Remark
| 1) Why was this study conducted? |
| This study was conducted to examine the indications for performing tuberculin skin tests in a tertiary hospital. |
| 2) What were the most relevant results of the study? |
| It was determined that the TST result of 50.2% of the immunosuppressive patients was anergic and therefore an additional test was required. |
| 3) What do these results contribute? |
| Since the TST may be anergic in immunosuppressed patients, IGRA tests can be performed in these patients. |
Introduction
Tuberculosis is a disease caused by a bacillus named Mycobacterium tuberculosis and has existed for tens of thousands of years. Around ten million people worldwide develop tuberculosis annually, and 1.2 million die from the disease 1. World Health Organization (WHO) recommends a single dose administration of the Bacillus Calmette-Guérin (BCG) vaccine during infancy in regions where the incidence of tuberculosis is more than 10 per hundred thousand. BCG vaccine has been applied in Turkey since 1953. In the very first years of the BCG vaccination, the incidence of TB was 177 per hundred thousand, and with the routine vaccination, the incidence was reported as 14.1 per hundred thousand in 2018 in Turkey 2,3.
TST is a delayed-type hypersensitivity reaction to antigenic components of the bacillus in individuals exposed to bacillus through infection or BCG vaccine 4. T cells that have been sensitized in a person previously infected with TB are recruited to the site where the TST is administered and release various lymphokines. The antigens (tuberculin and purified protein derivatives) used for TST are proteins found in the cell wall of the bacillus and are obtained by precipitation of M. tuberculosis bacilli produced in liquid media 3-5. In Turkey, where BCG is widely used, an induration ≥15 mm is considered positive in those inoculated with BCG, while an induration ≥10 mm in those not vaccinated with BCG and an induration ≥5 mm in those with suppressed immunity is considered positive 2,3,6.
Although an underlying autoimmune disease increases the risk of TB, this risk increases even more with anti-tumor necrosis factor (TNF) therapies. The risk varies in proportion to the incidence of TB in the relevant country and community. Anti-TNF therapy is contraindicated in the presence of active TB. Therefore, TB should be eliminated prior to anti-TNF therapy. Considering patients with active TB disease being excluded, latent tuberculosis infection (LTBI) screening should be performed before initiation of anti-TNF therapy 7,8.
LTBI screening provides resources for future tuberculosis cases. It is recommended that individuals with LTBI be treated. Although there is currently no problem in excluding tuberculosis disease, it is difficult to determine LTBI, especially in immunosuppressed individuals. Evidence suggests that screening for LTBI benefits patients receiving anti-TNF therapy, candidates for solid organ and hematological stem cell transplantation, people with AIDS, children in contact with patients with bacteriologically proven tuberculosis, and patients receiving dialysis 9.
There is no gold standard for the diagnosis of LTBI. However, TST and Interferon-Gamma Release Assays (IGRA) can be used to screen for LTBI 7,8. TST has had an important place in the diagnosis of LTBI for nearly a century. TST is widely used in tuberculosis dispensaries and authorized institutions in Turkey. This study aimed to investigate the general characteristics of patients tested with TST in a university hospital within two years.
Materials and Methods
This is a cross-sectional study. The study included patients tested with TST between March 15, 2019, and March 15, 2021, at Akdeniz University Pulmonary Medicine Clinic. After taking the medical history and physical examination of all patients who underwent TST, chest X-ray was requested. The presence of findings related to pulmonary and extrapulmonary tuberculosis was investigated. BCG scars were checked. TST indication, TST value, BCG vaccination history, and whether they received tuberculosis prophylaxis following TST were recorded in the data collection form. While all patients that were tested with TST were included in the study, patients with symptoms of pulmonary tuberculosis, patients under the age of 18, and patients with suspected pregnancy and inadequate medical records were excluded from the study.
The Mantoux method was used for the administration of TST 3,7. Based on this method, the purified protein derivative (PPD) of a dose of five tuberculin units, i.e., 0.1 mL, obtained from M. tuberculosis, was administered intradermally on the volar aspect of the forearm. 1-mL injectors with a 27-gauge needle were used for the administration. Results were read after 48-72 hours. The patients were informed that the concerned site should remain dry after administration. The diameter of induration at the test site was measured to determine the response. Test results were recorded in millimeters (mm). All tests were performed and interpreted by the same person. Accordingly, an induration ≥15 mm in those inoculated with BCG, an induration ≥10 mm in those not vaccinated with BCG, and an induration ≥5 mm in those with suppressed immunity are considered positive 7.
