at 100% of the prescribed dose to cover at least 99% of the PTV and 100% of the GTV for treatments through single-fraction SRS, patients treated with fSRS received a dose of 98% of PTV and 98% of GTV. The duration of each single-fraction SRS procedure was analysed. The number of patients requiring additional cone bean computer tomography (CBCT) and the number of isocentres used in each treatment were noted.
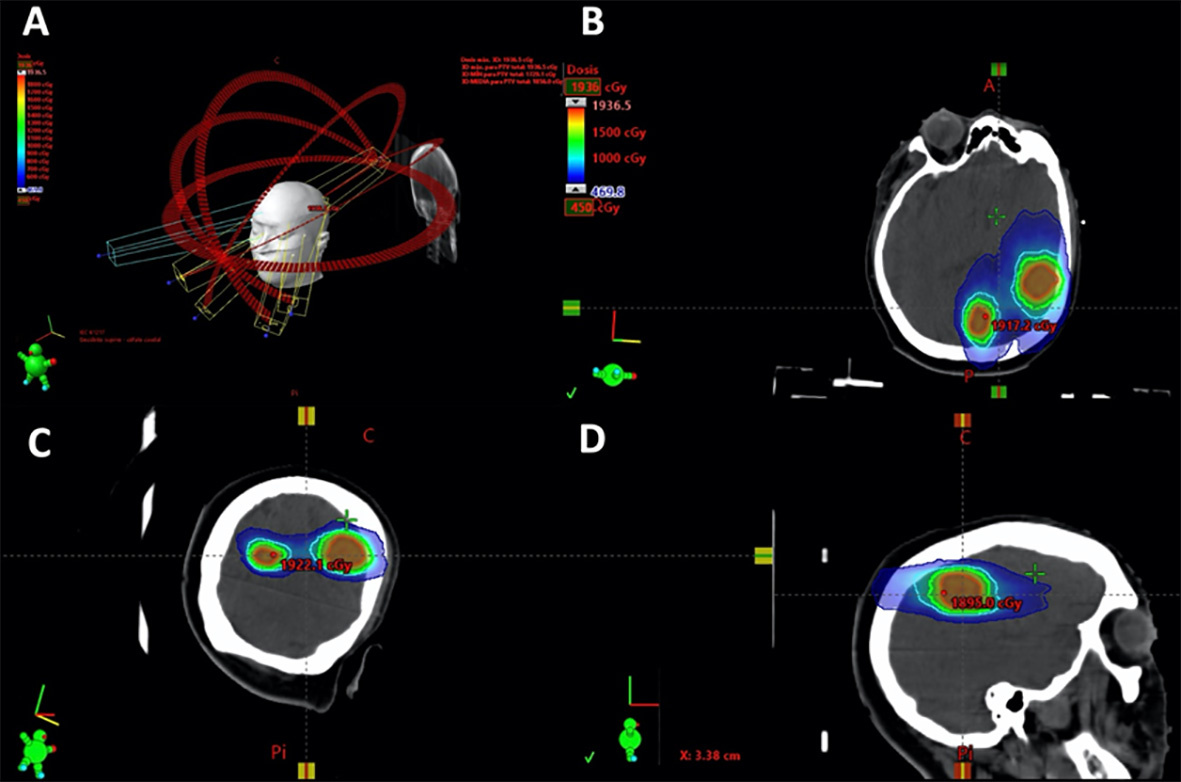
Figure 1 Planning example of a patient with two brain metastases, treated with 18 Gy. The images B, C and D, represent the axial, coronal and sagittal plane rotations respectively. Red PTV outline, green 12 Gy isodose, light blue 9 Gy isodose and dark blue 4.5 Gy. The 18 Gy isodose is not shown since it practically coincides with the PTV outline and would otherwise hinder viewing.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 25 (IBM, New York, USA) for Mac. Survival curves were performed according to the Kaplan-Meier model. Overall survival was computed from first SRS. The non-parametric log-rank test was applied in order to determine if differences existed in survival between patient sub-groups in terms of the following variables: KPS groups, ECOG groups, class of modified breast-GPA index, molecular subtypes of breast cancer, number of BMs, presence of some infratentorial brain metastases and total lesion volume. The Mann-Whitney U test was used to compare the diameters of the largest BMs and total gross tumour volume according to the different molecular subtypes of breast cancer. The chi-square test and Fisher's exact test were used to compare group categorical data. Significant differences were reached when the p values were lower than 0.05 (p <0.05).
Results
Patients
All 18 patients studied were women with BMs from BC and treated by means of VMAT-RS. Total treated metastases were 41, and the median number of BMs per patients were 2 (range: 1 - 4 BMs). Patients had a median age of 53 years (range: 34 - 81 years). Tables 2-3 summarises the main clinical characteristics and local treatment of BMs received by patients.
