Remark
| 1) Why was this study conducted? |
| This study was conducted in order to better analyze the mobility status of patients undergoing cardiac surgery. |
| 2) What were the most relevant results of the study? |
| The mobility status of patients admitted to intensive care units undergoing cardiac surgery gradually increases in the postoperative period. |
| 3) What do these results contribute? |
| The results of this study contribute to a closer look so that mobility and rehabilitation activities in the postoperative period of surgery are performed as soon as there are clinical conditions on the part of patients in recovery. |
Introduction
Coronary artery bypass grafting (CABG) is still the most common cardiac surgery worldwide and is associated with valve replacement; it stands out for its significant economic and social impact 1,2. Several studies have demonstrated that CABG induced lean tissue mass reduction, loss of handgrip strength, and pulmonary complications 3,4. In addition, during the first three days after CABG surgery, a significant inflammatory reaction and insulin resistance occurred, and during the first week after CABG, slight vital capacity decreased by 30-60% 5,6. Previous studies have also described significant muscle mass loss early after CABG 7,8. Considering these early consequences on respiratory, metabolic, and musculoskeletal systems due to surgical procedures and prolonged immobility have been supporting the growing interest in measures of physical function for critically ill adults.
In this context, physical function assessment is relevant to evaluate ICU recovery and assess the effectiveness of a structured early mobilization protocol on the mobility status of patients admitted to the ICU 9,10. Recently, new measures have been specifically developed to evaluate functional outcomes in the ICU setting 11. Parry et al. conducted a systematic review, presenting 26 measurement instruments of functional impairment that had established clinimetric properties designed for use in critically ill patients 12. The Perme Intensive Care Unit Mobility Score (Perme Score) is listed as one. The Perme Score was developed to measure patients' mobility status, starting with the ability to follow commands and culminating in the distance walked in two minutes 13. It indicates functional performance, particularly the patients' walking capacity, in the ICU at a specific time 14. Most previous studies have used Perme Score at a specific moment in time and specific patient populations 15-17. However, it is still unclear whether the Perme Score predicts the length of stay in the cardiovascular ICU and the possible influence of respiratory muscle strength, pulmonary function, and handgrip strength during the preoperative period on the mobility status of patients undergoing CABG and valve replacement. Several studies have demonstrated that the ICU length of stay (LOS) is a significant predictor of physical status, functional disability, nosocomial infections, and increased hospital costs. In the same way that the ICU LOS is a predictor of physical status after ICU discharge 18-21, we hypothesized that the physical function during ICU hospitalization might influence the ICU LOS. In addition, it remains unclear whether early mobility status, through Perme Score, is associated with reduced ICU LOS.
Therefore, this prospective study aims to assess the influence of Perme Score on ICU length of stay in patients after coronary artery bypass grafting (CABG) or valve replacement surgery. As a secondary objective, we aimed to investigate whether respiratory muscle strength, pulmonary function, and handgrip strength during the preoperative period were predictors of mobility status.
Materials and Methods
Study design and participants
This prospective, single-center, observational study was conducted in an 8-bed ICU in a university hospital in Santa Maria - Rio Grande do Sul state, Brazil. The ethics committee approved the study (process no.1.461.674). In addition, all subjects provided a written informed consent form before enrollment in the study. This study is reported by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement 22.