The test was repeated ten days later in patients showing no reaction to determine the booster effect 3,7. Results 6 mm higher than the first test or over 10 mm are considered positive.
All patients were screened for LTBI. In all patients with fibrotic lesions suggestive of tuberculosis sequelae observed on chest X-ray, three consecutive sputum examinations (direct and with culture) were performed to search for acid-fast bacilli (AFB). Fibrotic lesions were evaluated as sequelae in AFB-negative patients. Nine months of chemoprophylaxis with 5 mg/kg/day (maximum 300 mg/kg) of isoniazid was carried out for patients who had TB sequelae on their chest X-rays, who had contact with a patient with pulmonary tuberculosis in the last one year, whose first TST value prior to the anti-TNF therapy was ≥5 mm, and the healthcare personnel at high risk for TB. This therapy was given on the condition that it was started one month before the anti-TNF-α medication. In addition, rifampicin therapy was recommended for patients who developed isoniazid hepatotoxicity 3,7.
Regarding pulmonary tuberculosis, active tuberculosis diagnosis was made by AFB examination for three consecutive days and growth of the bacillus in tuberculosis culture.
Ethics committee approval for the study was given with the decision, numbered 580 and dated 18/08/2021, by Akdeniz University’s clinical research committee. In addition, verbal consent was obtained from the patients.
Statistics
Data obtained from the study were evaluated with the software package “SPSS (Statistical Package for the Social Sciences) for Windows 11.5.0” and p <0.05 value was considered statistically significant. In the statistical evaluation of the data obtained from the study, categorical data were summarized by frequency (n) and percentage (%), and continuous data were summarized by mean standard deviation. In addition, the Chi-square test, Fisher’s exact test, and likelihood ratio test from among cross-tabulation statistical methods were used for statistical evaluation between categorical variables. Finally, to compare the two groups in terms of numeric variables, the Independent Samples t Test was used to compare the two groups in terms of numeric variables.
