Introduction
Patients with rheumatoid arthritis have a higher risk of cardiovascular disease than the general population. This is thought to be about a higher prevalence of traditional cardiovascular risk factors and the effects of uncontrolled inflammatory disease over vascular endothelium. Cardiovascular disease is the first cause of death in this group of patients 1,2. It is believed that greater cardiovascular risk comes with greater disease progression and activity, with the possible development of subclinical atherosclerotic disease. Effective treatment of inflammation could decrease the burden of cardiovascular disease. However, certain biologics, such as infliximab, a chimeric anti-TNF monoclonal antibody, may lead to cardiovascular adverse effects, such as congestive heart failure or ischemic events, due to interference with endothelial function and coagulation, especially in patients with a history of heart disease or cardiovascular risk factors 3,4.
Despite some studies suggesting anti-TNFs have a lower risk of acute coronary syndrome in the medium term compared with conventional synthetic disease-modifying antirheumatics 5, according to observational population studies, rates of acute coronary syndrome remain high in patients receiving biologic therapy compared with the general population 6. In a retrospective observational cohort study of 47,193 patients with rheumatoid arthritis, authors found anti-TNF might be associated with higher rates of coronary events (adjusted HR 1.3; 95% CI: 1.0-1.6) compared with abatacept (adjusted HR 0.64; 95% CI: 0.41-0.99) 7.
Incidence of acute coronary syndrome as an adverse effect of infliximab is uncommon, but there are numerous case reports, most of them of patients with cardiovascular risk factors who develop coronary disease with the drug application. However, nearly all have been isolated non-consecutive events without clearly established causality, and most have been in patients with other indications for biological therapy, such as ankylosing spondylitis or Crohn's disease 6,8-12. We present the case of a patient with three episodes of acute coronary syndrome after infliximab infusion, further building up the evidence for a causality relationship between this medication and a dangerous cardiovascular adverse event.
Clinical case
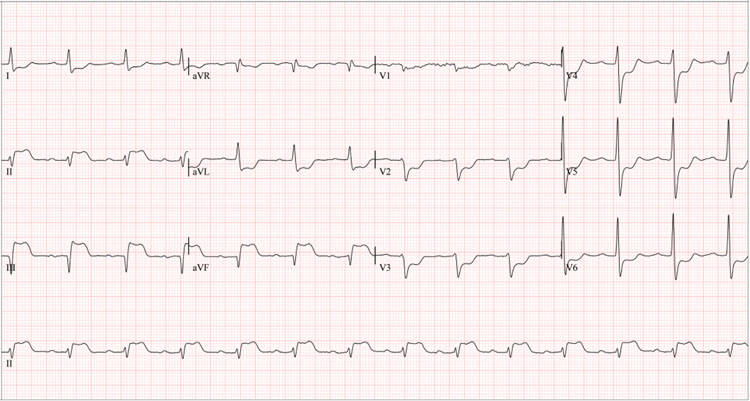
A 61-year-old man with a history of heavy smoking (22-year pack index, suspended 27 years ago), arterial hypertension and rheumatoid arthritis (since the age of 48), treated with methotrexate 15 mg/weekly, folic acid 1mg/daily, infliximab 3mg/ kg (Remsima dose adjusted to their weight) every eight weeks and amlodipine 5 mg/daily. He first visited the hospital for anginal chest pain 24 hours after administering intravenous infliximab 300 mg (Figure 1). Vital signs on admission were blood pressure 103/60 mmHg, heart rate 74 bpm, respiratory rate 20 rpm, saturation 92%, body mass index 24 kg/m2, rhythmic heart sounds without murmurs, and signs of heart failure. Electrocardiogram showed ST segment elevation in the inferior wall (Figure 2), with elevated troponin I (Table 1), consistent with acute myocardial infarction. After an attempt at pharmacological reperfusion using tenecteplase proved unsuccessful, a rescue coronary catheterization was promptly conducted, finding a 70% lesion in the middle segment of the anterior descending artery, high subocclusive thrombotic load in the mid-distal segment of the right coronary artery, and total occlusion in the posterior descending artery. The coronary intervention was performed with thrombus aspiration, tirofiban infusion, coronary balloon angioplasty, implantation of two medicated stents in the right coronary artery, and balloon angioplasty in the posterior descending artery. A drug-eluting balloon angioplasty was performed on the anterior descending artery in a subsequent staged intervention. An echocardiogram after revascularization revealed a left ventricular ejection fraction of 52%, akinesia of the basal inferoseptal segment and basal and mesial segments of inferior and inferolateral walls. Due to dyspnea following the initiation of ticagrelor, antiplatelet therapy was switched to clopidogrel and aspirin.
