Remark
| 1) Why was this study conducted? |
| To update information on the cancer situation in the city of Quito, as an instrument in the planning of public policies and their continuous monitoring, to evaluate their compliance. |
| 2) What were the most relevant results of the study? |
| Our results show a sustained increase in cancer incidence and mortality rates throughout the study period. The stagnation in the decrease of cancers of the cervix and stomach is worrying. The sustained increase in the incidence of thyroid, breast, prostate and colon-rectal cancer is striking, a situation that makes the sustainable implementation of the National Strategy for Comprehensive Cancer Care in Ecuador urgent. |
| 3) What do these results contribute? |
| Information that allows evaluating cancer control policies and assessing their health impact on the burden of this disease. |
Introduction
Cancer represents a challenge for global public health, not only because of the continuous increase in its incidence and mortality rates over the last decades, which place it among the main causes of morbidity and mortality, but, above all, because it requires a comprehensive, transdisciplinary, multisectoral, participatory and sustainable plan for its control, which seeks to impact all stages of cancer continuum: promotion, primary prevention, detection, diagnosis, timely treatment and palliative care 1; while reducing the social inequality gap related to this disease 2.
Worldwide, there are an estimated 19.3 million new cancer cases (18.1 million not including non-melanoma skin cancer) and almost 10 million cancer deaths (9.9 million not including non-melanoma skin cancer) in the year 2020 3. The global cancer burden is expected to be 28.4 million cases in 2040, an increase of 47% from 2020 4. To address this growing burden of disease, the World Health Assembly recommends the development of National Cancer Control Plans (NCCPs) in all countries, as strategic public health documents that serve as a roadmap for cancer control 4.
In this context, population-based cancer registries (PBCR) as continuous surveillance systems that collect new cancer cases in a defined population, for the calculation of incidence and mortality, are key and reliable actors for tailor-made policy planning, as well as to evaluate and monitor its progress 5,6.
In Quito, in 1984, the National Tumor Registry (RNT) was created as a PBCR to regularly collect, process, analyse, and disseminate information on new cancer cases diagnosed in the city 7. Its implementation was delegated to the “Sociedad de Lucha contra el Cáncer” (SOLCA) through Ministerial Agreement 6345 8. In order to provide updated information on the profile of cancer in the city and thus contribute to the monitoring of control policies, this study analyses the behaviour of the main types of cancer in the city of Quito, in terms of their incidence and mortality, from 1985 to 2017.
Materials and Methods
Population and registration area
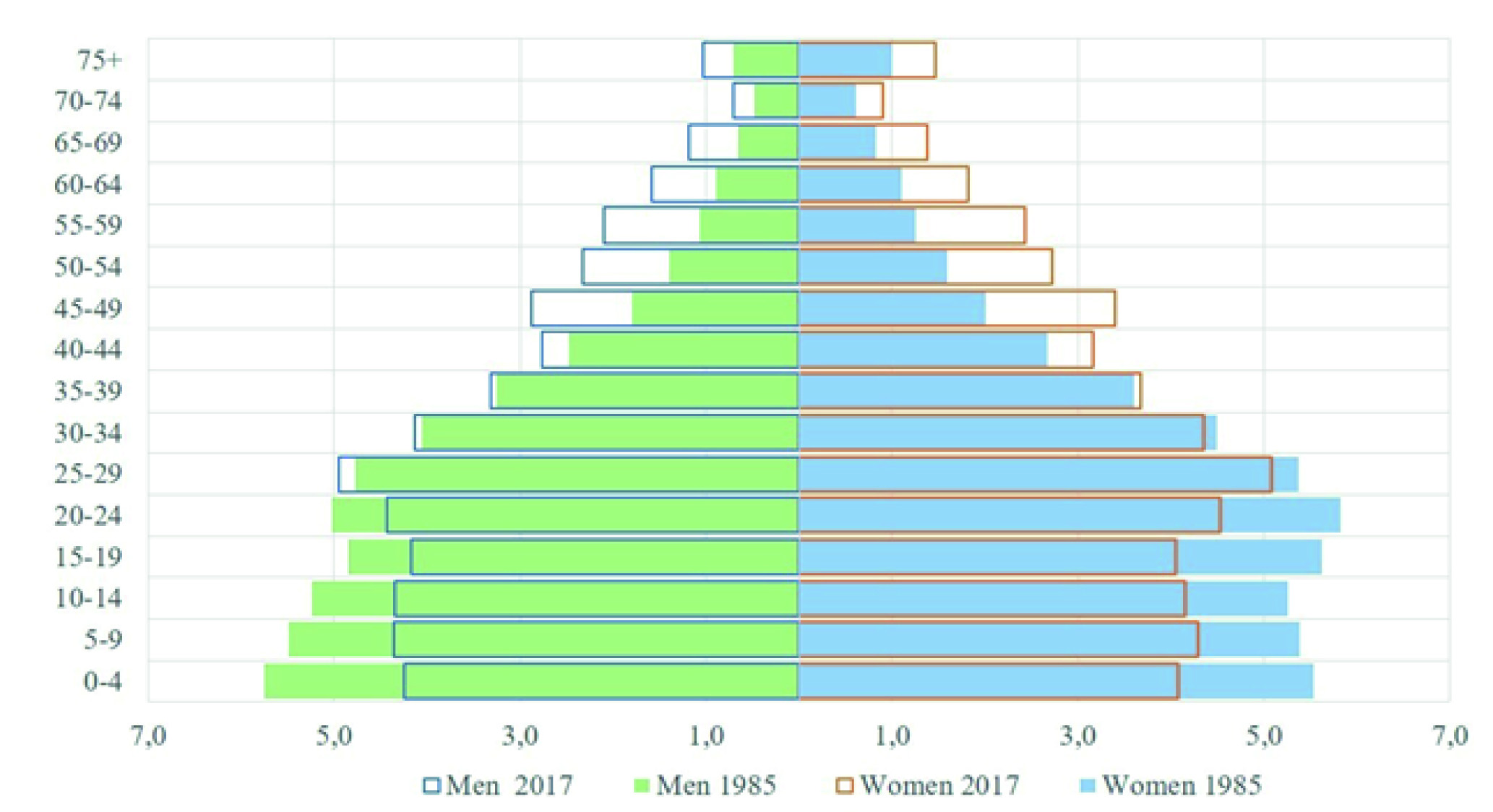
The city of Quito is the capital of Ecuador, it is the second largest and most populous city in the country, its population for the year 2017, according to the projections of the 2010 census, is 1,911,966 inhabitants 9. In the context of the demographic transition, the population has experienced changes in the population structure, where the increase in the older adult population and the reduction in the population under 15 years of age stand out. In 1985, those over 65 years of age represented 4.3% and in 2017, 6.7% (Figure 1).
