Remark
| 1) Why was this study conducted? |
| Triphasic waves constitute an electroencephalographic pattern associated with various types of acute encephalopathy. The reason why these waves appear only in some patients with encephalopathies is still controversial. Brain atrophy could predispose to its appearance. The objective of this work was to compare the degree of cerebral atrophy and white matter lesion in patients with acute encephalopathy with and without triphasic waves. |
| 2) What were the most relevant results of the study? |
| 16 patients with triphasic waves and 30 without triphasic waves matched according to age and sex were identified. The degree of brain atrophy was higher in patients with triphasic waves (10.43 vs 6.9, p= 0.03). There were no significant differences in relation to the degree of white matter lesion between both groups. Mortality was higher in the group with triphasic waves (31.25 vs 6.66% p= 0.02). |
| 3) What do these results contribute? |
| The presence of cerebral atrophy could be related to the appearance of triphasic waves in patients with acute encephalopathy. There were no significant differences in the degree of white matter injury. Mortality in the group with triphasic waves was high. Studies are needed to determine its prognostic value. |
Introduction
Acute encephalopathy is a state of cognitive dysfunction or altered sensorium that occurs as the result of brain injury or systemic disease, and it is usually transitory. The presence of acute encephalopathy is associated with a significant mortality rate.
Triphasic waves (TW) constitute a periodic and generalized electroencephalographic pattern characterized by a typical complex of three waves appearing at 1 to 3 Hz frequency 1.
Since its initial description in patients with hepatic encephalopathy, by Foley et al in 1950 and Bickford & Butt in 1955, Triphasic waves have been associated with various types of acute encephalopathy, including those of toxic, metabolic origin, secondary to sepsis, anoxia, among others 2,3. However, the reason why these waves appear only in some patients with acute encephalopathy is still controversial.
Brain atrophy is defined as the decrease in the brain parenchyma volume. It constitutes a classic but nonspecific biomarker of neurodegeneration and it is a common finding in patients with acute encephalopathy. It has been suggested that the degree of cerebral atrophy and other morphological alterations, such as white matter lesions, could predispose to the appearance of triphasic waves in patients with toxic-metabolic disorders 4. However, the available information is not conclusive, and it has not been replicated in the latinamerican population.
The hypothesis of this study is that patients with acute encephalopathy and triphasic waves have a higher degree of cerebral atrophy and/or white matter lesions than those patients with acute encephalopathy without triphasic waves. Therefore, our main objective was to compare the degree of brain atrophy and the magnitude of white matter injury in patients with acute encephalopathy with and without triphasic waves. The secondary objectives were to describe the clinical and electroencephalographic characteristics and the in-hospital mortality rate in this population.
Materials and methods
We conducted a cross-sectional descriptive study. We included patients older than 18 years with clinical diagnosis of encephalopathy admitted to Sanatorio de la Trinidad Mitre in Buenos Aires-Argentina between January 2016 and July 2019. To be eligible the patients must have been studied with brain imaging and electroencephalogram (EEG) during the course of encephalopathy.
The patient's selection strategy was done in two databases: 1) The digital database from the neurology department and 2) the electronic database from the neurophysiology area. In the first, we identified those patients with a diagnosis of encephalopathy. In the second, we selected those patients with abnormal EEG reports.
Encephalopathy was defined based on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (DSM-V), as an alteration in sensorium and/or attention associated with a dysfunction in another cognitive domain that it occurs in a short space of time and is not better explained by a pre-existing neurocognitive disorder 5. The medical records of the selected subjects were evaluated, verifying that they met the aforementioned clinical criteria. Information was collected from medical records of each patient, including age, sex, medical history, clinical status and complementary studies. Mortality rate and length of hospital stay were recorded.
Neurophysiological data were obtained based on the EEG tracing of each patient. EEGs were performed using ATI Vertex® (Argentina) or Harmonie Stellate® (Canada) digital equipment. The recorded signals included: Electroencephalography (8 to 20 channels), with electrodes placed according to the 10-20 international system. Monopolar and bipolar montages were used for signal analysis. Complete EEG tracings were retrieved from printed medical records or electronic files of the neurophysiology department. They were reviewed by an expert neurologist (NP) blinded to the patients clinical data.
