Remark
| 1) What is the reason for this study? |
| There are several hypotheses about the etiology of gastroschisis, without a consensus having been reached to date. In addition, the prevalence of this congenital abnormality has shown in recent decades a worrying increase in most of the countries where it has been studied. |
| 2) What are the most important results of the study? |
| Unlike previous research that has addressed the great diversity of risk factors involved in the etiology of gastroschisis individually, this manuscript collects evidence that supports the existence of a pathogenic pathway shared by these risk factors and possibly related to induction of stress response. |
| 3) What do these results provide? |
| We report recent information about the pathogenesis of gastroschisis that includes both genetic and non-genetic factors, in addition to the traditionally accepted theories. This contribution is significant because it presents new criteria for the medical approach to such entity. |
Introduction
Gastroschisis is a congenital anomaly of the development of the ventral body wall, first described in 1733 1,2, characterized by the presence of a hole less than 2 cm in diameter in the abdominal wall, which allows the intestinal loops to eviscerate, and sometimes part of the colon and other organs. Because the amnion does not surround it, the intestine is directly exposed to amniotic fluid, with consequent swelling and possible damage to the seromuscular layer. Evisceration also explains the high maternal serum levels of α-fetoprotein, even higher than in omphalocele cases 3. Depending on the extent of the defect, a surgical reduction can be carried out immediately after birth, in order to avoid thermal and evaporative loss through the exposed organs 4, or gradually, until their complete closure, a modality that has shown an overall survival rate of more than 90% 5.
Gastroschisis occurs predominantly to the right of the umbilical cord insertion. On rare occasions, it may be located on the left side 6,7, but always in contrast to the location on the midline of other abnormalities of the ventral body wall, such as omphalocele, ectopia of the heart, exstrophy of the bladder and cloaca, Pentalogy of Cantrell, and limb-body wall complex 3,8,9.
Other malformations can co-occur with gastroschisis -in 10-20% of cases- especially in the gastrointestinal tract, such as malrotation, volvulus, stenosis and atresia 3,10. Even rarer, they are other types of comorbidities, such as neural tube or diaphragm defects, ectopia cordis, or congenital heart disease.
In order to explain why the prevalence of the malformation has shown an increasing global trend in recent decades, especially in young mothers and mothers with a history of alcohol and tobacco consumption during pregnancy, multiple studies have been carried out that suggest the participation of various environmental factors and genetic predisposition as important causes of risk 8,10-14. However, and despite the diversity of factors involved, there is no conclusive evidence to date about the cause of this malformation.