Registry and analysis of information
Quito's PBCR captures all cancer cases diagnosed in the city. The sources of information are pathology and haematology laboratories, both from public and private health services, which are complemented by hospital discharges and deaths from cancer. The mortality data are obtained from the official reports of the National Institute of Statistics and Censuses (INEC), and are validated with the information captured by the PBCR in the primary sources (medical history), so it constitutes a corrected mortality data.
The Quito Registry is one of the 5 PBCRs in the country considered to be of high quality by the International Association of Cancer Registries (IACR), together they cover 41.4% of the population of Ecuador 10. Information about its history, objectives and methodology has been widely described in previous publications 7,11. Regarding quality indicators, for the period 2013-2017 these reach adequate values: 89.6% of histological verification (HV), 5.1% of cases that were admitted only by death certificates (ODC) and a Mortality/Incidence ratio (M/I) of 52.6% for both sexes (Table 1).
Table 1 Quito, Ecuador. Quality indicators for selected locations. 2013-2017
| Location | Histological verification | Death certificate only | Mortality incidence ratio |
|---|---|---|---|
| Percentage | |||
| Stomach | 85.8 | 9.7 | 85.1 |
| Colorectal | 91.4 | 4.5 | 61.4 |
| Lung | 76.1 | 13.0 | 88.7 |
| Breast | 97.1 | 1.5 | 36.4 |
| Cervix | 97.1 | 1.9 | 53.4 |
| Prostate | 92.6 | 5.3 | 46.3 |
| Thyroid | 97.3 | 0.5 | 7.8 |
| Non-Hodgkin lymphoma | 96.8 | 1.5 | 59.2 |
| All cases (-C44) | 89.6 | 5.1 | 52.6 |
Cancer data are presented according to the International Classification of Diseases ICD 10. The standardized rates by age (ASR) and sex, of incidence and mortality for the period 2013-2017, were calculated by the direct method using the world standard population of Segi 12. Crude rates (CR), cumulative rates (CuR) and the relative frequency (RF) of the most frequent locations are also presented. Non-melanoma skin cancer (C44) data are shown but excluded in the overall rate results.
To study the trend in incidence and mortality rates, the annual percentage change (APC) was estimated from 1985 to 2017 in selected locations. Joinpoint regression models were used to identify points of change in trends, so the terms ''increase'' or ''decrease'' were used when the APC was significantly different from zero (p-values <0.05); otherwise, the term "stable" was used. Significance tests were performed using the Monte Carlo permutation technique. All analysis were performed in the Joinpoint Regression Program version 4.9.0.0, of the Surveillance Research Program of the National Cancer Institute of the United States 13. APC and joinpoints were determined based on log-transformed TEEs and their standard errors. A maximum of four joinpoints with at least five observations were specified.
Results