Table 1 Summary of laboratory findings
| First event: | Second event: | Third event: | |
|---|---|---|---|
| Total Cholesterol (mg/dl) | 152 | 162 | 137 |
| LDL cholesterol (mg/dl) | 80 | 85 | 28 |
| Triglycerides (mg/dL) | 189 | 165 | 335 |
| Troponin (ng/L) (Normal value 0-26) | 144 | <10 | initial 27- control 1,210 |
| Hb1Ac | 5.8% | 5.8% | 5.4% |
| TSH mU /L | 1.33 | 1.13 | 1.22 |
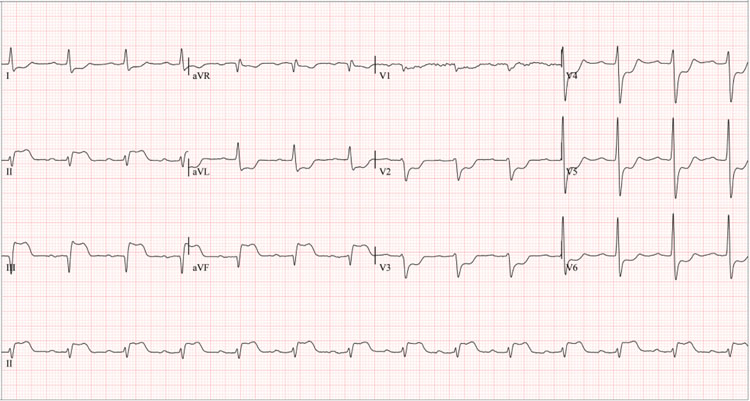
Figure 2 12-lead surface electrocardiogram of the first event, 24 hours after the first infliximab infusion. *Significant ST-segment elevation in leads DII, DIII, and aVF, and ST depression in precordial leads V1-V6 suggest inferior wall transmural infarction with possible posterior extension.
Five months later, treatment with infliximab was restarted, and 48 hours after the next consecutive dose of biologic, he developed anginal chest pain and was readmitted to the emergency room for a second acute coronary event. (Figure 1). Electrocardiogram showed first-degree atrioventricular block, T wave inversion in leads V4-V6 compared with the last known electrocardiogram (Figure 3), and the cardiac biomarker was negative (Table 1). Transthoracic echocardiogram showed altered segmental contractility: basal and mesial akinesia of the inferior and inferoseptal walls. Myocardial perfusion showed mild lateral ischemia involving 3% of the left ventricular mass and inferior, inferolateral, and inferoseptal necrosis involving 30% of the left ventricle. It was deemed an episode of unstable angina-type acute coronary syndrome, characterized by insignificant ischemia that did not warrant coronary arteriography. Cardioprotective pharmacological treatment (beta-blocker, statin, angiotensin II receptor antagonist, dual antiplatelet therapy with clopidogrel and aspirin) was optimized.
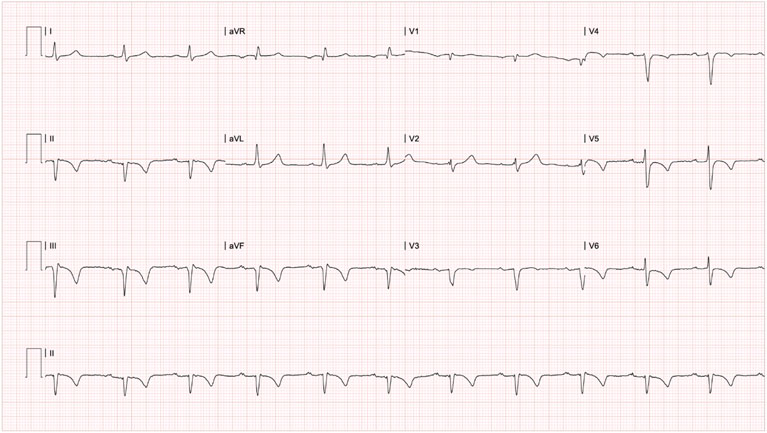
Figure 3 12-lead surface electrocardiogram of the second event, five months after the first event and 48 hours after applying the second consecutive dose of infliximab. *PR in 220ms, pathological Q wave in leads DII, DIII and AVF, and T wave inversion in leads DII, DIII, AVF, V4, V5 and V6, compatible with first-degree atrioventricular block, inferior necrosis and possible inferior and anterolateral subepicardial ischemia.