Results
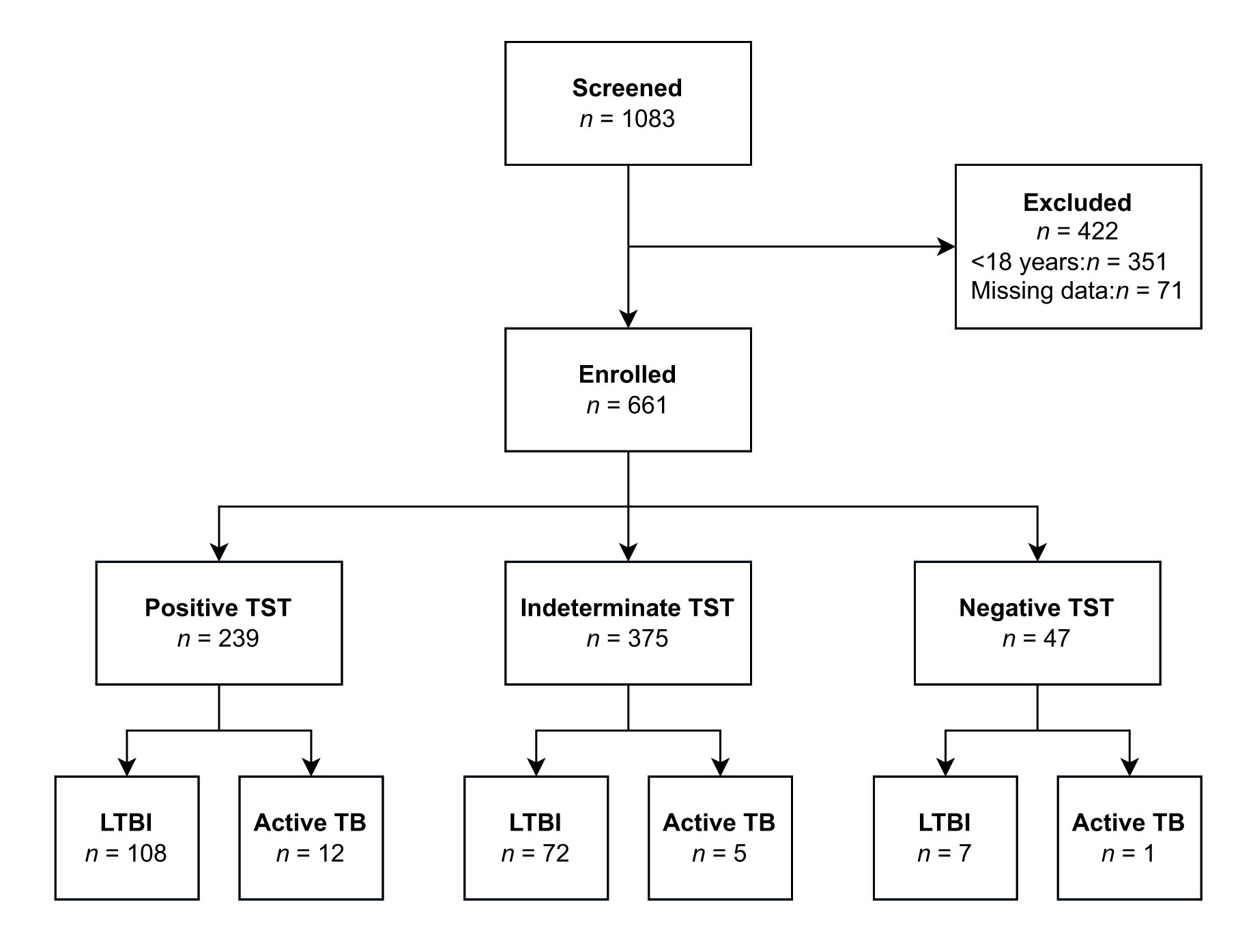
A total of 1,083 patients were screened. Four hundred twenty-two patients were excluded due to various reasons (<18 age (351), missing data (71)). A total of 661 patients (Figure 1), 316 (47.8%) women, with a mean age of 43±15.9 years, were included in the study. The mean TST score was 3.8 (0-27) mm. 90.3% (597) of the patients had at least one BCG scar. The most common comorbid conditions were hypertension (15.9%, n=105), diabetes mellitus (8.0%, n=53), and coronary artery disease (5.3%, n=35). The general characteristics of the patients are presented in Table 1.
Table 1 General characteristics of patients
| n | % | ||
|---|---|---|---|
| Age | (years) | 43.0 ± 15.9 | |
| TST | (mm) | 3.8 (0-27) | |
| Sex | Woman | 316 | 47.8 |
| BCG scar | Yes | 597 | 90.3 |
| Booster effect | Checked | 159 | 24.1 |
| Smoking | Smoker | 90 | 13.6 |
| Ex-smoker | 152 | 23.0 | |
| Non-smoker | 252 | 38.1 | |
| TST indication | Biological agent | 331 | 50.0 |
| Prior to the organ transplant | 135 | 20.4 | |
| Tuberculosis contact | 166 | 25.1 | |
| Healthcare professional screening | 7 | 1.1 | |
| Medical report (job application, trip abroad) | 22 | 3.3 | |
| TB diagnosis | Pulmonary TB | 14 | 2.1 |
| Extrapulmonary TB | 4 | 0.6 | |
| TST results | 0 mm | 375 | 56.8 |
| 1-4 mm | 47 | 7.2 | |
| 5-9 mm | 115 | 17.3 | |
| 10-14 mm | 92 | 13.9 | |
| >14 mm | 32 | 4.8 | |
| QFT test (due to anergic TST) | Done | 332 | 50.2 |
| TB prophylaxis | Done | 187 | 28.2 |
TST: Tuberculin skin test, BCG: Bacillus Calmette-Guérin, TB: Tuberculosis, QFT: QuantiFERON-TB Gold
TST was performed before anti-TNF biological agent therapy for 50% (331) of the participants, for LTBI screening before solid organ and hematological stem cell transplantation for 20.4% (135), for screening following contact with tuberculosis for 25.1% (166), for screening of healthcare professionals for 1.1% (7), and medical report for 3.3% (22).