Formation of the ventrolateral body wall and the primitive intestine
During the third week of development, the lateral body folds that give rise to the ventrolateral body wall are formed from the somatopleura. Externally, the folds are covered by the ectoderm that rests on the somatic sheet of the lateral mesoderm, in which somatic cells are found. Internally the folds are lined by a fine and thin mesothelium 15 (Figure 1 A-B).
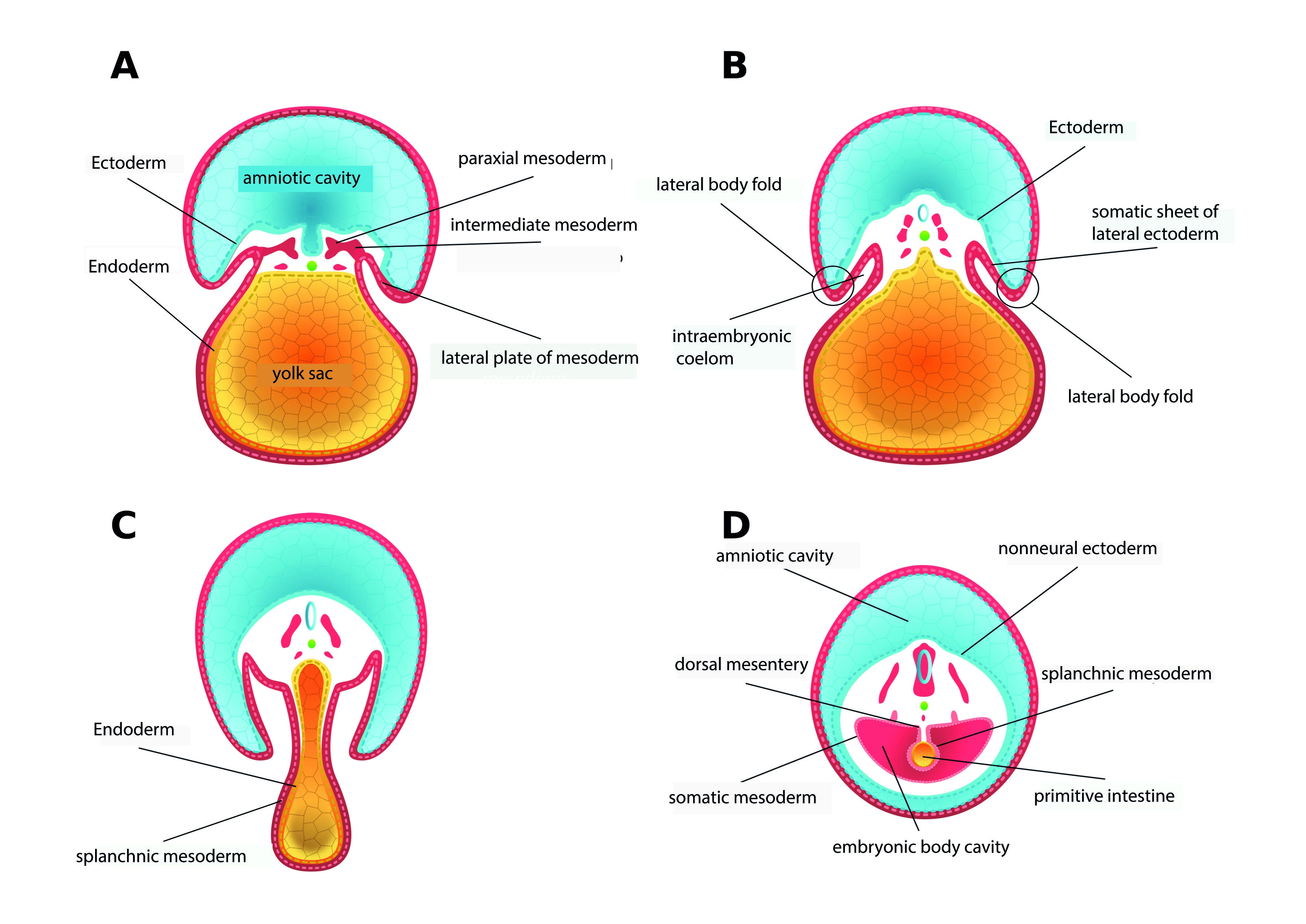
Figure 1 Embryonic development during the third and fourth weeks. Cross-sections. A. Towards the end of the third week of development, the lateral plate of the mesoderm bifurcates into two leaves: one underlying the non-neural ectoderm and the other around the endoderm that lines the yolk sac. B. The space delimited by the two leaves derived from the lateral plate of the mesoderm gives rise to the intraembryonic coelom. At this time, beginning of the fourth week of development, it is possible to observe the two lateral body folds, each constituted by the somatic sheet of the lateral mesoderm and by non-neural ectoderm. C. The growth of the two lateral body folds and their displacement in a ventral direction causes them to approach the midline by the middle of the fourth week. D. At the end of the fourth week, the two folds fuse and form the ventrolateral body wall. As a result of the folding, the yolk sac enters the body cavity of the embryo and gives rise to the primitive intestine.
At the end of the third week, the folds begin to grow in a ventral direction to approach the midline, where they fuse at the end of the fourth week (Figure 1 CD), in a process that involves forces resulting from the proliferation and growth of the non-neural ectoderm, and the cell division and the production of mesodermal extracellular matrix 16. In addition, some cells of the sclerotome and myotome that have migrated from the somitic abaxial region to the somatic sheet of the lateral mesoderm differentiate to give rise to the primordium of the costal cartilages and the muscles of the extremities and the ventral wall, generating forces that contribute to the fusion of the lateral folds and, therefore, to the formation of the ventrolateral body wall 17.
Simultaneously with the appearance and subsequent fusion of the lateral body folds, the splanchnopleura originates, tissue derived from the endoderm and the splanchnic leaf of the lateral mesoderm that lines the yolk sac. When this sac is introduced into the intraembryonic coelom to form the primitive intestine, the splanchnopleura differentiates into the tissues that make up the intestinal wall and the mesentery 9,16 (Figure 1 C-D).