TW were defined, according to the standardized terminology of the American Society of Clinical Neurophysiology, as complexes of two or three phases with each one of longer than the previous one and whose positive phase is the one with the greatest amplitude, respecting a polarity pattern negative-positive-negative or positive-negative 6. The distribution of background rhythm or predominant pattern in the EEG was classified as 1. generalized if it was bilateral, bi-synchronous and symmetric; 2. lateralized in case it was unilateral or bilateral synchronous but asymmetric in voltage; 3. Independent bilateral, when there were at least two lateralized patterns or rhythms asynchronously between the two hemispheres or 4. Multifocal when there were at least three independent lateralized patterns or rhythms with at least one in each hemisphere. Additional information on location was collected for each type of distribution as specified in the terminology of the American Society for Clinical Neurophysiology 6.
Patients with TW were identified according to the EEG analysis. Patients with incomplete or insufficient data from the medical records were excluded (Figure 1). Each patient with encephalopathy and TW was matched for age and sex with two patients with encephalopathy without TW based on the closest date to that of the patient with TW.
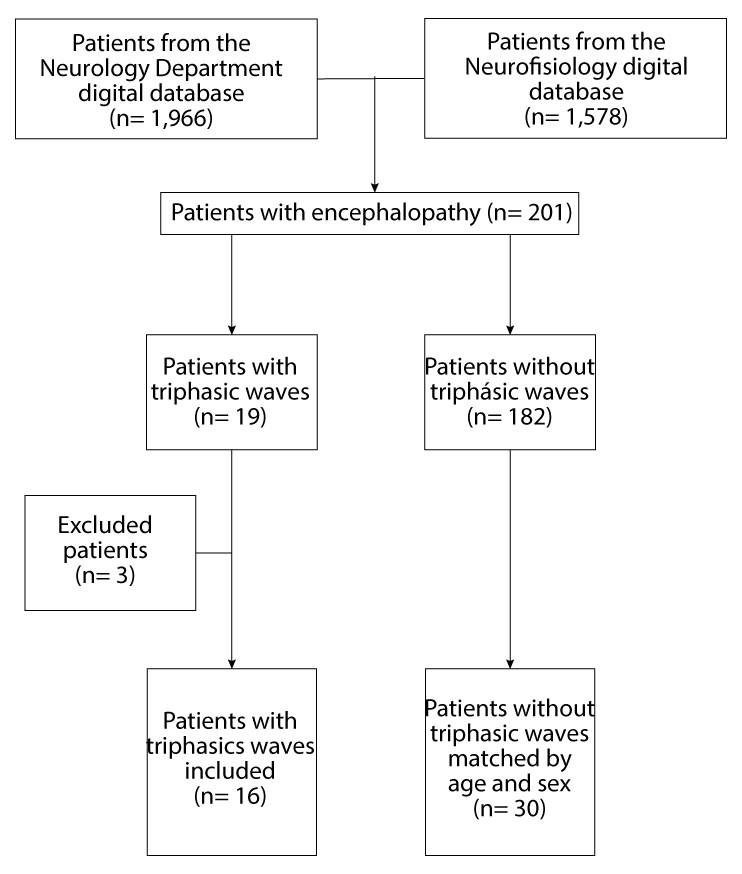
Figure 1 Flowchart shows the selection, exclusion and matching process of patients included in the statistical analysis.
The computed tomography (CT) scans and magnetic resonance imaging (MRI) were evaluated by two specialists (GP, GR) blinded to the clinical or electroencephalographic information of the patients. CT scan images were obtained with 16-row General Electric® (United States) equipment and MRI with 1.5 Tesla SIGNA Explorer® General Electric (United States) equipment. In MRI, only FLAIR, T1 and T2 weighted sequences were evaluated.
The degree of white matter lesion was defined using the visual scale Age-Related White Matter Changes (ARWMC) 7. This scale assesses the magnitude of white matter damage in five areas of the brain: frontal, parietal-occipital and temporal lobes, basal ganglia, and infratentorial parenchyma. White matter lesion is defined as an area ≥5 mm hypodense on CT or hyperintense on T2 or FLAIR MRI sequences, stipulating a value between 0 (no lesion) and 3 (confluent lesions for the basal ganglia or diffuse lesion with or without U-fibers involvement for the other four brain regions) according to the number, size, and distribution of the lesions. The total value is obtained from the sum of the scores of each right and left areas resulting in a final score between 0 and 30. The higher the score, the greater the white matter damage.
In order to define the degree of cerebral atrophy, the scale Global Cortical Atrophy (GCA)8 was used. It evaluates 13 brain regions and assigns a score between 0 (absent) and 3 (severe) according to the degree of ventricular dilatation, sulci widening and loss of gyral volume using the brain window on CT or with T1-weighted sequence on MRI. The evaluated regions are as follows: the third ventricle, the right and left frontal, occipital and temporal ventricular horns, and the right and left frontal, parieto-occipital and temporal lobes. The total score is obtained after the sum of the 13 sub-scores (range: 0-39). The higher the score, the greater the atrophy degree. This scale has a substantial intra and interobserver agreement (Kappa 0.65 and 0.67, respectively) 8. GCA and ARWMC are simple and can be applied on CT or MRI.