From 1985 to 2017, 93,821 new cases of cancer were diagnosed among residents of Quito (Figure 2). The number of cases went from 1,355 in 1985 to 5,846 in 2017. The trend throughout the entire period was towards an increase, with different patterns in two periods, changing from an APC of 3.1 (Confidence Intervals at 95% (CI 95%): 2.2-4.1) in the period 1985-1995, to an APC of 5.6 (CI 95%: 5.3-6.0) from 1996 (Figure 2).
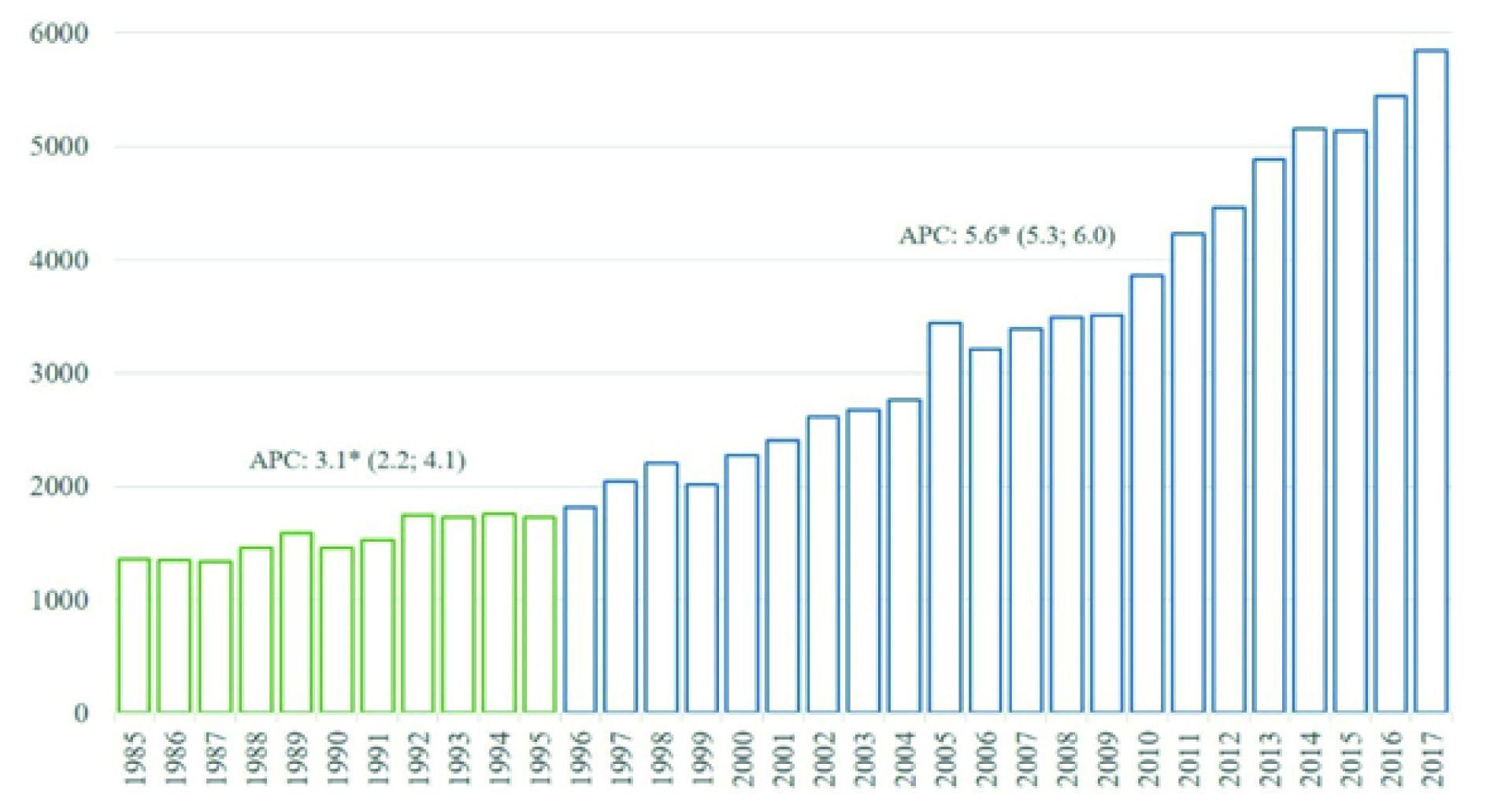
Figure 2 New cases diagnosed in residents of Quito, 1985 - 2017. Y: Number of cases; X: Year of diagnosis. CPA: Annual percentage change obtained from Joinpoint regression models, the figures in parentheses are Confidence Intervals at 95% of CPA; a star indicates a statistical significance of 0.05.
Incidence
The standardized incidence rates for all cancer locations, in the last period 2013-2017, were 212.2 in men; and 227.3 in women (Table 2). In men, the five most frequent locations were: prostate (ASR: 63.0), stomach (ASR: 19.5), non-Hodgkin's lymphoma (ASR: 15.2), colon-rectum (ASR: 14.3), and thyroid (ASR: 10.1). In women, the most frequent locations were: thyroid (ASR: 47.0), breast (ASR: 41.8), cervix (ASR: 17.5), stomach (ASR: 13.5), and colon-rectum (ASR: 12.5).
Table 2 Standardized incidence rates by sex. Quito, 2013 - 2017
| Code | Location | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | CR | ASR | CuR % | RF % | Cases | CR | ASR | CuR % | RF % | ||
| C00-C14 | Lip, oral cavity and pharynx | 91 | 2.0 | 2.2 | 0.2 | 1.0 | 113 | 2.4 | 2.2 | 0.2 | 1.0 |
| C15 | Esophagus | 72 | 1.6 | 1.7 | 0.2 | 0.8 | 31 | 0.7 | 0.6 | - | 0.3 |
| C16 | Stomach | 802 | 17.9 | 19.5 | 2.1 | 9.2 | 708 | 14.9 | 13.5 | 1.4 | 6.2 |
| C17 | Small intestine | 68 | 1.5 | 1.7 | 0.2 | 0.8 | 68 | 1.4 | 1.4 | 0.2 | 0.6 |
| C18-C20 | Colon-rectum | 594 | 13.3 | 14.3 | 1.5 | 6.8 | 641 | 13.5 | 12.5 | 1.4 | 5.6 |
| C21 | Anus and anal canal | 22 | 0.5 | 0.5 | 0.1 | 0.3 | 50 | 1.1 | 0.9 | 0.1 | 0.4 |
| C22 | Liver and intrahepatic bile ducts | 236 | 5.3 | 5.9 | 0.7 | 2.7 | 257 | 5.4 | 4.9 | 0.5 | 2.2 |
| C23-C24 | Gallbladder and bile ducts | 165 | 3.7 | 4.1 | 0.5 | 1.9 | 282 | 5.9 | 5.4 | 0.6 | 2.5 |
| C25 | Pancreas | 146 | 3.3 | 3.6 | 0.4 | 1.7 | 227 | 4.8 | 4.3 | 0.4 | 1.9 |
| C30-C31 | Nose, sinuses, etc. | 28 | 0.6 | 0.6 | 0.1 | 0.3 | 13 | 0.3 | 0.2 | 0.0 | 0.1 |
| C32 | Larynx | 58 | 1.3 | 1.5 | 0.2 | 0.7 | 17 | 0.4 | 0.3 | - | 0.1 |
| C33-C34 | Trachea, bronchi and lung | 333 | 7.4 | 8.2 | 1.0 | 3.8 | 342 | 7.2 | 6.8 | 0.8 | 2.9 |
| C40-C41 | Bone | 81 | 1.8 | 1.8 | 0.2 | 0.9 | 61 | 1.3 | 1.3 | 0.1 | 0.5 |
| C43 | Skin melanoma | 195 | 4.4 | 4.7 | 0.5 | 2.2 | 212 | 4.5 | 4.1 | 0.4 | 1.9 |
| C44 | Other malignant skin tumors | 1,813 | 40.5 | 43.7 | 4.7 | - | 2,018 | 42.5 | 38.2 | 3.9 | - |
| C45 | Mesothelioma | 13 | 0.3 | 0.3 | - | 0.1 | 9 | 0.2 | 0.2 | - | 0.1 |
| C46 | Kaposi sarcoma | 67 | 1.5 | 1.4 | 0.1 | 0.8 | 13 | 0.3 | 0.2 | - | 0.1 |
| C47+C49 | Connective and soft tissue | 150 | 3.4 | 3.6 | 0.4 | 1.7 | 157 | 3.3 | 3.2 | 0.3 | 1.4 |
| C50 | Breast | 23 | 0.5 | 0.6 | 0.1 | 0.3 | 2,057 | 43.3 | 41.8 | 4.8 | 17.9 |
| C51 | Vulva | - | - | - | - | - | 30 | 0.6 | 0.5 | 0.1 | 0.3 |
| C52 | Vagina | - | - | - | - | - | 24 | 0.5 | 0.5 | 0.1 | 0.2 |