As the intestine grows ventrally within the intraembryonic coelom, the lateral body folds to fuse in the midline, closing the thoracic and abdominopelvic walls and leaving outside the embryo the omphalomesenteric duct, which is the remnant of the yolk sac. This conduit is then incorporated into the fixation pedicle close to the umbilical vessels. The umbilical cord thus formed comprises two compartments: a left vascular one for the umbilical vessels and a flaccid right one for the omphalomesenteric duct 18. This last compartment into which the primitive intestinal loop protrudes during the sixth week, in the so-called physiological umbilical hernia. Inside it, the primitive intestinal loop grows and develops, resulting in the formation of the distal part of the duodenum, the jejunoileal loops, and most of the colon. Simultaneously, the primitive intestinal loop must initially rotate 90° counterclockwise and, towards the twelfth week, when the intestine is fully formed and begins its return to the abdominal cavity- 180° more, counterclockwise again allows mesenteric placement within that cavity 15.
The ventral body wall closure process may be interrupted between the third and fourth week of development due to a defective fusion of the lateral body folds in the midline 3,8,16. However, such interruption must occur between the eighth and the eleventh week, considering that for gastroschisis to occur, the midgut must be previously herniated and the umbilical cord fully formed, especially in its vascular compartment 18.
Origin of gastroschisis
Anatomical events
Until the last decade, it was thought that gastroschisis was an independent malformation of the umbilical cord. It was also thought that the cause of gastroschisis was a common embryonic mechanism associated with defective fusion of the lateral body folds that caused an abnormal closure of the thoracic and abdominopelvic cavities, which was shared with other congenital anomalies of the ventral body wall, except the omphalocele. Regarding omphalocele, it was posited that there was a failure in the return of the intestinal loops from the umbilical coelom to the abdominal cavity after the physiological herniation that usually must occur between the sixth and twelfth week of development 9,16.
According to those premises, the affected somatopleura was not normally developed due to a teratogenic exposure during the fourth week; consequently, an orifice devoid of ectoderm appeared in the abdominal wall through which the intestine protruded. At that time, an implicated teratogen was not proposed, nor was it explained why it exclusively affected an area as small as the orifice. Later on, a common mechanism was proposed to explain the origin of gastroschisis and omphalocele. It was proposed that, although both entities appeared as a consequence of physiological umbilical herniation, in the case of gastroschisis, the amnion ruptured, therefore causing exposure to amniotic fluid; it was also noted that in gastroschisis, the rectus abdominis muscles are in a normal position on each side of the central location of the defect 20.
Vascular alterations have also been postulated as the cause of gastroschisis. For example, de Vries 21) suggested that the defect could be generated by weakness and consequent rupture of the somatopleure, due in turn to abnormal involution of the right umbilical vein; while for Hoyme et al. (22, the defect and subsequent intestinal herniation were due to the rupture of the right vitelline artery in the umbilical region, which would produce infarction and necrosis at the base of the cord. Considering that the umbilical vein does not drain into the mesoderm of the umbilical region and that it is the dorsolateral aortic branches and not the vitelline arteries which supply the abdominal wall, both hypotheses were discarded. However, Lubinsky 23proposed a dual vascular and thrombotic model. The normal involution of the right umbilical vein leaves space to the umbilical ring's right, susceptible to a thrombotic event when estrogen levels are elevated, as occurs in very young mothers. In this case, the thrombus could damage the cell growth of the adjacent tissue and allow herniation of the abdominal viscera.
Although Shaw's hypothesis 20) did not take into account the genetic and epidemiological differences between the two malformations, nor did it resolve questions such as the reason for the presence of normal skin between the umbilical cord and the wall defect, nor the causes of the rupture, it was retaken in 1996 by Kluth and Lambrecht 24. They argued that gastroschisis resulted from the rupture of a small omphalocele.