Six months after the first event, infliximab was administered for the third time, and again 24 hours after administration (Figure 1), the patient presented a new episode of anginal chest pain. Electrocardiogram showed ST-segment elevation in the inferior wall (Figure 4), ultrasensitive troponin I with positive delta (Table 1); thus, a diagnosis of acute coronary syndrome (ST-segment elevation acute myocardial infarction) was made. Coronary arteriography revealed a complete occlusion of the right coronary artery. Attempts to perform angioplasty on the occlusion proved unsuccessful. A transthoracic echocardiogram estimated the left ventricular ejection fraction to be 51% and identified akinesia in the basal, inferior mesial, and inferoseptal segments, along with the formation of an inferior aneurysm. Thrombophilia and secondary antiphospholipid syndrome were ruled out through further studies. Considering the consistent occurrence of coronary events following drug administration, the decision was made to discontinue infliximab. As of today, the patient has not been admitted at our institution.
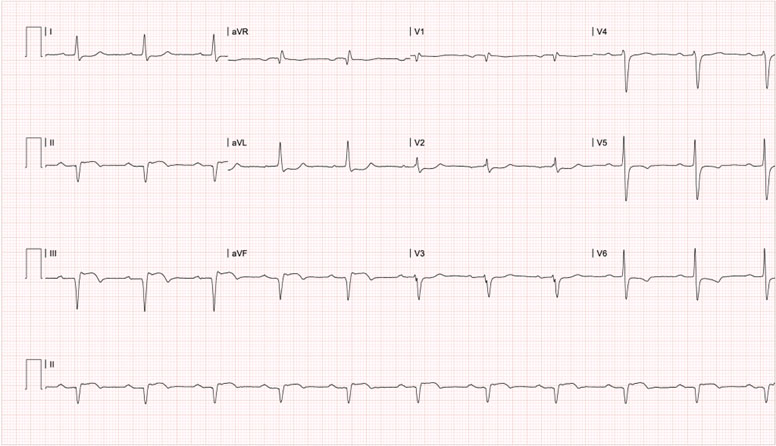
Figure 4 12-lead surface electrocardiogram of the Third event, six months after the first one, 24 hours after the third consecutive infliximab administration. * PR in 240ms, ST elevation and pathological Q wave in leads DII, DIII and aVF, along with T wave inversion in leads V5 and V6, compatible with first-degree atrioventricular block, evolved inferior transmural infarction and possible lateral subepicardial ischemia.
Ethical considerations
The authors declare that this article contains no personally identifiable information about the patient and that the patient's written informed consent was obtained to publish this case report and accompanying images. A copy of the written consent is available from the editor-in-chief of this journal for your review.
Discussion
We report the case of a patient with long-standing rheumatoid arthritis (13 years) and poorly controlled disease, leading to significant joint deformities. In 2018, biological therapy with infliximab was initiated. However, following consecutive administrations of this anti-TNF agent, the patient experienced three episodes of acute coronary syndrome. These episodes included two ST-elevation myocardial infarction incidents and one non-ST-elevation acute coronary syndrome episode. Before the first coronary event, he had controlled cardiovascular risk factors but had a high rheumatic disease burden with DAS28 in high activity. The algorithm developed by Naranjo et al. 13,14 determined that the adverse reaction was probably related to the drug (Table 2). Thus, it was suspended.
Table 2 Naranjo algorithm for estimating causality between infliximab and acute coronary syndrome.
| Question | Yes | No | Not Applicable | Answer |
| Are there previous conclusive reports on this reaction? | 1 | 0 | 0 | 1 |
| Did the adverse event appear after the suspected drug was administered? | 2 | - 1 | 0 | 2 |
| Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | 1 | 0 | 0 | 0 |
| Did the adverse reaction reappear when the drug was readministered? | 2 | - 1 | 0 | 2 |
| Are there alternative causes (other than the drug) that could on their own have caused the reaction? | - 1 | 2 | 0 | -1 |
| Did the reaction reappear when a placebo was given? | - 1 | 1 | 0 | 0 |
| Was the drug detected in the blood (or other fluids) and concentrations known to be toxic? | 1 | 0 | 0 | 0 |
| What is the reaction more severe when the dose was increased, or less severe when the dose was decreased? | 1 | 0 | 0 | 0 |
| Did the patients have a similar reaction to the same or similar drugs in any previous exposure? | 1 | 0 | 0 | 1 |
| Was the adverse event confirmed by an objective evidence? | 1 | 0 | 0 | 1 |
| Total punctuation | 6 | |||
* The algorithm was modified from the original version by Naranjo et al. 13,14, and classifies adverse drug reactions as improbable (0), possible (1-4 points), probable (5-8 points) and definite (≥9 points).