| C53 | Cervix uteri | - | - | - | - | - | 905 | 19.0 | 17.5 | 1.8 | 7.8 |
| C54 | Corpus uteri | - | - | - | - | - | 308 | 6.5 | 6.3 | 0.8 | 2.7 |
| C55 | Uterus; unspecified part | - | - | - | - | - | 46 | 1.0 | 0.9 | 0.1 | 0.4 |
| C56 | Ovary | - | - | - | - | - | 448 | 9.4 | 8.9 | 0.9 | 3.9 |
| C58 | Placenta | - | - | - | - | - | 12 | 0.3 | 0.2 | - | 0.1 |
| C60 | Penis | 47 | 1.1 | 1.1 | 0.1 | 0.5 | - | - | - | - | - |
| C61 | Prostate | 2,472 | 55.3 | 63.0 | 8.0 | 28.3 | - | - | - | - | - |
| C62 | Testis | 280 | 6.3 | 5.6 | 0.4 | 3.2 | - | - | - | - | - |
| C64 | Kidney | 260 | 5.8 | 6.5 | 0.8 | 2.9 | 182 | 3.8 | 3.8 | 0.4 | 1.5 |
| C65 | Renal pelvis | 3 | 0.1 | 0.1 | - | 0.0 | 2 | - | - | - | 0.0 |
| C67 | Bladder | 272 | 6.1 | 6.7 | 0.7 | 3.1 | 95 | 2.0 | 1.8 | 0.2 | 0.8 |
| C69 | Eye and adnexa | 33 | 0.7 | 0.8 | 0.1 | 0.3 | 29 | 0.6 | 0.6 | - | 0.3 |
| C70-C72 | Brain and nervous system | 257 | 5.7 | 5.9 | 0.5 | 2.9 | 248 | 5.2 | 5.2 | 0.5 | 2.1 |
| C73 | Thyroid | 443 | 9.9 | 10.1 | 1.1 | 5.1 | 2,402 | 50.5 | 47.0 | 4.8 | 20.9 |
| C81 | Hodgkin lymphoma | 65 | 1.5 | 1.5 | 0.1 | 0.7 | 50 | 1.1 | 1.0 | 0.1 | 0.4 |
| C82-C86,C96 | Non-Hodgkin lymphomas | 641 | 14.3 | 15.2 | 1.6 | 7.3 | 595 | 12.5 | 11.9 | 1.3 | 5.1 |
| C90 | Multiple myeloma | 147 | 3.3 | 3.6 | 0.4 | 1.7 | 104 | 2.2 | 2.1 | 0.3 | 0.9 |
| C91 | Lymphoid leukemias | 217 | 4.9 | 5.2 | 0.3 | 2.5 | 161 | 3.4 | 3.6 | 0.3 | 1.4 |
| C92 | Myeloid leukemias | 178 | 4.0 | 4.2 | 0.4 | 2.0 | 168 | 3.5 | 3.4 | 0.3 | 1.5 |
| All sites | 10,543 | 235.7 | 255.9 | 28.4 | - | 13,485 | 283.7 | 265.5 | 28.0 | - | |
| All Sites - C44 (Skin) | 8,730 | 195.2 | 212.2 | 23.7 | 100 | 11,467 | 241.3 | 227.3 | 24.1 | 100 | |
CR: crude rate; ASR: age-standardized rate; CuR: cumulative rate; RF: relative frequency
Mortality
The standardized mortality rates for all cancer locations, in the last period 2013-2017, were 123.6 in men; and 107.9 in women (Table 3). In men, prostate (ASR: 27.7) and stomach cancer (ASR: 16.8) had the highest mortality rates; in women, they were breast cancer (ASR: 15.1), stomach (ASR: 11.3) and cervical (ASR: 9.6).
Table 3 Standardized mortality rates by sex. Quito, 2013-2017.
| Code | Location | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | CR | ASR | CuR % | RF % | Cases | CR | ASR | CuR % | RF % | ||
| C00-C14 | Lip, oral cavity and pharynx | 53 | 1.2 | 1.2 | 0.1 | 1.0 | 64 | 1.3 | 1.2 | 0.1 | 1.2 |
| C15 | Esophagus | 68 | 1.5 | 1.6 | 0.1 | 1.3 | 28 | 0.6 | 0.5 | 0.1 | 0.5 |
| C16 | Stomach | 690 | 15.4 | 16.8 | 1.8 | 13.5 | 595 | 12.5 | 11.3 | 1.2 | 10.8 |
| C17 | Small intestine | 39 | 0.9 | 1.0 | 0.1 | 0.8 | 49 | 1.0 | 1.0 | 0.1 | 0.9 |
| C18-C20 | Colon-rectum | 364 | 8.1 | 8.7 | 0.9 | 7.1 | 394 | 8.3 | 7.5 | 0.8 | 7.2 |
| C21 | Anus and anal canal | 11 | 0.2 | 0.3 | - | 0.2 | 28 | 0.6 | 0.5 | 0.1 | 0.5 |
| C22 | Liver and intrahepatic bile ducts | 218 | 4.9 | 5.5 | 0.6 | 4.3 | 249 | 5.2 | 4.7 | 0.5 | 4.5 |
| C23-C24 | Gallbladder and bile ducts | 139 | 3.1 | 3.4 | 0.4 | 2.7 | 254 | 5.3 | 4.9 | 0.5 | 4.6 |
| C25 | Pancreas | 135 | 3.0 | 3.4 | 0.4 | 2.6 | 196 | 4.1 | 3.7 | 0.4 | 3.6 |