In 2007, almost a decade later, Feldkamp et al. (8, proposed that gastroschisis resulted from the abnormal fusion in the midline of the asymmetric body folds, which prevented the incorporation of the yolk sac into the fixation pedicle and, therefore, the formation of the intestine within the abdominal cavity. Furthermore, they explained the right lateral position of gastroschisis based on the tendency of the yolk sac to shift to the right of the fixation pedicle. Later, although Stevenson et al.10, affirmed that gastroschisis was independent of the umbilical cord, they contested that its cause was a defective folding and postulated that the non-fusion of the yolk sac and its vessels with the fixation pedicle caused that, at the right side of the umbilical ring, a new perforation was created through which the intestine was extruded into the amniotic cavity. They also explained this right lateral position of the defect as a result of the slight displacement of the yolk sac to this side, as well as the presence of the heart and the more accelerated growth of the left lateral wall.
More recently, while investigating stillbirths with gastroschisis, Rittler et al. (25, reported detachment of the umbilical cord towards the right side of the ring, leaving a space for the gut evisceration. The malformation was caused by the defective union between the cord and the ring, possibly due to amnion weakness, vascular alterations, or insufficient tissue development in the periumbilical region.
Returning to the ideas of Shaw 20, Bargy and Beaudoin 18) proposed that the cause of gastroschisis was the rupture of the amnion that surrounds the eviscerated intestine during physiological umbilical hernia, which could be caused by teratogenic agents, following the observation of the vacuolar changes in the amniotic cells of the human embryos that they examined. The prenatal rupture of the physiological hernia was once again emphasized as the cause of gastroschisis by Beaudoin 26, who additionally discarded the hypothesis that it was independent of the umbilical cord.
Although Bargy and Beaudoin 18, Beaudoin 26 and Opitz 27considered that gastroschisis, rather than being due to a defect in the abdominal wall, was caused both by a failure in the closure of the umbilical ring, prior to the return of the physiological hernia to the abdominal cavity, as for the rupture of the amniotic membrane, it has not been possible to reach a conclusive agreement about its true origin (Table 1).
Table 1 Summary of the most relevant hypotheses about the origin of gastroschisis. The data is displayed chronologically
| Author | Hypothesis |
|---|---|
| Duhamel 19 | Altered differentiation in somatopleural mesenchyme and subsequent defective growth of the lateral abdominal wall and intestinal herniation, caused by teratogenic exposure at week 4 |
| Shaw 20 | Rupture of the amnion at the base of the umbilical cord during the period of physiological herniation (weeks 6-10); or delayed closure of the umbilical ring |
| De Vries 21 | Abnormal differentiation of the mesenchyme of the umbilical region and subsequent rupture of the adjacent body wall, caused by abnormal involution of the right umbilical vein |
| Hoyme et al. (22 | Infarction and subsequent necrosis at the base of the umbilical cord, after disruptive changes in the right vitelline artery |
| Kluth and Lambrecht 24 | Altered development of the umbilical cord, which causes the appearance of a small omphalocele and its subsequent rupture |
| Feldkamp et al. (8 | Defect resulting from abnormal folding of the ventral body wall, which allows extrusion of the intestine |
| Stevenson et al. (10 | Failure to incorporate the yolk sac and its vessels into the umbilical cord so that they are included within a second hole, outside the area of closure of the abdominal wall |
| Rittler et al. (25 | Defective umbilical ring, through the margin of which evisceration of the abdominal organs occurs, probably due to a deficiency in the deposition of cells at the site of transition between the amnion and the abdominal wall |
| Lubinsky 23 | Thrombotic event adjacent to the umbilical ring, located in the space generated by the atresia of the right umbilical vein |
| Bargy and Beaudoin 18 | Rupture of the amnion due to teratogens, located at the level of the flaccid part of the umbilical cord, during the period of physiological umbilical hernia |
| Beaudoin 26 | Rupture of the amnion of multifactorial origin, which likely occurs at week 8 |
| Opitz et al. (27 | Midline defect involving the canal and umbilical ring, with an absence of closure or rupture of the amnion that covers the area between the cord and the edge of the ring |
Etiology
In the last decade, many reports have been published on the role of genetic and non-genetic factors in the genesis of gastroschisis. Although there is no absolute certainty about the genetic origin of the malformation, information is available about family cases of twins and distant relatives 26,28,29. Through a systematic review of population studies, Salinas-Torres et al. (12, found associated chromosomal anomalies and defects, with an isolated frequency of 82.1%. The prevalence of concurrent anomalies was 17.9%, the most frequent cardiovascular and digestive ones. Regarding chromosomal abnormalities (trisomies 13, 18, and 21), the prevalence was more significant than 3%. In the involved chromosomes, they postulated some regions significantly associated with critical biological processes for the pathogenesis of the malformation, such as vascular alterations, thrombosis and mesodermal deficiency. On chromosome 13, they identified the regions 13q12.3 (FLT1), 13q22.1 (KLF5), 13q22.3 (EDNRB) and 13q34 (COL4A1, COL4A2, F7, F10), involved in the regulation of blood pressure, angiogenesis and coagulation; on chromosome 18, the 18q21.33 (SERPINB), 18q22.1 (CDH7, CDH19) (KRTAP (21q22.11 and 21q22.3) regions, involved with the regulation of endopeptidase activity and with calcium-dependent intercellular interactions; and on chromosome 21, regions 21q22.11 and 21q22.3 (KRTAP), related to keratinization processes.