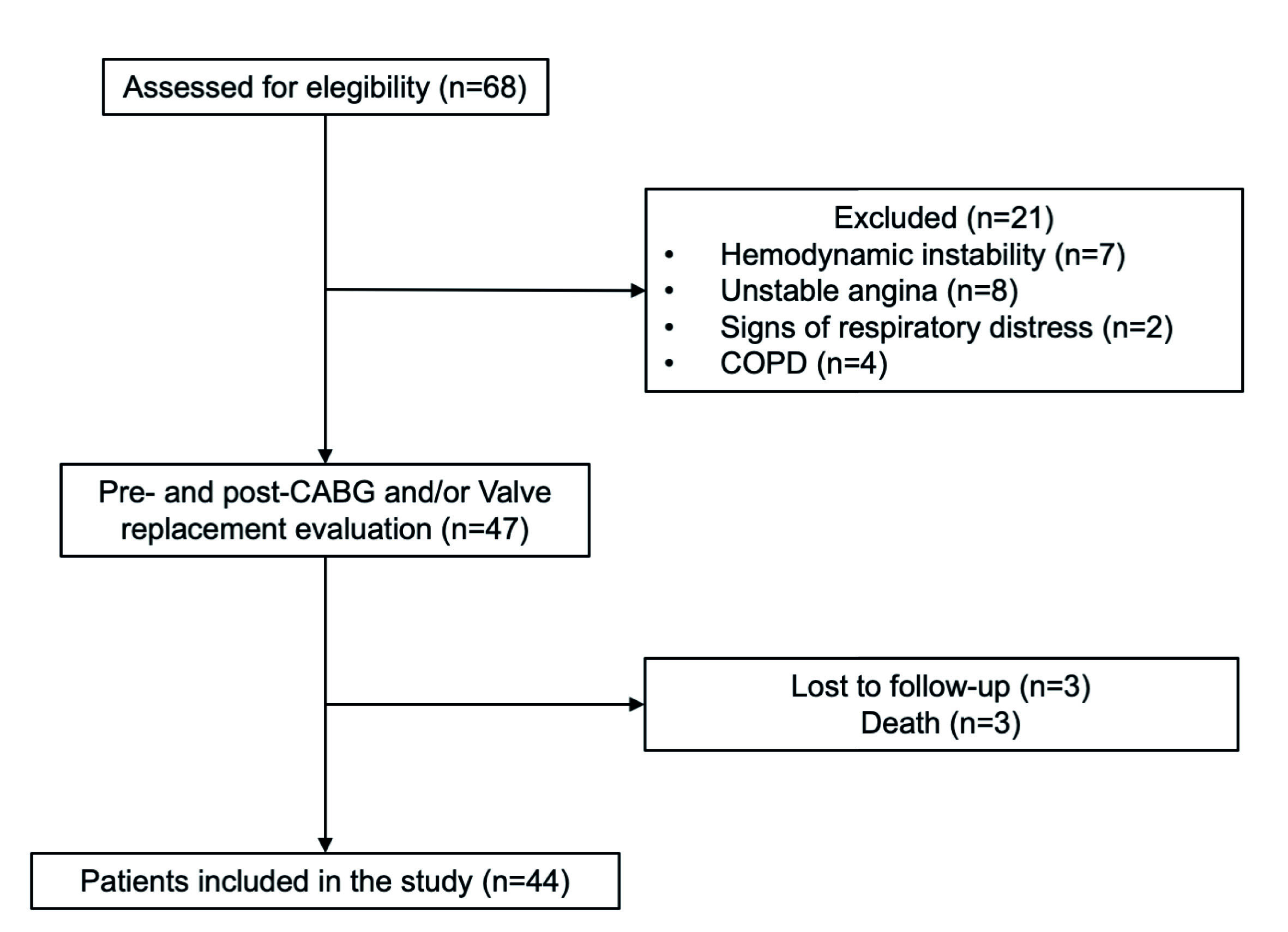
Patients admitted to the hospital for elective cardiac surgeries, age ≥18 years, referred to physical therapy assessment, were initially considered eligible for the study. The following inclusion criteria were used: 1) on-pump CABG and valve replacement surgery, and 2) admission to the cardiovascular ICU after surgery. In addition, we excluded patients diagnosed with chronic obstructive pulmonary disease (COPD), unstable angina, hemodynamic instability, signs of respiratory distress, and death.
Data collection and study variables
All study data were retrieved from the medical record. Collected variables included demographics, comorbidities, surgical procedures, medications, ICU and hospital stay, respiratory muscle strength, pulmonary function, handgrip strength, and mobility status. Respiratory muscle strength, pulmonary function, and handgrip strength were assessed one day before surgery.
Respiratory muscle strength
The maximum inspiratory pressure and maximum expiratory pressure (MEP) were evaluated with an MVD-300 digital manometer (MDI®, Rio Grande do Sul, Brazil). According to the American Thoracic Society and European Respiratory Society (2002) recommendations, all measurements were performed 24. The highest measured value was considered for analysis and compared to predicted values 23.
Pulmonary function
The assessment of the pulmonary function was performed according to American Thoracic Society and European Respiratory Society guidelines 24 with a portable digital spirometer (One Flow FVC KIT, Clement Clarke International, United Kingdom). The following measures were collected: 1) peak expiratory flow, 2) forced vital capacity (FVC), 3) forced expiratory volume in one second (FEV1), and 4) forced expiratory volume in one second divided by the forced vital capacity ratio (FEV1/FVC). The results obtained were compared with the predicted values 25,26.
Handgrip strength
The handgrip strength was measured according to the protocol recommendations 27 using a portable dynamometer (TKK 5401 GRIP-D; Takei Scientific Instruments Co. Ltd., Tokyo, Japan). The grip strength was defined as the highest value measured on the dominant hand compared to predicted values 28.