| C30-C31 | Nose, sinuses, etc. | 22 | 0.5 | 0.5 | 0.1 | 0.4 | 14 | 0.3 | 0.3 | - | 0.3 |
| C32 | Larynx | 41 | 0.9 | 1.0 | 0.1 | 0.8 | 11 | 0.2 | 0.2 | - | 0.2 |
| C33-C34 | Trachea, bronchi and lung | 300 | 6.7 | 7.4 | 0.8 | 5.9 | 299 | 6.3 | 5.8 | 0.6 | 5.4 |
| C40-C41 | Bone | 46 | 1.0 | 1.0 | 0.1 | 0.9 | 42 | 0.9 | 0.9 | 0.1 | 0.8 |
| C43 | Skin melanoma | 92 | 2.1 | 2.2 | 0.2 | 1.8 | 86 | 1.8 | 1.7 | 0.2 | 1.6 |
| C44 | Other malignant skin tumors | 595 | 13.3 | 14.1 | 1.2 | - | 563 | 11.8 | 9.7 | 0.7 | - |
| C45 | Mesothelioma | 8 | 0.2 | 0.2 | - | 0.2 | 8 | 0.2 | 0.1 | - | 0.1 |
| C46 | Kaposi sarcoma | 25 | 0.6 | 0.5 | - | 0.5 | 2 | - | - | - | - |
| C47+C49 | Connective and soft tissue | 97 | 2.2 | 2.3 | 0.2 | 1.9 | 84 | 1.8 | 1.7 | 0.2 | 1.5 |
| C50 | Breast | 12 | 0.3 | 0.3 | - | 0.2 | 745 | 15.7 | 15.1 | 1.8 | 13.6 |
| C51 | Vulva | - | - | - | - | - | 15 | 0.3 | 0.2 | - | 0.3 |
| C52 | Vagina | - | - | - | - | - | 14 | 0.3 | 0.3 | - | 0.3 |
| C53 | Cervix uteri | - | - | - | - | - | 483 | 10.2 | 9.6 | 1.1 | 8.8 |
| C54 | Corpus uteri | - | - | - | - | - | 121 | 2.5 | 2.5 | 0.3 | 2.2 |
| C55 | Uterus; unspecified part | - | - | - | - | - | 35 | 0.7 | 0.7 | 0.1 | 0.6 |
| C56 | Ovary | - | - | - | - | - | 248 | 5.2 | 4.9 | 0.5 | 4.5 |
| C58 | Placenta | - | - | - | - | - | 14 | 0.3 | 0.3 | - | 0.3 |
| C60 | Penis | 20 | 0.4 | 0.5 | - | 0.4 | - | - | - | - | - |
| C61 | Prostate | 1,145 | 25.6 | 27.7 | 2.7 | 22.4 | - | - | - | - | - |
| C62 | Testis | 58 | 1.3 | 1.2 | 0.1 | 1.1 | - | - | - | - | - |
| C64 | Kidney | 115 | 2.6 | 2.9 | 0.3 | 2.2 | 75 | 1.6 | 1.5 | 0.2 | 1.4 |
| C65 | Renal pelvis | 4 | 0.1 | 0.1 | - | 0.1 | - | - | - | - | - |
| C67 | Bladder | 148 | 3.3 | 3.6 | 0.4 | 2.9 | 60 | 1.3 | 1.1 | 0.1 | 1.1 |
| C69 | Eye and annexes | 13 | 0.3 | 0.4 | - | 0.3 | 12 | 0.3 | 0.2 | 0.0 | 0.2 |
| C70-C72 | Brain and nervous system | 196 | 4.4 | 4.6 | 0.4 | 3.8 | 168 | 3.5 | 3.5 | 0.3 | 3.1 |
| C73 | Thyroid | 63 | 1.4 | 1.6 | 0.2 | 1.2 | 158 | 3.3 | 3.2 | 0.4 | 2.9 |
| C80 | unspecified sites | 94 | 2.1 | 2.2 | 0.2 | 1.8 | 164 | 3.5 | 3.3 | 0.4 | 3.0 |
| C81 | Hodgkin lymphoma | 30 | 0.7 | 0.7 | 0.1 | 0.6 | 21 | 0.4 | 0.4 | - | 0.4 |
| C82-C86,C96 | Non-Hodgkin lymphomas | 370 | 8.3 | 8.9 | 1.0 | 7.2 | 343 | 7.2 | 6.7 | 0.7 | 6.2 |
| C90 | Multiple myeloma and malignant plasma cell tumors | 104 | 2.3 | 2.6 | 0.3 | 2.0 | 82 | 1.7 | 1.7 | 0.2 | 1.5 |
| C91 | Lymphoid leukemias | 137 | 3.1 | 3.2 | 0.2 | 2.7 | 95 | 2.0 | 2.0 | 0.2 | 1.7 |
| C92-C95 | Myeloid leukemias | 144 | 3.2 | 3.4 | 0.3 | 2.8 | 110 | 2.3 | 2.2 | 0.2 | 2.0 |
| All sites | 5,712 | 127.7 | 137.6 | 13.9 | - | 6,061 | 127.5 | 117.6 | 12.3 | - | |
| All Sites - C44 (Skin) | 5,117 | 114.4 | 123.6 | 12.7 | 100 | 5,498 | 115.7 | 107.9 | 11.6 | 100 | |
CR: crude rate; ASR: age-standardized rate; CuR: cumulative rate; RF: relative frequency
Main types of cancer
The analysis of the main types of cancer diagnosed in the city over time can be seen in Figure 3, built from an incidence comparison of the period 1985-1989 and the last 2013-2017. Significant changes are evident in the location that will be discussed later on.