Using bioinformatics tools and from genes co-segregated with gastroschisis, it has also been possible to recognize pathogenetic pathways involved in the closure of the ventral abdominal wall, which suggests that the abnormality could result from the interaction between biological and molecular mechanisms and genetic predisposition during the first ten weeks of development 13. In this same line of reasoning, Feldkamp et al. 14, through shared genomic segment analysis, identified heritable chromosomal segments in high-risk multigenerational pedigrees, thus supporting the concept of the existence of genetic susceptibility.
Early maternal age appears to be the main non-genetic risk factor. However, taking into account that until a few decades ago women were mothers at an early age but there was no growing global prevalence that the malformation currently shows 30, maternal age must be considered simultaneously with other prevalent factors in very young women, related to exposure to various exogenous agents such as tobacco, alcohol or illicit drugs, as well as the early initiation of sexual activity and the acquisition of genitourinary infections. In this regard, epidemiological findings have shown that infections acquired shortly before or during the first trimester of gestation, such as urinary and sexually transmitted infections, are similarly associated with gastroschisis, possibly through a pathway that involves the immune response 31-33. In a case-control study, Feldkamp et al. (31, reported that the risk of malformation doubled and even increased up to 25 times in the case of maternal age less than 20 years or smoking. Consequently, they associated the increased risk of gastroschisis with genitourinary infections and young maternal age.
Infectious agents
Feldkamp et al. 31 reported that, among the pathogens that cause sexually transmitted infections, Chlamydia trachomatis was the most frequent in the mothers of the group of cases they studied, which they explained as the result of the particular affinity of the pathogen for columnar epithelial cells of cervical ectropion in adolescent women, pregnant women and those taking estrogen contraceptives. It is worth noting that these columnar cells become squamous with increasing age, which decreases the pathogen-cell affinity and the probability of infection. The results of Feldkamp et al. (31differ from those previously published by Parker et al. (33, for the first trimester of gestation, in that the latter found no association between reactivity in terms of IgG against the pathogen or against the chlamydial heat shock protein CHP60, which should reflect the chronicity of the infection with Chlamydia trachomatis.
Ahrens et al. (34, reported an elevated risk of gastroschisis during the first trimester of pregnancy, associated with the use of antiherpetic drugs such as acyclovir, valacyclovir or famciclovir, a risk that was similar in magnitude to that which has been associated with genital herpes untreated. However, they noted that the observed medication-risk association was possibly affected by the infection itself as a confounding factor. Later, Werler et al. (35, postulated that the genuine risk factor for gastroschisis was maternal reactivity to herpes simplex viruses 1 and 2, Epstein Barr, and cytomegalovirus during early gestation. They showed that the risk of gastroschisis was not increased in the presence of IgG against herpes simplex virus 1, nor of IgM or IgG against cytomegalovirus and that, although primary infection with Epstein Barr was not associated with gastroschisis, it did occur between IgM and IgG against Epstein Barr viruses and herpes simplex 2. Based on these findings, they suggested that primary infections with Epstein Barr viruses, herpes simplex 1 or 2, or cytomegalovirus during the first trimester of pregnancy did not appear to be associated with the risk of gastroschisis, and that the genuine risk factor for the development of the malformation was the reactivation of infections with the Epstein Barr virus and herpes simplex 2.