Publication of adverse events plays a crucial role in closely monitoring drug safety in real-world scenarios, in this case allowing to raise awareness regarding potential links between infliximab and cardiovascular events 15. While it is not feasible to determine the exact proportional risk attributed to infliximab, it does provide grounds to entertain the hypothesis of an associated adverse effect 15. Despite the controversial connection between infliximab and ischemic heart disease, the existing literature and our specific case provide evidence supporting a causal relationship between infliximab and coronary events.
Of the anti-TNF drugs, infliximab has the highest rate of adverse effects, including cardiovascular (1%-10%), with bradycardia and arterial hypertension as the most frequent 16,17. In an open-label study to evaluate the safety of infliximab in patients with RA 18, 60% of patients reported some adverse effects, with heart failure and cardiovascular events only reported by 0.9%. Within FDA warnings 19, new-onset heart failure or worsening symptoms are documented, but not acute coronary syndrome. However, case reports documented in the literature specifically mention this complication.
Among them, it is noteworthy that some exhibit uncontrolled cardiovascular risk factors; recurrence of coronary events in such cases raises concerns regarding the use of infliximab in patients with pre-existing cardiovascular risk factors 8. One of them describes a male patient, also in the seventh decade of life (63 years), with rheumatoid arthritis and pre-existing cardiovascular risk factors, such as type 2 diabetes mellitus, arterial hypertension, and hypercholesterolemia. He presented with acute coronary syndrome on two occasions (ST-segment elevation acute myocardial infarction and then non-ST-segment elevation myocardial infarction), showing obstructive coronary disease in 2 vessels and requiring angioplasty with stenting, comparable to what was described in our case report. Events occurred in the first 72h after applying infliximab (dose not described); however, not with consecutive applications as in our case, since they occurred after the first and eighth infusion, with a period in which infliximab was applied and did not present the adverse reaction.
In another report 9, a woman younger than our patient (49 years old), also with rheumatoid arthritis and arterial hypertension, presented acute coronary syndrome without ST-segment elevation with infliximab infusion (dose of 4.2 mg/kg every eight weeks). Unlike our case, the event occurred 10 minutes after the fifteenth infusion of infliximab, and the arteriography did not find obstructive coronary disease.
There are also reports in entities other than rheumatoid arthritis. One of them describes a 41-year-old man with ankylosing spondylitis who, after the 18th infusion of infliximab (unspecified dose), presented acute myocardial infarction with ST-segment elevation of the anterior wall, with elevation of cardiac enzymes 10. Unlike our case, the patient was younger, had no other cardiovascular risk factors, and no obstructive coronary disease was found. In contrast to our report, the event occurred a few minutes after the infusion, and it was not in the first but in the eighteenth application of the medication. Another report also describes a 50-year-old male patient with ankylosing spondylitis, with no other associated cardiovascular risk factors, who, a few minutes after starting the fifth infliximab infusion (at a dose of 3 mg/kg every eight weeks), presented angina and elevated ST on the electrocardiogram, unlike our case, with a negative biomarker and no significant coronary artery disease on arteriography, with complete reversal of electrocardiographic changes, deemed as vasospastic angina 11. Additionally, we found a report on Crohn's disease, also of a male patient, in this case, 40 years old and without cardiovascular risk factors, who also presented within the first 72 hours after the first infliximab infusion (dose of 15 mg/kg), non-ST-segment elevation myocardial infarction with troponin elevation, but there was no description of the coronary anatomy. He also presented other complications not seen in our case, with atrial fibrillation and ventricular dysfunction, describing hypokinesis of the interventricular septum on the echocardiogram and ejection fraction of 45% 12.