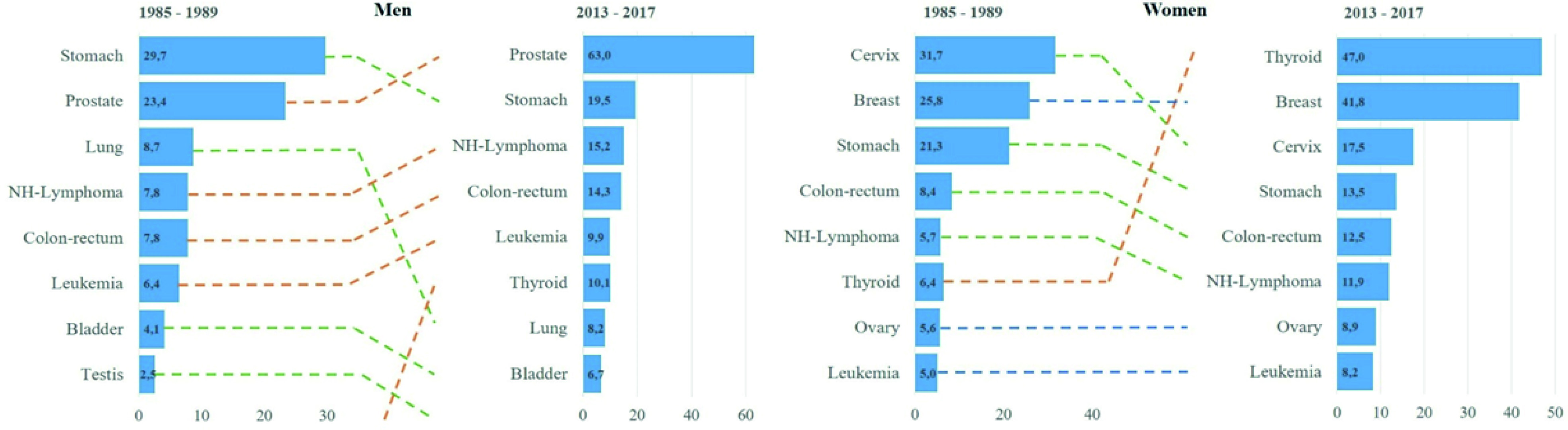
Figure 3 Changes in the distribution of the most frequent cancers in two diagnostic periods from 1985-1989 to 2013-2017, Quito by sex. Y: types of cancer; X: standardized rates per 100,000 person years.
Trend analysis
A significant increase in incidence rates was found for all cancer sites combined, beginning in 2001 in women and 1996 in men. In relation to overall mortality, a significant increase was observed throughout the analysis period, with an APC of 1.3% in women and 2.0% in men (Figure 4).
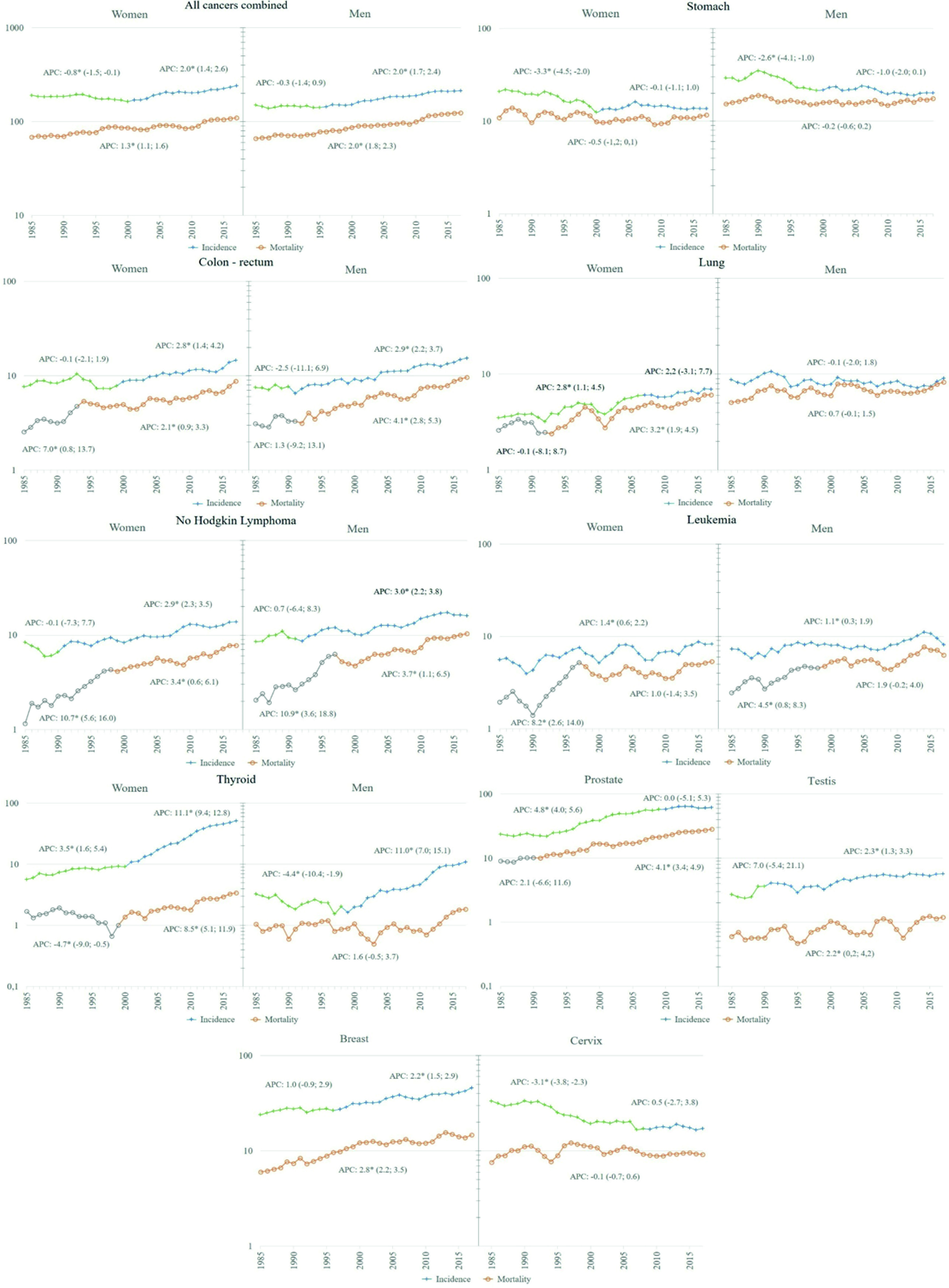
Figure 4 Trend of incidence and mortality in Quito 1985 - 2017, selected locations. Y: Standardized rate per 100,000 person years; X: year of diagnosis/death. APC: Annual percentage change obtained from Joinpoint regression models, the figures in parentheses are Confidence Intervals at 95% of APC; a star indicates a statistical significance of 0.05.