Stress as an etiological factor
In addition to genetic and chromosomal alterations and infectious agents, other factors have been involved in the pathogenesis of the malformation. Apparently, there is an association with exposure to herbicides 36,37 or pesticides 38,39, radiation 40, use of drugs such as opioids 41,42, antihyperthyroid 43) or antiasthmatics 44, nutritional factors such as high preconception caloric intake plus a deficiency of methionine and threonine 45, a deficient intake of folic acid 46, and even with adverse maternal psychosocial conditions 47,48.
As a consequence of the great diversity of risk factors that have been implicated in the etiology of gastroschisis, the idea of a possible existence of a shared pathogenic pathway is better supported, the activation of which could induce oxidative damage in response to the stress generated by these factors 47-49. It has been shown that the oxidative imbalance resulting from excessive production of reactive oxygen species or a weakness of the antioxidant system can negatively affect the early development of the embryo since recently formed and actively proliferating cells are especially vulnerable to deleterious effects of these chemicals on DNA, which can cause not only congenital abnormalities, but also early abortion, preeclampsia, and intrauterine growth restriction 47,50.
Considering that the risk of suffering from the malformation seems to increase with the sum of exposures to different causative factors, Werler et al. 51 evaluated 16 different stressors associated with the risk of gastroschisis in a case-control study during the first trimester of pregnancy, such as fever, genitourinary infections, medications (anti-herpes, bronchodilators, opioids, aspirin, ibuprofen, oral contraceptives), illicit drugs, alcohol and cigarettes, among others; they found that, in the group of cases, the number of exposures was significantly higher, with an estimated risk up to 3.6 times higher than in the control group, which supports the hypothesis that the common denominator of gastroschisis risk factors is the induction of the inflammatory and oxidative response. It is worth noting that in this study, the accumulation of exposures did not take into account the strong inverse association that had previously been described between maternal age and the risk of malformation.
Some epidemiological considerations
The frequency of gastroschisis has shown an increasing global trend during the last decades. In the 1960s, the incidence was 0.06-0.8/10,000 52; and currently, it has reached values of 4.5-5.1/10,000 53. In the United States of America, Stallings et al. (54, by analyzing the data provided by the National Birth Defects Prevention Network (NBDPN), during the period 2012-2016, reported a global prevalence of 4.3/10,000 live births, almost three times more than that corresponding to the period 1998-2013. However, although the global trend has continued to rise over time, some studies indicate that it could reverse a decrease in some countries. In this sense, in the United States of America, Clark et al. (55, estimated a decrease to 3.3/10,000, noting that they only examined data from infants hospitalized in pediatric care units. Similarly, Li et al. (56, reported that, in 14 cities of a province of China, the prevalence decreased to 2.30/10,000 in 2006-2015, after having increased from 1.6 in 1986-1987 to 2.54/10,000 in the period 1996-2007. In Latin America, it is estimated that in Colombia the prevalence has shown a decreasing trend, from 3.26 in 2018, to 2.09 in 2020, despite that in the 2015-2017 period it had increased from 1.92 to 3.4/10,000; this in contrast to the reported prevalence of 9.59/10,000 for other countries in the region 57,58.
The strengthening of the surveillance programs for congenital malformations in all the countries of the world will make it possible to confirm whether the decrease above in prevalence corresponds to an isolated event or if, on the contrary, it can be attributed to more remarkable regional development, associated with less risk of harmful environmental exposures or, with socioeconomic conditions of better access to health and education.
Conclusions
The information obtained in both human beings and animal models indicates that the origin of gastroschisis must be sought in the defective closure of the umbilical ring, as well as in the rupture of the amnion; and, that these two events could occur as a consequence of the resulting oxidative damage of the activation of a common pathogenic pathway in which more than one factor with stress inducing potential converges and, perhaps, simultaneously with some type of genetic predisposition in the affected embryos. It is therefore feasible that the deepening of the biochemical and immunological aspects of this stress-inducing pathogenic pathway, as well as the search for regulatory genes and proteins, may shortly contribute to clearing up the uncertainty about the etiology of the malformation, especially in cases of family recurrences.
It is also worthwhile that the decrease in the global prevalence rate of the malformation reported in some countries be an object of further study to establish whether there are population factors involved.











 text in
text in 