The most common reasons for the use of a biological agent by patients who took TST for anti-TNF biological therapy were as follows: 24.2% (159) rheumatological diseases (rheumatoid arthritis (71), ankylosing spondylitis (69), mixed connective tissue disease (14), systemic lupus erythematosus (5)), 12.5% (83) dermatological diseases (psoriasis, Behçet’s disease), 6.6% (44) inflammatory bowel diseases (ulcerative colitis, Crohn’s disease) and 3.2% (21) neurological diseases (multiple sclerosis).Patients who took TST due to future transplantation procedure were going to undergo liver transplantation (11.5% (76)), kidney transplantation (6.5% (43)), and bone marrow transplantation (2.9% (19)). The TST distribution was given in Table 2.
Table 2 Distribution of TST
| Variable | Tuberculin skin test (diameter of induration at the test site) | ||||
|---|---|---|---|---|---|
| 0 mm | 1-4 mm | 5-9 mm | 10-14 mm | >14 mm | |
| Sex | |||||
| Male | 195 (56.5) | 21 (6.1) | 67 (19.4) | 42 (12.2) | 20 (5.8) |
| Female | 179 (56.6) | 26 (6.1) | 48 (15.2) | 50 (15.8) | 13 (4.1) |
| Smoking | |||||
| Smoker (current/former) | 126 (52.1) | 13 (5.4) | 48 (19.8) | 37 (15.3) | 18 (7.4) |
| TST indication | |||||
| Biological agent therapy | 179 (54.1) | 26 (7.9) | 56 (16.9) | 55 (16.6) | 15 (4.5) |
| Prior to organ transplant | 92 (68.1) | 7 (5.2) | 24 (17.8) | 5 (3.7) | 7 (5.2) |
| Tuberculosis contact | 92 (55.4) | 12 (7.2) | 26 (15.7) | 28 (16.9) | 8 (4.8) |
| Healthcare professional screening | 2 (28.6) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 2 (28.6) |
| Medical report (job application, trip abroad) | 9 (40.9) | 1 (4.5) | 8 (36.4) | 3 (13.6) | 1 (4.5) |
| Chest X-ray findings | |||||
| Normal) | 333 (56.0) | 45 (7.6) | 104 (17.5) | 83 (13.9) | 30 (5.0) |
| Sequelae findings | 5 (62.5) | - | - | 2 (25.0) | 1 (12.5) |
| Infiltration | 8 (61.5) | 1 (7.7) | 1 (7.7) | 2 (15.4) | 1 (7.7) |
| Pleural effusion | 4 (56.7) | - | 1 (14.3) | 1 (14.3) | 1 (14.3) |
| Other (nodule, mass, atelectasis, mediastinal enlargement, diaphragm height, etc.) | 16 (69.6) | - | 6 (26.1) | 1 (4.3) | - |
Data are presented as n (%)
38.1% (126) of those who had TST before to anti-TNF therapy, 26.7% (36) of those who had TST before transplantation, 21.7% (36) of those who had contact with tuberculosis, and 18.2% (4) of those who needed a health report were considered TST-positive (p <0.001).
A detailed analysis of the TST-positive patients on anti-TNF drugs showed that 28.2% (20) of patients with rheumatoid arthritis, 55.1% (38) of patients with ankylosing spondylitis, 42.1% (8) of patients with a mixed connective tissue disease, 38.1% (8) of patients with multiple sclerosis, 43.4% (36) of the patients with a dermatological disease, and 17.8% (8) of the patients with inflammatory bowel disease were TST-positive.
2.7% of the TST patients were diagnosed with active tuberculosis (14 with pulmonary tuberculosis and 4 with extrapulmonary tuberculosis). On the other hand,1.5% (2) of the patients undergoing organ transplantation, 1.2% (4) of the patients on anti-TNF drugs, and 6.5% (12) of the patients with contact with an active tuberculosis patient were diagnosed with active tuberculosis (p <0.001). Therefore, among the patients with active TB, the mean TST value of the patients on anti-TNF medications was determined to be 9.7 (5-14) mm while the TST value of the patients undergoing organ transplantation was 0 mm, and the mean TST value of the patients with contact with a TB patient was 9.4 (0-22) mm. Specificity, sensitivity, and negative predictive value are given in Table 3.