Mobility status assessment
The mobility status was assessed by the Perme Intensive Care Unit Mobility Score (Perme Score) 13, and was collected by a single rater previously trained in evaluating the patient using the Perme ICU Mobility Score. The rater is a physical therapist, a member of a research team who was not part of the interdisciplinary team of the ICU, and has more than five years of clinical experience. The Perme Score was collected daily from the moment at ICU admission - within 24 hours until ICU discharge - performed in the same ICU discharge. Starting with the ability to follow commands and culminating with the distance walked in two minutes, the score objectively measures the mobility status of patients admitted in ICU. It consists of 15 items grouped into seven categories, as follows: 1) mental status, 2) potential mobility barriers, 3) functional strength, 4) bed mobility, 5) transfers, 6) gait, and 7) endurance. The total score ranges from 0 to 32 points, with higher scores indicating better mobility.
Physical rehabilitation intervention
The patients received general ward rehabilitation comprising chest physiotherapy and a progressive five steps of active-assistive exercises of lower/upper limbs. Each step corresponds to one day of postoperative intervention 29. The ICU physical therapists provided the interventions twice daily, for approximately 30 minutes, seven days per week. The rehabilitation started on the first postoperative day until discharge.
Sample size calculation
The sample size was calculated using the GPower (version 3.0), based on the data from a pilot study composed of the first ten patients included in the present study. To find a coefficient of determination (R2) of 0.185, with an effect size of (i2) 0.22, power of 80%, and α level of p: <0.05, having as an independent variable the Perme score on day 3 and the dependent variable the ICU length of stay, a total of 38 patients were required.
Statistical analysis
Continuous variables are reported as mean, standard deviation (SD) or median, interquartile range (IQR, 25-75th percentile) values, and categorical variables are presented in absolute and relative frequencies. The Shapiro-Wilk test assessed the normality of the variables.
To assess the influence of Perme Score on ICU length of stay, we performed a simple linear regression where the dependent variable was ICU length of stay. The independent variables we individually tested, the Perme Score obtained on the first three days of stay in the ICU (D1, D2, and D3), were the days that the entire sample (n= 44) was still hospitalized in the ICU. From the fourth day of hospitalization, the number of patients gradually reduced because patients began to be discharged from the ICU. The significance of the final model was assessed by the ANOVA F test and the quality of the adjustment by the adjusted determination coefficient (adjusted R2). In order to eliminate a possible bias that the type of surgery (CABG, valve replacement, or CABG and valve replacement) could cause on the variables analyzed, we used the regression model. The residues were evaluated according to the assumptions of normality, constant variance, and independence. To compare the behavior of the Perme score on the first three days of ICU stay and the types of surgery, we performed a 2-way ANOVA followed by Bonferroni's posthoc. The effect size for simple linear regression was calculated using R-squared (ρ), with values interpreted as “very high” (0.90 to 1.00); “high” (0.70 to 0.90); “moderate” (0.50 to 0.70); “low” (0.30 to 0.50); “small” (0.10 to 0.30) 30.
We also performed a regression analysis, where initially, a pre-selection of the variables was performed through simple linear regression analysis. Then, a multiple linear regression model was performed to assess the effect of preoperative independent variables (pulmonary function, respiratory muscle strength, and handgrip strength), age, left ventricular ejection fraction (LVEF), and cardiopulmonary bypass time (CPBT) on mobility status [Perme Score at day 1 (D1), day 2 (D2), and day 3 (D3)]. All analyses were conducted in IBM SPSS Statistics for Windows (version 26.0), and the significance level was set at p: <0.05.
Results
Participants
A total of 68 patients were screened, and 47 patients met the inclusion criteria. Three patients were excluded due to death. Of the 44 patients studied, the mean (SD) age was 62.3 (10.8) years, and 28 (%) were enrolled in the study (Figure 1). Baseline characteristics and clinical outcomes of polled patients are shown in Table 1. The median [IQR] of the mobility status at ICU admission and ICU discharge to 6 [3-7] points and 27 [23-30] points, respectively (p < 0.001) (Figure 2).