Distribution of BMs in the nervous system
Regarding the distribution in the CNS of BMs treated in our study group, we observed a prevalent location in the cerebellum (Figure 2), in accordance with previous studies (25,26. On the one hand, the analysis of molecular subtypes shows that infratentorial region is the most frequent location for BMs from HR-/HER2+ patients, although no significant differences were found between subgroups. On the other hand, when analyzing the distribution of brain metastases according to only the HER2 biomarker, and clustering into HER2+ and HER2- BMs, a higher frequency of HER2+ BMs was determined in the brain stem, cerebellum or occipital lobes when compared to HER2- BMs (p= 0.048).
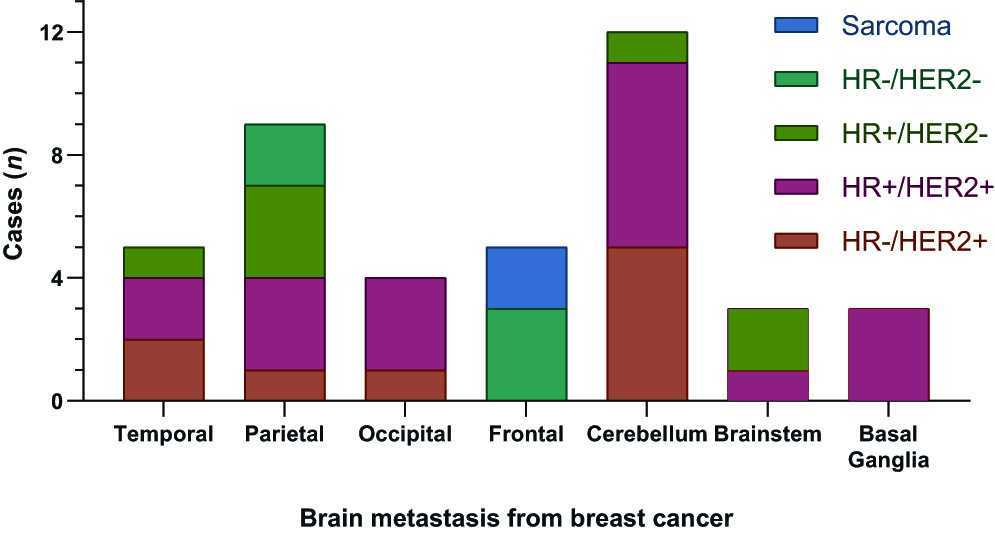
Figure 2 Distribution of BMs from BC treated by VMAT-RS in the central nervous system. Stratifying BMs by the histology and molecular subtypes of the breast cancer.
Both the cumulative tumor volume and the diameter of the largest BM suggest larger sizes for HER2+ than for HER2- (Table 3), although no significant differences were reached in our study.
Survival analysis
Survival analysis according to the Kaplan-Meier model is shown in Figure 3. The median survival time (MST) from the date of radiosurgery was 19.7 months (95% confidence interval CI: 14.9-24.5 months). The overall survival rates were 83.3% at 6 months, 65.5% at 12 months and 44.9% at 24 months. Significant differences in patient OS were determined in the following variables: firstly, among patients with KPS ˂70 versus those with KPS ≥70 (p= 0.02). Patients with KPS ≥70 had a MST of 31.7 months (95% CI 10.8 - 52.7 months), while those with KPS ˂ 70 had a MST of 6.3 months (95% CI: 4.4-8.1 months). Secondly, on the one hand, significant differences were obtained according to the HER2 status (p= 0.004). (fig. 3). Thus, HER2 + patients had a MST of 43 months (95% CI: 20.8-65.2), while HER2 - patients had a MST of 5.1 months (95% CI: 2.3-7.9). On the other hand, analyzing both biomarkers (HER2 and HR), significant differences were also determined between the four molecular subtypes of BC (p= 0.042). Patients with HR+/HER2+ molecular subtype and triple negative breast cancer presented the longest and shortest MSTs, respectively. Thirdly, significant differences were also obtained according to the modified breast-GPA class (p= 0.004). However, given the small size of the sample, these last results should be taken carefully. Finally, no statistically significant differences were found in the number of BMs (single versus multiple metastases), in the total lesion volume (<2 versus (2 cc), or in the presence of any BM in the infratentorial region (Table 4).