Table 3 Specificity, sensitivity and negative predictive value of TST
| Sensitivity | Specificity | Negative predictive value | |
|---|---|---|---|
| Vaccinated | 85.7% (95% CI, 57.2-98.2%) | 68.1% (95% CI, 64.1-71.9%) | 99.5%(95% CI, 98.1-99.9%) |
| Unvaccinated | 50.0% (95% CI, 6.8-93.2%) | 91.7% (95% CI, 81.6-97.2%) | 96.5%(95% CI, 91.1-98.7%) |
| Entire cohort | 66.7% (95% CI, 40.9-86.7%) | 70% (95% CI, 66.3-73.5%) | 98.7% (95% CI, 97.5-99.3%) |
Furthermore, a QFT test was also performed in 332 (50.2%) patients with anergic TST results. QFT test was required in 67.7% (222) of the patients receiving anti-TNF therapy, 50.4% (66) of the patients with organ transplants, and 27.1% (42) of those in contact with active TB patients (p <0.001). QFT test was positive in 13.3% (44) of the patients taking QFT test.
The most common findings in chest radiogram were sequela findings (1.2%, n= 8), infiltration (2%, n= 13), and pleural effusion (1.1%, n= 7). Chest X-rays were negative for TB in 92.1% of the patients. The booster effect was evaluated in 42 (6.3%) patients with 0 mm TST result.
According to TST and QFT test results, 28.3% (187) of the patients were started on tuberculosis prophylaxis. On the condition that it was started one month before the anti-TNF therapy, isoniazid 5 mg/kg/day (maximum 300 mg/kg) was given to patients requiring tuberculosis prophylaxis for nine months. Rifampicin treatment was applied for 13 patients who developed side effects from INH treatment.
Discussion
A crucial public health problem, tuberculosis is one of the oldest diseases. Widespread use of immunosuppressive medications due to the modernization in the health sector may lead to tuberculosis in different patient groups. While diagnosing active tuberculosis is accessible in the presence of appropriate symptoms and radiological imaging, diagnosing LTBI may be challenging to some extent. Although IGRA has commonly been used for LTBI in recent years, TST is still in use as the initial test in many places. In this study which included the evaluation of TSTs performed in a tertiary hospital, we determined that TSTs are most commonly utilized to screen for LTBI before to biological agent therapy and almost half of the patients required a second test, such as QFT test, in addition to TST.
TNF-alpha is a proinflammatory cytokine that plays a significant role in the pathogenesis of many inflammatory diseases. An increase in the incidence of tuberculosis has been observed after anti-TNF medications that antagonize the biological activities of TNF-alpha have been used in the treatment of rheumatic diseases. The reactivation of latent tuberculosis infection poses an important problem for anti-TNF therapy, especially in countries with high prevalence. Screening patients with chest X-rays, TST, and IGRA prior to anti-TNF therapy is generally accepted 3,7. According to the national guidelines, isoniazid prophylaxis is recommended against latent tuberculosis in patients with a TST result ≥5 mm 7,10,11. Half of our patients took the TST test due to anti-TNF use in our study. Approximately one-third of the TST performed in patients receiving anti-TNF therapy was positive. In the systemic review by Pyo et al. 12, TST was found to be 29% positive in rheumatology patients receiving anti-TNF therapy. In a study conducted in Turkey investigating the incidence of tuberculosis in patients on anti-TNF drugs, the rate of patients with TST ≥5 mm was found to be 70.5% and the mean TST value was 10.9 mm. In two studies from Turkey, 1.1%-1.5% of patients using anti-TNF were diagnosed with active tuberculosis 8,13. Similarly, in our study, the rate of active tuberculosis was 1.2% in patients using anti-TNF drugs. This rate is higher than the incidence of tuberculosis in Turkey. According to the data from the Ministry of Health, the incidence of tuberculosis in Turkey was 14.1 per hundred thousand in 2018 2.
It was denoted that the rate of development of TB in patients undergoing solid organ transplantation is 50 times higher than in the average population 14. TB is a serious infectious complication in solid organ transplant candidates. In this group of patients, the mortality rate due to TB, despite the treatment, has been reported to be between 6-22% 15,16. The use of immunosuppressive agents can increase the mortality rate to 40% in these patients 15,17. Screening for LTBI may help reduce morbidity and mortality in transplant candidates, both by way of prophylaxis and early initiation of treatment 16,18. In transplant patients, TB or other infections are primarily due to an infected donor or new organ. However, the recipient patient may also develop LTBI. The risk of latent reactivation of TB is 20-74 times higher in transplant patients compared to the general population. Determining the risk of development of LTBI in transplant patients is thus critical. Current guidelines, therefore, recommend pre-transplant screening for LTBI in transplant candidates 16,18,19. A literature review demonstrated that the risk of TB was found to be 0.7-2.3% in liver transplant recipients, 0.5-15% in kidney transplant recipients, and 0.2-0.7% in bone marrow transplant recipients 17. In our study, 1.5% (2) of the organ transplants patients had active TB, and both were liver transplant recipients. In the study by Ahmadinejad et al., TST was positive in 15.9% of patients with solid organ transplants 18. In our study, on the other hand, this rate was 26.7%. Such differences between countries may be linked to the BCG vaccination program.