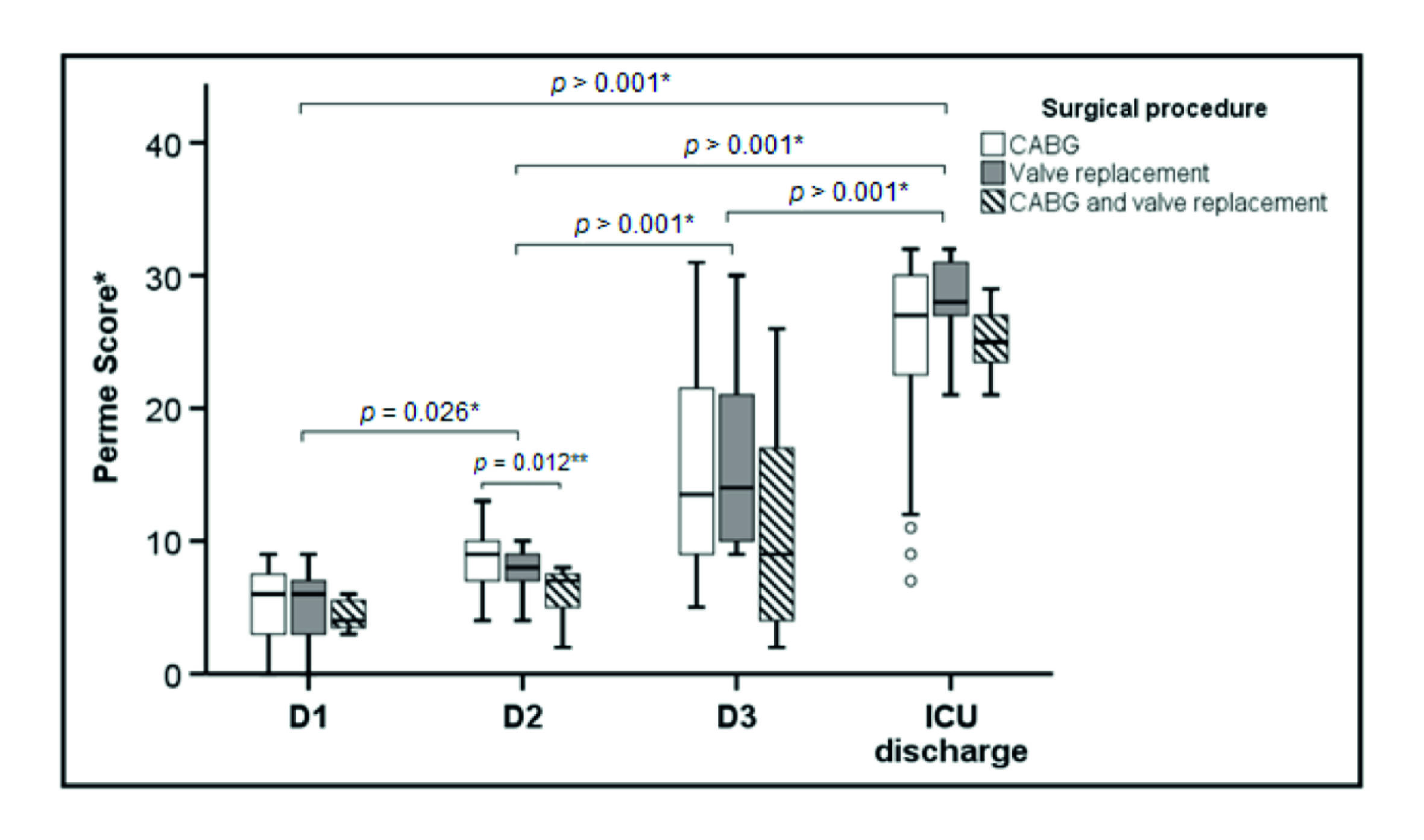
Figure 2 Boxes represent median and interquartile range and the open circles represent outliers. Definition of abbreviations: D1 = first postoperative day; D2 = second postoperative day; D3 = third postoperative day; ICU = intensive care unit; Perme Score = Perme intensive care unit mobility score. *Comparison between times (days of ICU stay). **Comparison between CABG and CABG + valve replacement.