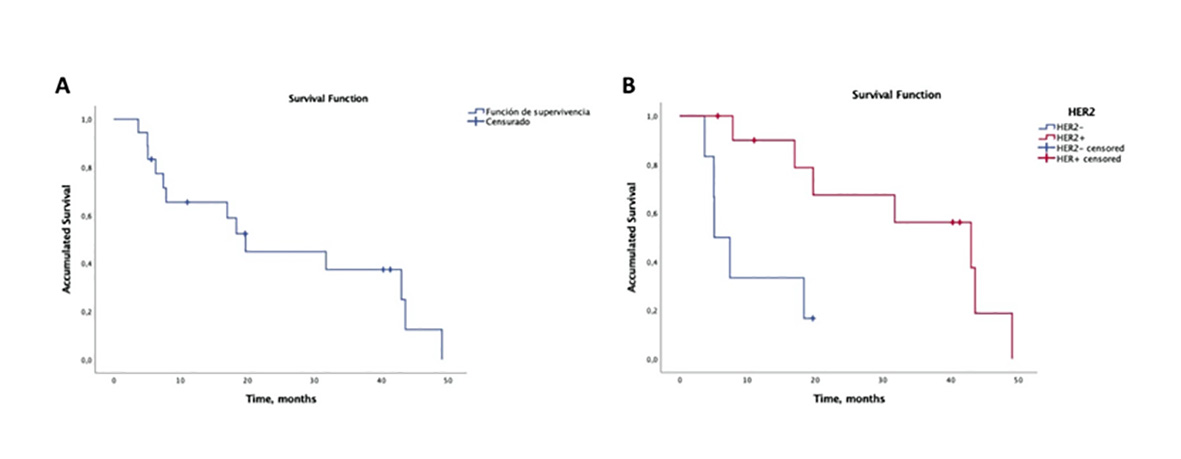
Figure 3 Kaplan-Meier curves for: a) overall probability of survival post-SRS/fSRS, b) survival according to HER2 status.
Table 4 Log-rank test (Mantel-Cox) results for the Kaplan-Meier model.
| Log-rank test (Mantel-Cox) results for the Kaplan-Meier model. | |
|---|---|
| Variable | p-value |
| KPS: <70 versus ≥70 | 0.020 |
| Molecular Subtypes of Breast Cancer (HER2+ versus HER2-) | 0.004 |
| Number of single versus multiple metastases | 0.222 |
| Total lesion volume: <2 versus ≥ 2 cc | 0.110 |
| Presence of some infratentorial brain metastases | 0.924 |
GPA: Graded prognostic assessment, HER2: human epidermal growth factor receptor 2, KPS: Karnofsky Performance Status.
Local control of BMs
The control of BMs was individually analysed for each BM. The first neuroimaging control (MRI) was performed within a median time of 3 months (IQR: 1 month). The local response was not known in 13.6% of the BMs, since they did not receive the first MRI control. The rest presented a local control proportion of 90.9%, with each individual BM fulfilling the criteria of: PD 9.1%, SD 48.5%, PR 27.8% and CR 15.2%. The second MRI control was performed in a median time of 5 months (IQR: 2 months). The local response of the BMs was unknown in 24% of cases. In the rest, 88.5% of individual local control was achieved.
Discussion
Radiosurgery treatment of BMs in BC patients with up to 4 BMs 3, as well as, in selected patients with 5-10 BMs 16, has become a current treatment modality of choice as it provides advantages over conventional whole-brain radiation therapy, especially due to its lower neurocognitive effects 1,11-14,27. This is of particular importance given the increased survival of these patients 28. In our study, we employed the VMAT-RS which is enjoying increasing acceptance and usage 29. Its advantages over other SR techniques (such as GK-RS) include the following: shorter treatment time 30, use of single isocenter for cases with multiple metastases (provided that the relative location of the brain metastases and their size allow for it) (Fig.1) 31, and not needing an invasive fastening system (frameless). This reduces discomfort and affords a better integration into the departments of Radiation Oncology.
The primary endpoint of the present study was to analyse the response to VMAT radiosurgery treatment in terms of overall survival and local control in patients with brain metastases from breast cancer. Although we have not evaluated the specific treatment toxicity, we have used both survival and local control as a surrogate for the treatment efficiency in terms of enabling a comparison with other radiosurgical techniques.
Firstly, regarding the overall survival, an MST of 19.7 months was obtained, which is comparable with what is portrayed in different studies of radiosurgery treatment using GK-RS: Jo et al (MST 18.2 months) 32, Matsunaga et al (MST 13 months) (33 or Abu-Khalaf (13.1 months) 34, although the sample size of our study is smaller. Among the variables that we determined to be significant for the overall survival of the patients in our study are the KPS, the molecular subtype and the modified breast-GPA. KPS has been shown to be a significant variable in assessing survival of patients with brain metastases, using different treatment techniques 15,35-37. In our study, we determined differences between both subgroups by establishing a KPS cut-off point of 70 (KPS <70 versus KPS (70).