Statistical analysis was done using Epidat v4.2 software. The descriptive analysis was carried out using percentages and frequencies for qualitative variables and through means and standard deviation or median and quartiles for numerical variables according to their distribution. The comparison between percentages was performed using the z test. Numerical variables were compared using Student's t-test in the case of normal distribution or the non-parametric Mann-Whitney U test for variables without normal distribution. Analysis was performed using an alpha value of 0.05.
The study was approved by the institutional teaching and research committee. All data was anonymized. Informed consent was waived given the retrospective nature of the study.
Results
Two hundred and one patients with a clinical diagnosis of acute encephalopathy and abnormal EEG were identified during the study period. Of these, 19 patients had TW. Three patients were excluded due to missing or insufficient information, resulting in a final number of 16 patients with encephalopathy and TW included. These patients were randomly matched according to age and sex with 30 patients with acute encephalopathy without triphasic waves (Figure 1).
The clinical and demographic characteristics of the patients are detailed in Table 1. The mean age of the patients with TW was 80 years (SD ±10.2); 87.5% were women. In the TW group, multifactorial encephalopathy was the most frequent diagnosis (37%), followed by metabolic encephalopathy (25%) and central nervous system infections (18.7%). The combination of a metabolic disorder and systemic infection accounted for half of the cases of multifactorial encephalopathy in both groups. Metabolic disorders were found in 62% of cases with TW, either as an isolated cause or as part of a multifactorial encephalopathy.
Table 1 Population characteristics
| Triphasic waves n= 16 | Without triphasic waves n=30 | p* | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Women | 14 | 87.5 | 23 | 76.6 | 0.3 |
| Medical history | |||||
| Hypertension | 12 | 75 | 23 | 76.6 | 0.9 |
| Diabetes | 2 | 12.5 | 11 | 36.6 | 0.08 |
| TBI | 4 | 25 | 6 | 20 | 0.6 |
| Epilepsy | 1 | 6.25 | 3 | 10 | 0.6 |
| Atrial fibrillation | 2 | 12.5 | 7 | 23.3 | 0.3 |
| Coronary heart disease | 2 | 12.5 | 4 | 13.3 | 0.9 |
| Cognitive decline | 5 | 31.2 | 16 | 53.3 | 0.1 |
| Stroke | 3 | 18.7 | 1 | 3.33 | 0.07 |
| Chronic kidney disease | 5 | 31.2 | 6 | 20 | 0.3 |
| Cancer | 6 | 37.5 | 10 | 33.3 | 0.7 |
| Encephalopathy type | |||||
| Multifactorial | 6 | 37.5 | 10 | 33.3 | 0.7 |
| Metabolic † | 4 | 25 | 9 | 30 | 0.7 |
| CNS infection | 3 | 18.7 | 2 | 6.67 | 0.2 |
| Systemic infection ‡ | 2 | 12.5 | 5 | 16.6 | 0.7 |
| Structural § | 1 | 6.25 | 1 | 3.3 | 0.6 |
| Toxic | 0 | 0 | 3 | 10 | - |
| Mean | SD | Mean | SD | p‖ | |
| Age (years) | 80 | 10.2 | 79 | 9.7 | 0.8 |
| Glasgow coma scale | 11.25 | 2.08 | 11.86 | 2.48 | 0.1 |
| Laboratory | |||||
| Hemoglobin (g/dL) | 11.1 | 1.26 | 10.9 | 1.98 | 0.7 |
| White blood cells count (/mm3) | 10.2 | 4.9 | 10.2 | 5.57 | 1 |
| GFR (mL/min/1.73 m2) | 43.4 | 30.9 | 61.6 | 31.6 | 0.09¶ |
| Urea (mg/dL) | 84.8 | 56.6 | 58.3 | 39 | 0.1 |
| Sodium (mEq/L) | 137.4 | 7.4 | 134 | 6.7 | 0.3 |
CNS: Central Nervous system. GFR: Glomerular filtration rate. SD: Standard deviation. TBI: Traumatic brain injury.
* Calculated with Z test
† Includes: Uremic encephalopathy, hyponatremia, hypernatremia, dialytic disequilibrium syndrome, hypokalemia, hypercalcemia.