Discussion
During the 33 years of analysis, cancer incidence and mortality rates in Quito have increased continuously. In 2017, the Ecuadorian Ministry of Health developed a national strategy for cancer care 14, with the aim of reducing morbidity and mortality and improving the quality of life of people affected by cancer, but also to guide the National Health System (NHS) in its commitment to promote, prevent and control cancer and seek coordinated actions of the different segments. Although evaluating the impact of this strategy is not possible, it is necessary to place it as a reference in this discussion space, given the dynamic epidemiological profile of cancer.
Throughout the entire period evaluated, the number of cancer cases has increased steadily. This increase is a product, in part, of the demographic transformation of the Ecuadorian population that has doubled and aged, increasing its life expectancy from 66 years in 1985 to 76 years in 2017 9.
Cancer among older adults (over 65 years of age) represented 43.7% of the total cases diagnosed in Quito for the period 2013-2017. Similar behaviour was observed in the Latin American and Caribbean region, where new cancer cases among older adults accounted for almost half (48%) of the total incidence burden (43% in Central America, 49% in South America, and 52% in % in the Caribbean) in 2018. According to updated GLOBOCAN estimates for 2018, the number of new cancer cases among older adults in the Latin American region is expected to double by 2040 15.
Worldwide, there has been a reduction in cancers related to infections (stomach and cervical cancer) and an increase in cancers related to westernized lifestyles (breast, prostate, and colorectal cancer). In Quito, the trend analysis allowed us to identify this pattern of behaviour. Prostate, breast, and colorectal cancer showed increasingly faster growth rates, while stomach and cervix initially decreased followed by a period of stagnation.
Stomach
The decrease in incidence and mortality rates is part of the historical behaviour of reduction during the last half century in most populations. This pattern is attributed to the unplanned effect of improved hygienic-sanitary conditions, including a decrease in the prevalence of Helicobacter pylori and improvements in food preservation and storage 3. In Quito, however, the significant decrease occurred until the year 2000, after which the rates no longer decreased.
Recent research contributes to explain this stagnation, probably the increase in the diffuse histological type compared to the intestinal one, whose natural history would have different connotations, particularly in the earlier age of presentation, a situation reported in countries such as the United States, Canada, the United Kingdom, Chile and Colombia 16,17. Another reason could be the impact of social inequalities in this type of cancer, where the risk is higher in people with a low socio-economic position (SEP). In the context of growing social inequities, less privileged people would benefit to a lesser extent from advances in the promotion, prevention, and treatment of the disease 18,19.
Despite the initial decrease in incidence rates, Quito continues, both in men and women, to be among the regions with the highest incidence rates in the world 3, with an M/I ratio of 85.1%, which reflects the high lethality of the disease.
The national strategy for cancer care establishes screening in the population over 50 years of age, by combining serological tests to determine Helicobacter pylori and pepsinogen combined with upper gastrointestinal endoscopy. The interval of the endoscopic studies would be determined by the ABC stratification method 14. Although these actions would contribute to the control of this type of cancer, it is necessary to rethink the strategies in light of these results, without losing sight of the determination that social inequities have in this disease 18.
Lung
Incidence rates remained stable in men throughout the period, and a significant growth pattern was seen in women between 1985 and 2005, which subsequently levelled off. This behaviour has had an impact on the male-to-female ratio, going from 2.4:1 in the first period to 1.2:1 in the last, probably reflecting the historical and gender-specific smoking profiles, with an earlier prevalence of smoking among men versus a later smoking rate among women 3.
Mortality rates have remained stable among men and there has been significant growth among women since 1993. In both men and women, mortality closely follows incidence, with an M/I ratio of 88.7% accounting for wrong prognosis of this cancer, as is known worldwide. In the global context, the rates found in Quito place us among the countries with the lowest incidence 3.
More than 70% of cases can be attributed to tobacco, so, without a doubt, tobacco control policies and the application of anti-smoking laws are key to reducing incidence and mortality rates. In Ecuador, anti-smoking measures began in the 1980s and were deepened after the ratification of the WHO Framework Convention on Tobacco Control (WHO FCTC) in 2006 11,20.
The most recent national health assessment shows that the prevalence of tobacco use in 2012 was 35.1% in the population aged 20 to 59 years, and 28.4% in the population aged 10 to 19 years 21. Reducing the prevalence of tobacco consumption has become a national objective, not only because it implies a health and social cost, but also because it is a risk factor that can be prevented.
Cervix
In Quito, the incidence rates decreased significantly until 2008, after which they stagnated. As in most countries, the initial decrease can be attributed, in part, to the change in the lives of women in urban contexts 3. It is also important to highlight the pilot screening program that remained active between 1996 and 2007 22. However, beyond the stagnation in the reduction of incidence rates, more worrying is the fact that mortality rates did not decrease in the 33 years of analysis, with an M/I ratio of 53.4%, which reflects the difficulties of access in a segmented National Health System, such as the Ecuadorian 23.
According to the national strategy, sexual and reproductive health should be promoted within the framework of human rights, and the universal immunization program against the Human Papillomavirus (HPV) should be strengthened from 9 years of age. Regarding screening, it should be performed in women between 21 and 65 years of age with a Pap smear every 3 years. In women aged 30 to 65 years, screen with cytology and molecular tests for HPV every 5 years 14.
In the context of the Global Strategy to accelerate the elimination of cervical cancer in the world, the high incidence rates observed in Quito, which place it among the highest incidence in the world 24, and a mortality that does not show significant changes in the 33 years of analysis; make sustained implementation of the National Strategy urgent 25.
Breast
Since the 1980s, there has been an increase in incidence rates worldwide, initially attributed to greater detection through the widespread adoption of mammography, and later to the obesity epidemic, given the stronger and more consistent association of excess body weight with oestrogen receptor-positive cancer 3.
In Quito, the increase in incidence has been significant since the end of the 1990s. Breast cancer has always ranked second among Quito women, in the eighties after cervical cancer, and currently after thyroid cancer. Mortality rates have increased steadily throughout the study period, currently occupying the first place as a cause of death. Worldwide, Quito presents intermediate rates in relation to the other countries 3.