Table 1 Baseline characteristics and clinical outcomes of the included participants.
| Variable | Overall participants (n = 44) |
|---|---|
| Age, years | 62.3 (59.0 to 65.6) |
| Male sex, no. (%) | 28 (63.7) |
| Body mass indexa, Kg/m2 | 26.7 (4.8) |
| Left ventricular ejection fraction, % | 57.4 (7.3) |
| Comorbidities | |
| Diabetes mellitus, no. (%) | 18 (40.9) |
| Dyslipidemia, no. (%) | 15 (34.1) |
| Hypertension, no. (%) | 36 (81.8) |
| Intensive care unit length of stay, days | 4 [4-7] |
| Hospital length of stay, days | 8 [6-12] |
| Respiratory muscle strength | |
| Maximum inspiratory pressure, cmH2O | 67 [43.2-84.7] |
| Maximum inspiratory pressure MIP, % pred. | 70.9 [46.6-90.3] |
| Maximum expiratory pressure, cmH2O | 83.5 [57.2-98.7] |
| Maximum expiratory pressure, % pred. | 86.4 [57.2-103.4] |
| Pulmonary function | |
| FEV1, L | 2.1 [1.4-2.7] |
| FEV1, % pred. | 77.7 [55.3-99.1] |
| FVC, L | 3.1 [2.1-3.9] |
| FVC, % pred. | 94.5 [60.2-103.6] |
| FEV1/FVC | 73.0 [57.7-80.7] |
| FEV1/FVC, % pred. | 98.1 [79.4-111.9] |
| Peak expiratory flow, mL | 252.5 [165.0-388.7] |
| Peak expiratory flow, % pred. | 70.1 [43.8-88.6] |
| Handgrip strength, kgF | 31.5 [23.2-10.0] |
| Handgrip strength, % pred. | 90.4 [80.4-101.3] |
| Surgical procedure | |
| Coronary artery bypass graft - no. (%) | 28 (63.6) |
| Valve replacement - no. (%) | 9 (20.5) |
| Coronary artery bypass graft and valve replacement - no. (%) | 7 (15.9) |
| Cardiopulmonary bypass, min | 94 [80-136] |
| Extent of disease - no. (%) | |
| 1-vessel | 5 (14.3) |
| 2-vessel | 11 (31.4) |
| 3-vessel | 15 (42.9) |
| 4-vessel | 4 (11.4) |
Data presented as mean and standard deviation (SD), median and interquartile range [quartile 25% - quartile 75%] or absolute and relative frequency (%). *Data presented as mean (95% Confidence Interval).
Definition of abbreviations:
FEV1 = forced expiratory volume in one second;
FEV1/FVC = forced expiratory volume in one second divided by the forced vital capacity ratio;
FVC = forced vital capacity;
aThe body-mass index is calculated by weight in kilograms divided by the square of the height in meters (Kg/m2).
The categories are the same for men and women of all body types and ages, as follows: below 18·5 - underweight, 18·5-24·9 - normal or healthy weight, 25·0-29·9 - overweight, and 30·0 and above - obese.
Influence of Perme score on ICU length stay
In the simple linear regression, no significant association was observed between the Perme Score on D1 with ICU length of stay [β = -0.30; (95% CI, -0.62 to 0.01); p = 0.064], even when the different surgical procedures were evaluated (Figure 3-A). However, in these different procedures, the effect size was larger in valve replacement, followed by the combination of CABG and valve replacement and CABG alone (ρ = 0.660; ρ = 0.421; and ρ = 0.216, respectively). Regarding D2, the Perme Score was associated with ICU length of stay [β = -0.76; (95% CI, -1.19 to -0.33); p = 0.001], explaining 22% of the variance, and when analyzing the three types of surgical procedure, only CABG showed a significant correlation (Figure 3-B). The effect size showed the following coefficients: CABG and valve replacement, ρ = 0.865; CABG, ρ = 0.663; valve replacement, ρ= 0.463.