‡ Includes: Urinary tract infection, septic arthritis, pneumonia, infectious endocarditis, primary bacteremia, celulitis.
§ Includes: Subdural Hematoma, brain metastasis, primary brain tumor, vasculitis, intraventricular hemorrhage, ischemic stroke
‖ Calculated with t-student test
¶ Calculated with Mann-Whitney U test
The mean triphasic waves frequency was 2.25 ± 0.62 Hz. The distribution of the TW was generalized with no-predominance in 31.25% (n= 5) of the patients, generalized with frontal predominance in 43.75% (n= 7), generalized with occipital predominance in 12.5% (n= 2) and lateralized in only one case (6.25%). The background EEG had a mean frequency of 4.9 ± 0.9 Hz and its distribution was generalized in 68.75% (n= 11) of the patients with TW. In patients without TW, the background EEG had a frequency of 5.5 ±1.8 Hz and its distribution was predominantly occipital in 40% (n= 12). The difference in background EEG frequency between the two groups was not statistically significant.
There were no significant differences in the percentage of patients who underwent MRI between patients with and without TW (56.2% vs 63.3% p= 0.3). Table 2 details the white matter lesion and brain atrophy scores. The degree of global atrophy was greater in the TW group than in the group without TW (10.43 vs 6.9 p= 0.03). The mean ARWMC score was 9.43 ±6.5 in the TW group and 8.5 ±7.89 in the non-TW group. This difference was not statistically significant (p= 0.5).
Table 2 Imaging characteristics
| With triphasic waves | Without triphasic waves | ||||
|---|---|---|---|---|---|
| n= 16 | n=30 | ||||
| x | SD | x | SD | p* | |
| White matter Disease | |||||
| Total ARWMC | 9.43 | 6.5 | 8.5 | 7.89 | 0.5 |
| Frontal (R) | 1.75 | 1.23 | 1.33 | 1.18 | |
| Frontal (L) | 1.81 | 1.22 | 1.53 | 1.27 | |
| Parieto-occipital (R) | 1.87 | 1.2 | 1.13 | 1.25 | |
| Parieto-occipital (L) | 1.68 | 1.35 | 1.13 | 1.27 | |
| Temporal (R) | 0.25 | 0.44 | 0.56 | 0.97 | |
| Temporal (L) | 0.31 | 0.6 | 0.46 | 0.86 | |
| Basal ganglia (R) | 0.18 | 0.4 | 0.5 | 0.9 | |
| Basal ganglia (L) | 0.18 | 0.4 | 0.5 | 0.9 | |
| Infratentorial (R) | 0.68 | 1.07 | 0.66 | 1.02 | |
| Infratentorial (L) | 0.68 | 1.07 | 0.66 | 1.02 | |
| Atrophy | |||||
| Total GCA | 10.43 | 7.45 | 6.9 | 6.9 | 0.03 |
| Frontal (R) | 1.31 | 0.87 | 0.83 | 0.98 | |
| Frontal (L) | 1.5 | 0.81 | 0.80 | 0.92 | |
| Parieto-occipital (R) | 1.06 | 0.92 | 0.73 | 0.94 | |
| Parieto-occipital (L) | 1.31 | 0.94 | 0.83 | 0.87 | |
| Temporal (R) | 0.93 | 0.92 | 0.86 | 0.81 | |
| Temporal (L) | 1.31 | 1.01 | 0.86 | 0.93 | |
| Frontal horn (R) | 0.31 | 0.87 | 0.26 | 0.69 | |
| Frontal horn (L) | 0.31 | 0.87 | 0.23 | 0.67 | |
| Occipital horn (R) | 0.5 | 0.96 | 0.30 | 0.72 | |
| Occipital horn (L) | 0.45 | 0.96 | 0.26 | 0.69 | |
| Temporal horn (R) | 0.43 | 0.89 | 0.26 | 0.69 | |
| Temporal horn (L) | 0.43 | 0,89 | 0.3 | 0.75 | |
| Third ventricle | 0.56 | 0.89 | 0.33 | 0.8 | |
ARWMC: Age-related white matter changes.GCA: Global Cortical Atrophy. L: Left. R: Right. SD: Standard deviation.
* Calculated with Mann-Whitney U test
The median length of hospital stay was 18 days (Interquartile Range -IQR 7-47) in patients with TW and 16 days (IQR 6-33) in patients without TW (p= 1). In-hospital mortality was higher in the TW group (31.25 vs 6.66% p= 0.02).