The official statement proposes screening healthy women, from 50 to 69 years of age with mammography, and starting earlier detection in women with a history, risk or symptoms every two years 14. Taking into account the magnitude and its behaviour over time, it is essential to move from the proposal to the implementation of the breast cancer screening program.
Thyroid
The increase in thyroid cancer is a worldwide phenomenon, which began in the late 1990s and early 2000s, whose initial explanation based on the increase in risk factors such as radiation and obesity, was not enough to understand the magnitude of the increase. Comparison of the incidence curves by age before and after the introduction of ultrasound shows an increase attributed to the intense search for nodules in middle-aged individuals. Several studies estimate that this increase is due to overdiagnosis 26-28 since it would be self-limited nodules that do not lead to death. The increase in overdiagnosis was initially observed in the most economically developed countries and has now spread to low- and middle-income countries, especially those in which an important component of health services is private 26.
In Quito, the incidence rates showed a continuous increase throughout the study period, which was accentuated in women from 2001 with an APC of 11.1 (95% CI: 9.4-12.8), and in men from 1999 with an APC of 11.0 (95% CI: 7.0-15.1). Over time, this type of cancer climbed positions among the most frequent types, moving to first place among women and sixth place in men. Mortality rates also increased in women from 2002, while in men they remained stable throughout the period.
The increase in thyroid cancer constitutes a serious public health problem that needs further study and decision-making, especially due to the economic implications, unnecessary treatments and undesirable effects that it may be causing in the population. Globally, Quito's rates are among the highest in the world. Although the national strategy for cancer care in Ecuador has not prioritized this type of cancer to issue comprehensive interventions, technical guidelines are required in this regard.
Prostate
Worldwide there are important differences in incidence rates, which range from 6 to more than 100 cases per 100,000 men. In most countries the trend is increasing, the great differences, it seems, are not only linked to the increase in risk factors, such as obesity, diet, but fundamentally to differences in prevention and diagnosis policies. The implementation of Prostate Specific Antigen (PSA) and ultrasound shot up the rates from the 1980s to the 2000s, when they made a downward curve attributable to the recommendation not to use PSA in asymptomatic patients 3,29.
In Quito, this phenomenon can be observed since the end of the century, ten years later than in countries with high Human Development Index (HDI), and it continues until 2010, after which it stabilizes. This behaviour has placed this tumour in first place among the most frequent cancers, distancing itself from stomach cancer, which until the beginning of the 1990s was in first place. The incidence rate went from 23.4 in the period 1985-1989 to 63.0 cases per 100,000 men in 2013-2017, the average value for Latin America 3.
To date, no consensus has been reached on screening and early detection of prostate cancer in the population. The World Health Organization has not yet developed specific recommendations in this regard. In Ecuador, the national strategy for cancer care states that generalized collective screening for prostate cancer is not appropriate because unnecessary diagnoses can be made and causes undesirable effects 7. Rather, this strategy focuses on promoting healthy lifestyles. Thus, an individual choice of men after receiving information about the uncertainties, risks and potential benefits associated with screening, as recommended by the United States Preventive Services Task Force, becomes an option to complement control of this type of cancer 30.
Colon-recto
At the global level, the increase in colorectal cancer rates has been linked to the increase in the HDI, which is why it is considered a marker of socioeconomic development 31. In Quito, during the period of analysis, a constant increase was evidenced both in its incidence and mortality rates, both in men and women, with the difference that the increase in incidence began earlier in men, 1991 and 2000 respectively. This increase in the number of new cases probably reflects changes in lifestyles, particularly in diet, that is, changes towards a higher intake of foods of animal origin and a more sedentary lifestyle, including excessive consumption of alcohol, smoking and consumption of red or processed meat 32.
In this regard, primary prevention remains the key strategy to reduce the growing burden of this type of cancer, current evidence suggests adding early detection with affordable and non-invasive methods (guaiac tests and faecal immunochemical tests), at least in some emerging economy settings, thus offering options to manage the growing burden of disease 3. In Ecuador, this less invasive methodology, after a pilot project developed in a public hospital in Quito 33-35, has been adopted throughout the country through the screening manual published in 2017 35.
Leukemias
Recently, the marked geographical disparities in the incidence of leukemia and its relationship with human development have been highlighted, explained in part by the quality and access to health systems, various etiological factors, including interactions between genes and the environment 36. In Quito, trends in incidence and mortality from leukemia, both myeloid and lymphoid, show a constant increase that places it among the main types of cancer in both men and women. Further study is required to understand the underlying factors and thus support future prevention strategies for this type of cancer.
Non-Hodgkin Lymphoma
Despite a growing understanding of the pathology and genetics of lymphomas, global reports of variations in incidence remain limited in number and scope 37. The marked differences in incidence rates by country and region may be related in part to contrasting levels of access to care and the availability of diagnostic services.
In Quito, Non-Hodgkin's lymphomas are the third most common cancer in men and the sixth in women, for the last study period. Its incidence and mortality show a continuous increase, starting in the 1990s, with higher figures in men than in women. In-depth analysis are necessary to justify control actions for this type of cancer.
Conclusion
This article describes the epidemiological profile of cancer in the city of Quito. Indispensable information, as a baseline, to formulate and provide feedback on strategies to control this disease. It is of the utmost importance to prioritize actions on types of cancer with a high incidence and likely to have an impact, such as cervical, stomach and breast cancer, to advance in colorectal cancer screening and to outline the diagnosis policy for thyroid and prostate cancers.
Promotion and primary prevention are a particularly effective way to control cancer, as they have several advantages: effectiveness could have benefits for people other than those directly targeted 38. Although it has been established that, based on current knowledge of risk factors, between one-third and one-half of cancers can be prevented; contrasts the little investment that has been given to this area, compared to others. Even in high-income countries, the budget allocated to cancer prevention barely reaches 10% 39.
A strength of this study lies in the consistency of the Quito PBCR as an information system that has been maintained over time and has received recognition from the IACR, qualifying it as a high-quality registry. A limitation could be that the data correspond to a region of the country, so the challenge to be met in the future will be to establish national estimates, based on the information of the 6 PBCR existing in the country.











 text in
text in