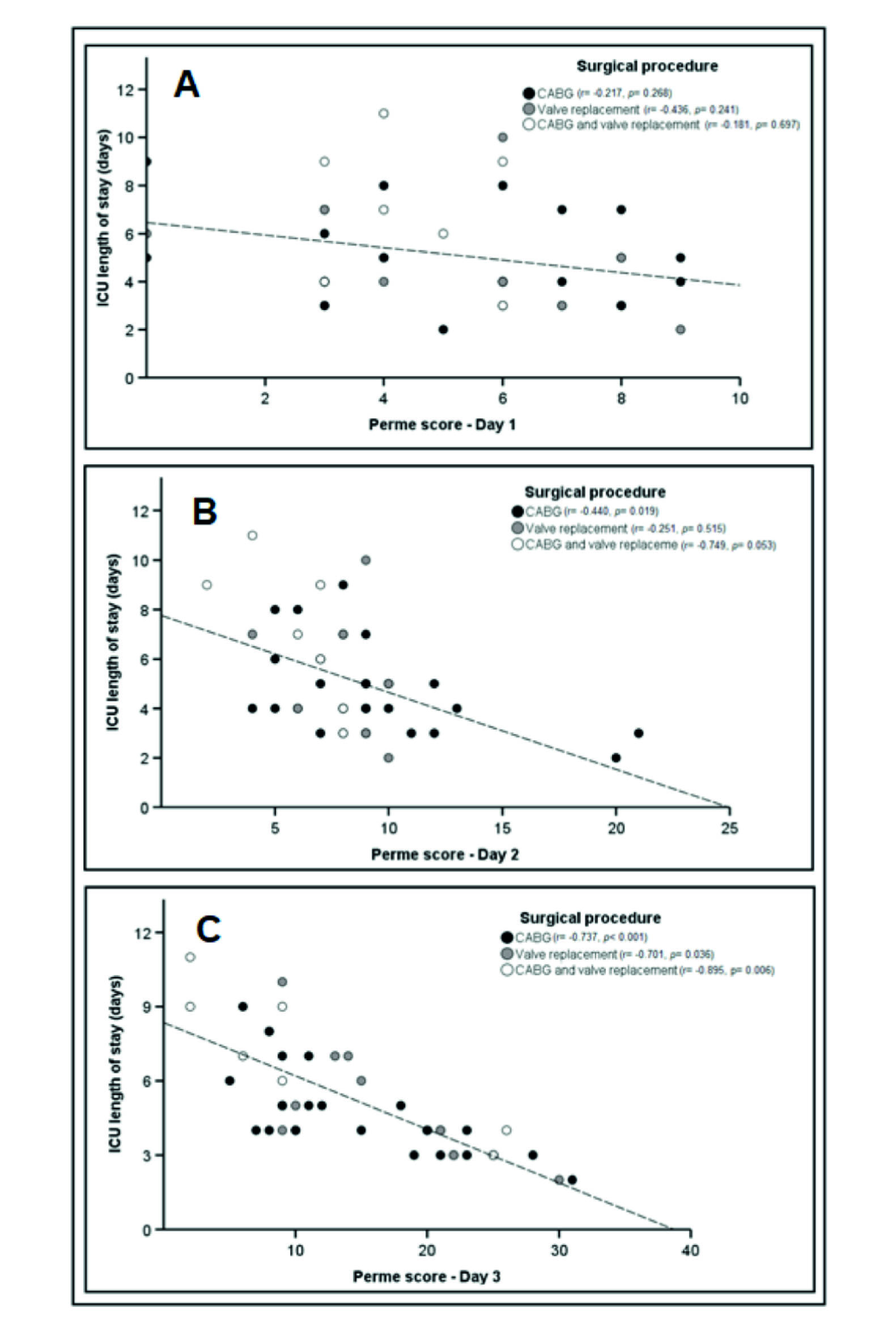
Figure 3 Association between the Perme Score and the first three days of ICU stay, according to the surgical procedure performed. Definition of abbreviations: Day 1 = first postoperative day; Day 2 = second postoperative day; Day 3 = third postoperative day; ICU = intensive care unit. *Perme ICU mobility scores range from 0 to 32, with higher scores indicating better mobility level.
In D3, as in D2, the Perme Score was associated with ICU length of stay [β= -2.67; (95% CI, -3.38 to -1.95); p < 0.001], explaining 57% of the variance and a 4.6-point increase in the Perme Score reduces ICU length of stay by one day, independent of surgical procedures. The effect size presented the following coefficients: CABG and valve replacement, ρ = 0.946; CABG, ρ = 0.858; valve replacement, ρ = 0.837 (Figure 3-C).
Influence of preoperative variables on the Perme Score
The multiple linear regression analysis identified that LVEF, CPBT, preoperative predicted peak expiratory flow and FEV1 values explained 51% (p= 0.006) of mobility status on D1. On the other hand, the variables age, LVEF and preoperative predicted values of FEV1/FVC and maximum expiratory pressure explained 71% (p= 0.002) of mobility status on D2. Likewise, only the preoperative predicted values of FEV1/FVC and maximum expiratory pressure explained 51% (p < 0.001) of the mobility status on D3 (Table 2).