Discussion
The main objective of the study was to compare the degree of cerebral atrophy and white matter lesion in patients with acute encephalopathy with and without triphasic waves. Our results showed that encephalopathic patients with TW had higher degree of cerebral atrophy than encephalopathic patients of the same age and sex without TW. Cerebral atrophy has been reported in between 44 and 55% of cases of acute encephalopathy with waves with triphasic morphology 9,10. However, to our knowledge only one study has shown an independent association between cerebral atrophy and TW 11.
The degree of brain atrophy might be involved in the genesis of TW. Based on a computerized model, van Putten & Hofmeijer 12 have postulated that the release of pyramidal cells, due to functional alteration or selective damage of inhibitory interneurons in situations of oxidative and metabolic stress, would produce generalized periodic discharges (GPD) in the EEG. Considering the constant morphology and synchronous character of TW, they can be considered as a type of GPD that are frequently observed with frontocentral predominance in the EEG 13. In our study, patients with TW had a greater degree of global brain atrophy, especially in the frontal area (Table 2), so it could be argued that this structural alteration predisposes the appearance of TW in patients with toxic-metabolic disorders or infections.
Although in our study we decided to include only patients older than 18 years, the youngest subject with TW was 60 years-old and the average age of this group was 80 years, which reveals that this electroencephalographic pattern is generally observed in elderly, as reported in other series 9-11. TW have not been described in young adult or pediatric patients 2. These findings are in concordance with the hypothesis that cerebral atrophy is involved in the appearance of TW, taking into account that cortical volume progressively reduces with advancing age and that the frontal and temporal lobes are particularly susceptible to these morphologic changes 14.
Another aspect to highlight is the considerable female predominance in patients with TW (87.5%). Although this finding should be interpreted cautiously, given the limited number of patients included in our study, there are other studies that show a higher percentage (57% to 64%) of women with TW than men 9-11.
Regarding the EEG, although we did not find a statistically significant difference, the background EEG had slower frequencies in patients with triphasic waves and a more generalized distribution. This could correspond to a deeper degree of encephalopathy 9.
On the other hand, our study showed that there were no significant differences in the magnitude of white matter lesion between encephalopathic patients with and without TW. However, the ARWMC scores in both groups were higher than those reported in healthy adults of the same age 15, so it could not be ruled out that, in the context of a metabolic disorder, infection or systemic disease, white matter lesion might be associated with a higher risk of developing acute encephalopathy regardless of the electroencephalographic pattern.
There were no significant differences in the length of hospital stay between both groups, however, the mortality was higher in patients with TW than without TW. This finding agrees with that described by Sutter et al .9, who found a high risk of death in patients with encephalopathy and TW (OR: 4.5, 95% CI: 1.57-12.7). Reported mortality in relation to TW is generally greater than 30% 2, which agrees with our results. In any case, although it is not possible to establish a direct relationship between mortality and TW, it is possible that the latter constitute a marker of severity in different clinical conditions. Additional studies are necessary to determine the value of TW as a potential prognostic biomarker.
This study has some limitations that include the small sample size, the clinical heterogeneity of the patients and the retrospective design that makes the study vulnerable to selection and/or collection bias. Another limitation is that a single EEG (generally the first since diagnosis) was taken as a reference to define the study groups; since acute encephalopathy is a dynamic entity, it is likely that the electroencephalographic pattern changes according to the evolution of the disease. Therefore, it is necessary to carry out studies analyzing electroencephalographic variation through serial EEG or continuous electroencephalographic monitoring.
The strengths of this study include: 1) the search strategy for selecting study patients was based on the double exploration of databases with clinical diagnoses, as well as electroencephalogram reports. It allowed a more accurate selection of the included subjects; 2) the evaluation of EEG and imaging studies were performed by specialists blinded to the clinical data or study reports, which reinforces internal validity, and 3) considering that there are few studies worldwide (and to our knowledge none in Latin America) that investigated the relationship between TW and brain atrophy in patients with acute encephalopathy, the information obtained in our study may serve as a basis for future studies to determine the degree of association between these variables, an important issue considering that these markers could help to predict which patients are more vulnerable to suffer acute encephalopathy.
Conclusion
In this study, the patients with acute encephalopathy and TW had a higher degree of brain atrophy than patients of the same age and sex with acute encephalopathy without TW. It is possible that such a structural alteration predisposes to the occurrence of TW in patients presenting with toxic-metabolic disorders, infections, or hypoxia. No significant difference in the degree of white matter lesion was found between the two groups. The mortality of the TW group was high, so future studies are necessary to determine the prognostic value of TW.











 text in
text in 