Regarding breast cancer biomarkers, we determined significant differences according to HER2 status. Thus, as in previous studies 38, the OS of HER2+ patients was longer than the OS of HER2- patients. We also determined significant differences between BC molecular subtypes (based on HR and HER2 status), with triple negative breast cancer and HR+/HER2+ having the shortest and longest median survival time, respectively. These significant differences between the different molecular subtypes of BC have also been described in previous studies, such as those conducted by Sperduto et al. (21, and Griguolo et al (22. However, given the small size of the sample, these results should be taken carefully.
We did not find differences between subgroups of patients depending on the total lesion volume, the BMs number, or the presence of infratentorial BMs in the patients. While previous studies have reported that the number of BMs is not a predictor of survival, cranial tumor volume appears to be 39. Yamamoto et al. (16, described that the cumulative tumor volume ((1.9 cc) and the diameter of the largest BM (( 16 mm) influence the survival of patients; while the BMs number (>4) is not significant. Bhatnagar et al. (40, in the treatment of patients with multiple BMs (4-18 BMs) with SRS, explained that smaller cranial tumor volume was associated with improved survival regardless of the number of BMs.
Secondly, for the assessment of the local response of the BMs to the radiosurgery treatment, the mRECIST criteria 23,24 were used, which represent an institutional modification of the RECIST 1.1 standard criteria 41. The main difference between the two criteria, as concluded in the study by Qian et al. (24, is in the definition of measurable lesions. Thus, with the mRECIST criteria, lesions with a minimum diameter of 5 mm can be included instead of 10 mm as postulated in the RECIST criteria 1.1. (41. This allows for the inclusion of more BMs in the study. In addition, it could be more sensitive to detect local disease progression since it does not require a minimum absolute increase of 5 mm (like the RECIST 1.1 criteria), but only an increase ≥20% in the sum of longest diameters compared to nadir 24. In our study, the percentage of local control using these mRECIST criteria, which was applied individually for each BM, was 90.9% in the first MRI control (3 months), and 88.5% in the second MRI control (5 months), which is higher than what was described in a previous study by our group 15 when conducting a single-fraction VMAT-RS, for which local control was 80% at 3 months. Becker et al.42, employing frame-based LINAC radiosurgery, found local control rates for brain metastases to be 74% at 6 months and 61% at 12 months. This was somewhat less than what has been reported by Bilger et al. (43, who using a frameless-SRS system, estimated local control of 83% at 12 months. In addition to the RECIST criteria, there are other criteria for assessing response to treatment, such as the MacDonald criteria (historically used for the assessment of high-grade gliomas), and the standardized response criteria proposed by the response assessment in neuro-oncology (BM-RANO) working group 44. Among other differences, the latter two also consider clinical status and corticosteroid use among the response criteria. Some recent studies, such as Qian et al, have observed a high degree of concordance between some of these criteria (mRECIST, RECIST 1.1 and BM-RANO) 24. Nevertheless, it would be advisable to consider the evaluation by metrics of Mc Donald, BM-RANO and mRECIST criteria as support and complement, because their isolated evaluation presents limitations and this may affect an adequate evaluation.
As a secondary endpoint, the distribution of brain metastases in the central nervous system and the possible influence of molecular biotype were analysed.
In relation to the distribution of BMs, Kyeong et al. (26, found that the BMs from triple negative breast cancer spreads evenly in the brain, whereas in the case of the HER2+ and HR+/HER2- subtypes, BMs are concentrated in the posterior circulation territories, such as the occipital lobes and the cerebellum. In our study, we determined that metastases of HER2+ patients are more frequent in the region comprised by brainstem, cerebellum and occipital lobes (posterior circulation), unlike HER2- BMs. However, unlike this study, BMs from HR+/HER2- subtype are more frequently located outside this region, although statistical significance was not established in the latter case, possibly due to the small sample size. In this sense, in agreement with Kyeong et al. (26, it seems that the spatial distribution of the BMs in the CNS could differ according to the genetic composition (molecular subtype) of the primary breast cancer, which could be of importance for the planning of different treatment strategies in the future.
Conclusion
Our results suggest that both the survival and local control of patients with brain metastases from breast cancer treated with VMAT is comparable to that of other radiosurgery techniques. Among the factors that influence the OS of patients, we should highlight the baseline situation at the time of treatment (KPS, ECOG) and the molecular subtype of the breast cancer. Finally, our results show that the cerebellum is the predominant site of breast cancer BMs, and also suggest that HER2+ BMs have a predilection for some structures of the posterior circulation, such as the cerebellum, brainstem and occipital lobes. These findings could be important for the planning of different treatment strategies in the future, although further work with larger sample sizes is needed.











 text in
text in 