Table 2 Multiple linear regression analysis for the Perme Score on the first three days of admission to the ICU.
| Dependent variable | Independent variables | R2 | R2 adjusted | β-non-standardized coefficient | β- standardized coefficient | p-value | CI 95% |
|---|---|---|---|---|---|---|---|
| Perme Score at D1 | LVEF, % | 0.65 | 0.51 | 0.143 | 0.693 | 0.017 | 0.032 to 0.255* |
| CPBP, min | -0.046 | -0.825 | 0.004 | -0.074 to -0.019* | |||
| Preop. PEF, % pred | 0.150 | 1.469 | 0.008 | 0.049 to 0.251* | |||
| Preop. FEV1, % pred. | -0.120 | 1.162 | 0.022 | -0.218 to -0.021* | |||
| Perme Score at D2 | Age, years | 0.79 | 0.71 | 0.283 | 0.431 | 0.035 | 0.023 to 0.542* |
| LVEF, % | -0.120 | -0.368 | 0.038 | -0.232 to -0.008* | |||
| Preop. FEV1/FVC, % pred. | -0.132 | -0.621 | 0.005 | -0.214 to -0.050* | |||
| Preop. MEP, % pred. | 0.128 | 0.992 | <0.001 | 0.079 to 0.176* | |||
| Perme Score at D3 | Preop. FEV1/FVC, % pred. | 0.58 | 0.51 | -0.210 | -0.502 | 0.025 | -0.388 to -0.031* |
| Preop. MEP, % pred. | 0.193 | 0.002 | 0.002 | 0.085 to 0.301* |
*Significant independent variable (p: <0.05). LVEF: left ventricular ejection fraction; CPBT: cardiopulmonary bypass time; Preop.: preoperative; D1: first postoperative day; D2: second postoperative day; D3: third postoperative day; FEV1: forced expiratory volume in one second; FEV1/FVC = forced expiratory volume in one second divided by the forced vital capacity ratio; MEP = maximum expiratory pressure; PEF = peak expiratory flow; % pred. = percentage of predict.
Discussion
This is the first study to demonstrate that the Perme Score is associated with the ICU length of stay in patients after CABG with or without valve replacement and an increase in the mobility status reduced the ICU length of stay. Additionally, our findings showed that the preoperative pulmonary function (D1, D2 and D3); LVEF and CPBT (D1); age and LVEF (D2) and maximum expiratory pressure (D3) were independent predictors of mobility status. It is known that physical function may decrease immediately after surgery, especially in elderly patients, and impairment of functional status is associated with prolonged ICU stay in patients after cardiovascular surgery 31. Different methods and instruments have been used to evaluate physical functioning in the critical care setting 12. Itagaki et al. used the gait speed test and identified age, estimated glomerular filtration rate, preoperative gait speed, and the postoperative day patients regained independent walking as predictors of a postoperative decline in gait speed 32. Thus, it is essential to emphasize that to identify physical function impairments, the use of physical functioning tools developed for the ICU stay is recommended 12. We used the Perme ICU Mobility Score to measure the mobility status. Due to the reason that this is the only instrument to consider in assessment, potential barriers to mobility may affect patients' performance in mobility activities 14,33. In the context of cardiovascular surgery, pain in the sternotomy area and chest tube site was one of the main obstacles to implementing mobility activities as part of routine clinical practice 34. The low mobility status observed on the first postoperative day can be associated with barriers (i.e., pain, catheters, drains, and tubes) after surgery rather than a functional decline, considering the patient's previous function before surgery. However, this is different when we consider the second day for CABG and valve replacement procedures. Recently Wu et al. 35, demonstrated that a combination of CABG and valve replacement was associated with worse in-hospital outcomes, including high in-hospital mortality and increased costs.
The time at which ambulation is first initiated in postoperative patients directly affects outcomes, which may enhance functional independence 34. The findings of our study demonstrated that patients with better mobility status on the second (only CABG alone) and third postoperative days (three types of intervention) presented a shorter ICU stay. This result agrees with the effect size analysis, where CABG alone on D2 and the three types of surgical procedures on D3 showed a moderate and high to very high effect size, respectively. It reinforces the importance of delivering mobility interventions and enhances patients' knowledge regarding the benefits of mobility activities to provide clinical improvements in physical function after cardiac surgery, reducing complications and ICU stay 36,37.
The minimal detectable change (MDC) of the Perme Score of 1.36 points was established, presenting evidence to be sensitive to detecting changes on patients' mobility levels over time 38. Furthermore, our study showed that an increase of 4.6 points on the Perme Score on D3 reduces ICU stay by one day, regardless of the type of surgery. This finding is clinically relevant since previous studies have reported that reducing ICU LOS significantly impacts the cost savings of all hospital expenditures 39-41. Besides this, the incidence of healthcare-associated infections remains frequent among cardiac surgery patients with prolonged ICU stay and are associated with high mortality 42.