TST and IGRA are used for latent tuberculosis screening. TST is an inexpensive and easily applicable method. IGRA requires laboratory conditions. It is also a more expensive method compared to TST. However, many factors affect TST results. BCG vaccine, in particular, affects TST results 20,21. The application of TST poses challenges such as the patient having to return to the clinic to evaluate the test result, variability in the application and evaluation of the test, and differences in interpretation.
Moreover, considering false-positive results due to BCG vaccination and non-tuberculous mycobacterial infections, it is essential to find antigens that will not cross-react with BCG and to start applying IGRA. It has been reported that the TST has low specificity for diagnosing active tuberculosis in BCG-vaccinated populations. In the study of Lee et al. 22, sensitivity was 94% and specificity 88%. In our study, the sensitivity was 66.7%, the specificity was 70%, and the positive predictive value was 98.7%. Even though IGRA is a more expensive test, it provides a lower rate of false positivity and negativity because it is completed in a single visit, is less affected by external factors, and is less affected by BCG and other mycobacterium species. Additionally, further testing may be required to confirm TST, hence the increase in cost 20,21. The American Centers for Disease Control and Prevention (CDC) guidelines published in 2010 recommended using IGRA for screening of tuberculosis infection in countries where the administration of the BCG vaccine is widespread.21 Since TST results are affected by other factors, unnecessary prophylaxis is initiated and drug load increases, thus the exposition to side effects. In Turkey, the BCG vaccine is included in the routine vaccination program. There is no enough data on the BCG coverage rate in Turkey. In the study by Ozcirpici et al. 23, the BCG coverage rate was found to be 98.5%. Considering the high number of BCG-vaccinated patients, using IGRA may be reasonable. Our study concluded that at least half of the patients taking TST required IGRA. Inquiring about the reasons behind this, we determined that the anergic TST result was the most important reason. Because most of the patients in our study were using anti-TNF drugs, they can be considered as immunosuppressed individuals. The TST result may be anergic in these patients since T cells cannot react to TST. Therefore, we think that using IGRA in the upcoming follow-up tests after the initiation of immunosuppressive therapy in this patient group may be advantageous for patient comfort and cost-effectiveness.
It is recommended that if a first TST in vaccinated persons gives negative results, but there is a BCG vaccination scar, TST is repeated one week later at the earliest and the second TST result is evaluated 4. In our study, TST measurement was 0 mm, although at least half of the patients had a BCG vaccination scar. Whereas some of these patients were tested for the booster effect, QFT test was performed on a significant portion of them.
Reviewing all published reports, Horsburgh calculated the risk of developing tuberculosis in people with a positive TST 24. Based on the conclusions of this study, it was determined that reactivation was significantly higher under the age of 35, regardless of the limit value accepted for TST positivity. In addition, this study established advanced HIV infection, chronic renal failure, and TNF alpha inhibitor therapy, respectively, as the conditions with the highest risk for TB reactivation 24. In our study, although most TST-positive patients requiring isoniazid prophylaxis were on anti-TNF medications, patients with active TB in their follow-up included mostly those having TB contact but not immunosuppressive. This finding indicates that patients who are not immunosuppressive but have contact with active TB are at high risk of developing TB.
The absence of a control group is the most significant limitation of our study. However, we believe it is important because it is a study conducted with a large TST-related patient population.
Conclusion
While TST is most performed for LTBI screening prior to biological agent therapy, almost one-fourth of TST patients require tuberculosis prophylaxis. On the other hand, about half of the patients require an additional QFT test. Therefore, although we recommend that TST can be used for LTBI screening before the start of the immunosuppressive state, we suggest that IGRA be performed primarily in patients who have already been started on immunosuppressive therapy because TST results of these patients may be anergic.