In our case, each of the events described has a clear temporal relationship. Reactions occurred each time the drug was administered, on three occasions with different periods. Infliximab doses differed in the cases described, and there is no established dose-response relationship. In some cases, it occurred in the first infusion; in others, it was after several previous cycles in which there had been no complications. Unlike the cases reported in the literature, our case is the only one where all the events occurred consecutively, presenting the reaction with each of the three applications. The magnitude of the impact of the reactions does not seem to be easily estimable, as described. Obstructive coronary disease was not present in all cases, and only in one of the cases in which the coronary anatomy was not described complications such as arrhythmias and ventricular dysfunction develop, which did not occur in our case. Even with repeated events, no ventricular dysfunction or arrhythmia was found, but obstructive coronary disease with thrombotic burden did persist.
Finally, in support of the causation hypothesis, the adverse event in our case was unequivocally confirmed through the utilization of multiple diagnostic methods (electrocardiogram, cardiac enzymes in blood, and arteriography). Several mechanisms are proposed to explain the association between acute coronary syndrome and anti-TNF therapy. Coronary arteriography findings, clinical and laboratory characteristics of our case (Table 1), and one of the reports described support the notion of the mechanism due to plaque rupture 8. This phenomenon is believed to be explained by matrix degradation secondary to an imbalance between the concentration and activity of extracellular matrix metalloproteinases and their inhibitor 8,20,21. The complication presented in our patient with inferior aneurysmal formation could also be related to this same mechanism. Other cases suggest the plausibility of different mechanisms for the acute coronary syndrome, including Kounis syndrome, characterized as an acute coronary syndrome that occurs associated with activation of basophils and mast cells, resulting in allergic/anaphylactic reactions, generating coronary vasospasm, plaque erosion and rupture with coronary thrombosis 22. The thrombotic load was significant in both arteriograms performed on our patient, so much so that even thrombolysis with tenecteplase in the first event and angioplasty in the third event were unsuccessful. This further supports the pathophysiological thrombotic component in these reactions. Likewise, acute vasoconstriction phenomena 9-11) and long-term lipid alterations have been described 10, which were not demonstrated in our case.
In terms of limitations concerning the causal association and the case description, there are several considerations to consider. The dose was not modified, so a clear dose-response relationship could not be established. No specific antagonist or placebo was administered to assess response. Blood levels of the drug were not determined.
Our patient had been receiving methotrexate, as it is known that the combination therapy with infliximab reduces the immunogenicity of the latter, thereby decreasing the risk of adverse reactions to infliximab 23. However, it is important to note that this combination therapy has been associated with increased plasma homocysteine levels 24. Hyperhomocysteinemia may be a consequence of vitamin B6 and folate deficiency. Elevated homocysteine levels are related to endothelial damage, increased oxidative stress and inflammation as possible underlying mechanisms in the worsening of atherosclerosis and generation of coronary disease 25. Patients with acute myocardial infarction in whom hyperhomocysteinemia is identified have worse short- and medium-term cardiovascular outcomes 26. Concomitant supplementation with folic acid, as received by the patient described, decreases homocysteine levels, protecting against possible cardiovascular risks 24. Of the drugs added to manage cardiovascular disease, only infliximab was found to lower blood statin levels 27) and increase the risk of peripheral neuropathy 28.
It is not possible to definitively exclude other potential causes that could have contributed to the occurrence of coronary events. It is important to consider that these events might have arisen spontaneously in a patient with pre-existing cardiovascular risk factors, and comprehensive investigations into non-traditional cardiovascular risk factors not thoroughly conducted, such as lipoprotein (a), homocysteine, uric acid, high-sensitivity C-reactive protein, interleukin 1, and tumor necrosis factor-α 29-30. Ultimately, there was a lack of patient follow-up between the events, leading to disregard of the preventable risk for the second event and, even more significantly, for the third event. This could be attributed to the initial low suspicion of this reaction associated with infliximab, mainly due to its low reported frequency in the literature. Additionally, at the outset, there was a higher likelihood that the events were related to the patient's existing cardiovascular risk factors.
Conclusion
Our case, along with those documented in the literature, provides even more robust evidence supporting a probable causal relationship between infliximab and acute coronary syndrome across its entire spectrum (ST-segment elevation acute myocardial infarction, non-ST-segment elevation acute myocardial infarction, myocardial infarction with non-obstructive coronary disease "MINOCA" and unstable angina), with a predominance of male patients, without necessarily the presence of other uncontrolled cardiovascular risk factors. Our findings underscore the significance of screening and managing cardiovascular risk in these patients. In addition, it is crucial to ensure proper control of rheumatoid arthritis and maintain close monitoring to promptly identify the risk of encountering such adverse events. This enables timely interventions and evaluation of alternative pharmacological approaches.











 texto en
texto en