Few earlier studies assess physical function early and longitudinally in the surgical ICU as a predictor for ICU LOS. Kasotakis and colleagues have described a model for predicting surgical ICU LOS and mortality based on the Surgical Intensive Care Unit Optimal Mobility Score (SOMS) 43. The authors conclude that in surgical ICU patients, the SOMS is a reliable and valid tool to predict hospital mortality and length of stay. Physical function monitoring in ICU is relevant to determine patients' risk for poor physical outcomes, intervention efficacy, and recovery trajectories 44-46.
A study conducted with patients undergoing cardiac surgeries determined the incidence and risk factors associated with mobility impairments in the postoperative period. The authors identified that age as a predictor of postoperative mobility impairment 47. The same pattern was observed in our study when we evaluated the predictors for mobility status on the first three days of ICU admission. In addition to age, preoperative pulmonary function, LVEF, CPBT, and maximum expiratory pressure were independent predictors of mobility status. Preoperative pulmonary dysfunction has been associated with increased operative mortality and morbidity after cardiac surgery 48. A study on patients undergoing elective cardiac surgery identified a significant number presenting preoperative respiratory muscle weakness 49. However, we did not observe respiratory muscle weakness in our study's sample. Different prediction equations likely explain a possible reason for these divergent findings 50. In a recent prospective cohort study conducted in patients undergoing elective cardiac surgery, Risom et al. found that the reduced preoperative FEV1 was a strong and independent predictor of postoperative complications, including the risk of death 51. In this context, our findings highlight the importance of preoperative pulmonary evaluation as a routine examination to provide important prognostic information in patients undergoing elective cardiac surgery.
In addition, the early consequences of cardiac surgeries on the respiratory system is a musculoskeletal system impairment due to surgical stress and a severe systemic inflammatory response 52-54. Furthermore, it causes dysregulation of protein synthesis and consequently loss of muscle mass, which is responsible for functional decline 53,54.
Our findings showed that the CBPT was an independent predictor of mobility status. In a study that examined the impact of CPB on postoperative outcomes, Madhavan et al. demonstrated that prolonged CBPT (>56 minutes) showed an indirect effect on mortality could be manifested through enhanced risks of complications, prolonged ICU stays (>48 hours), and prolonged mechanical ventilation (>24 hours) 55. In our study, the median CBPT was 94 minutes. Similar to our results, in a recent observational study that included 60 patients who underwent cardiac surgery, Sumin et al. demonstrated that CBPT was an independent predictor of functional state in patients with a complicated postoperative period upon discharge from the hospital 56.
Handgrip strength is considered a reliable measure to assess muscle strength, an indicator of overall muscle strength and function, and has recently been identified as an independent predictor of overall survival and cardiovascular events in patients with coronary artery disease 57,58. However, a study by Perry and colleagues presented that preoperative handgrip strength values for elective cardiac surgery patients were below predicted reference values 59. In our study, we did not observe abnormal values of preoperative handgrip strength measures. These conflicting findings are likely because, in the present study, we used a reference for predicted values consistent with the age group of the subjects included in our study, which is different from the previous study that used a reference value for healthy individuals over 20 years old.
Strengths and limitations
The results of our study also highlight the importance of daily assessment of the Perme Score in the postoperative period of cardiac surgery, allowing physiotherapists to assess changes in the ICU patients' mobility status over time. In addition, it is essential to note that preoperative assessment can provide additional clinical practice information for physiotherapists to identify patients at high risk for functional decline.
Although, the results of this study should be interpreted considering some limitations. First, the sudden variation of the score in such a few days may have occurred due to the few multidimensional barriers to mobilizing patients during the postoperative period. Second, we did not consider the preexisting impairment of functional mobility that could impact the mobility status during ICU stay. It is important to note that for patients with previous mobility impairment, the assessment by the Perme Score should be reconsidered since the patient would not be able to show the progression of the mobility status during the hospital stay. Finally, the Perme Score has a limitation in clinical practice when used to assess patients with preexisting total dependence for activities of daily living, which was not observed in our study.
Conclusion
Our findings indicated that the Perme Score influenced the ICU length of stay in patients after CABG and valve replacement, and an increase of 4.6 points in the Perme Score reduced the ICU length of stay by one day. In addition, preoperative pulmonary function (D1, D2, D3), LVEF (D1 and D2) and cardiopulmonary bypass time (D1), age (D2) and maximum expiratory pressure (D3) were independent predictors of mobility status. Further studies are needed to provide additional information regarding the impact of daily assessment of mobility status during ICU and hospital stays and its long-term outcomes.











 texto em
texto em