Remark
| 1) Why was this study conducted? |
| To describe the clinical characteristics, treatment patterns, and outcomes of patients with AL amyloidosis in the Colombian population through a retrospective analysis in three highly specialized centers in Medellín, Colombia. |
| 2) What were the most relevant results of the study? |
| The study found a higher frequency of renal involvement compared to cardiac involvement. Additionally, the frequency of multiorgan involvement was similar to other regions, such as Europe and Asia. The overall survival rate observed in this study was comparable to that reported in a recent European publication. The multivariate analysis conducted in the study identified cardiac involvement and consolidation with HDCT-AHCT as potential prognostic factors. |
| 3) What do these results contribute? |
| This study reports the characteristics and outcomes of Colombian patients with AL amyloidosis, identifies potential prognostic factors in our population and provides a basis for designing future studies with a broader scope. |
Introduction
Primary or amyloid light chain (AL) amyloidosis is the most common form of systemic amyloidosis 1. This disease originates from a clone of plasma cells or, less frequently, from a low-grade lymphoma such as chronic lymphocytic leukemia (CLL), lymphoplasmacytic lymphoma, or marginal zone lymphoma, capable of secreting monoclonal immunoglobulin light chains with unstable folding due to mutations in their variable region 2. In 80% of cases, the compromised light chain is the lambda chain due to a higher intrinsic amyloidogenic predisposition than the kappa chain 3,4. This amyloidogenic protein activates the formation, aggregation, and stabilization of insoluble amyloid fibrils that exhibit a high affinity for Congo red staining, characteristic of this disease 3. The presence of amyloid deposits in different tissues leads to organ dysfunction mediated by proteotoxicity and replacement of the typical architecture, eventually resulting in systemic signs and symptoms 5,6.
AL amyloidosis is a rare and challenging-to-diagnose disease, resulting in limited published data regarding its global epidemiology and clinical characteristics 7,8. Recently updated data from a patient cohort in Olmsted, Minnesota, estimate an incidence of 8.9 to 12 cases per million person-years 9,10; data from other regions of the world estimate an incidence of 3 to 12 cases per million person-years 11-13. A recently published systematic review of the literature used published data from North America, South America, Europe, and Asia to evaluate the global epidemiology of AL amyloidosis, estimating an incidence and 20-year prevalence rate of 10.44 and 51 cases per million person-years, respectively 14.
Except for the central nervous system, monoclonal immunoglobulin light chains can deposit virtually in any tissue, leading to a wide range of clinical manifestations and organ involvement, including the heart, kidneys, liver, soft tissues, peripheral nervous system, among others, which are also the main prognostic markers 8,15,16.
The current treatment of AL amyloidosis primarily focuses on suppressing the underlying malignant neoplasm to reduce the production of amyloidogenic light chains 2. There are no approved therapies for the treatment of AL amyloidosis, so the therapy for this disease has been heterogeneous, based on adapted combinations of treatments for multiple myeloma or low-grade lymphomas 17, including regimens such as MDex (melphalan and dexamethasone), VMD (bortezomib, melphalan, and dexamethasone), CyBorD (cyclophosphamide, bortezomib, and dexamethasone) 18,19, and consolidation of response with high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (HDCT-AHCT) in eligible patients 20. Recently published data demonstrated promising results with the addition of Daratumumab to the CyBorD regimen, achieving better response rates and progression-free survival than CyBorD 21. The prognosis of AL amyloidosis is influenced by multiple factors, including the severity of cardiac involvement, which has been established as the primary determinant of survival 2,22,23, coexistence with other neoplasms such as multiple myeloma 24, depth of hematological response 25, among others.
Although some studies report data from Latin America, there is a lack of information about Colombian patients. This study aims to describe the clinical characteristics, treatment patterns, and outcomes of patients with AL amyloidosis in the Colombian population through a retrospective analysis in three highly specialized centers in Medellín, Colombia.
Materials and Methods
Population and sample
The study population consisted of patients over 18 years of age, of both sexes, diagnosed with AL amyloidosis, who had been seen as outpatients or hospitalized for this condition at Hospital Universitario San Vicente Fundación de Medellín, Hospital San Vicente Fundación de Rionegro, or Hospital Pablo Tobon Uribe between January 2012 and December 2022.
We included all patients who met the inclusion criteria during the study period, excluding only those who did not have information on the variables of interest.
The diagnosis of AL amyloidosis required histological demonstration of amyloid deposits by positive Congo red staining and apple-green birefringence under polarized light, associated with the presence of monoclonal gammopathy identified by serum/urine immunofixation, an abnormal kappa/lambda ratio indicating an excess of light chains, or the presence of clonal plasma cells in bone marrow biopsy 7,21. Other types of amyloidosis (AA amyloidosis, transthyretin amyloidosis, localized amyloidosis, hereditary amyloidosis, etc.) were excluded. Organ involvement classification, organ response, and hematological response were defined according to the criteria of the 10th International Symposium on Amyloid and Amyloidosis published in 2005 26 and the guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis published in 2012 27.
Variables
Demographic, clinical, laboratory, histological, functional status, treatment, and overall survival variables were evaluated. Univariate analysis was performed to determine the potential effect of associated neoplasms, renal involvement, cardiac involvement, and HDCT-AHCT on the overall survival of the evaluated population, and multivariate analysis was conducted to adjust for possible confounding variables. Clinical information was retrospectively obtained from electronic medical records and independently reviewed by six physicians.
Statistical Analysis
For categorical variables, absolute and relative frequency distributions were used for each category. For numerical variables, measures of central tendency such as mean with standard deviation or median with interquartile range were used.
Overall survival was analyzed using the Kaplan-Meier model with median survival and 95% confidence intervals (CI). Cox regression analysis assessed associated factors, verifying the proportional hazards assumption. Observed and adjusted hazard ratios (HR) along with 95% CI and p-values were estimated. Variables with p <0.25 in the multivariate analysis were included in the adjustment. Through post hoc analysis, it was established, with an alpha of 0.05, that the statistical power is greater than or equal to 0.80 for HR ≥2.7 or HR: ≤0.31.
Ethical considerations
The Research Directorate and the Ethics Committee of Hospital Universitario San Vicente Fundación de Medellín, Hospital San Vicente Fundación de Rionegro, and Hospital Pablo Tobon Uribe approved this study. Data confidentiality was ensured to prevent patient identification during the publication stages.
Results
Patient Characteristics
This study included 63 patients with AL amyloidosis, with demographic, clinical, histological, treatment, and survival outcomes evaluated. Table 1 provides details of the analyzed clinical and laboratory characteristics.
Table 1 Demographic and Clinical Characteristics of AL amyloidosis patients
| Characteristics | Number of patients (%) |
|---|---|
| Sex | |
| Male | 36 (57) |
| Age (years) | |
| Mean (Range) | 60 (34-87) |
| ECOG | |
| 0 | 4 (6.0) |
| 1 | 25 (40) |
| 2 | 14 (22) |
| 3 | 7 (11) |
| No data | 13 (21) |
| Thrombocytosis (PLTs >450,000/mm3) | 6 (9.5) |
| Anemia (Hb <10 gr/dL) | 16 (25) |
| Creatinine >1.5 mg/dL | 9 (14)a |
| Mean eGFR by CKD-EPI (ml/min/1.73 m²) | 73 (SD: 29.5)a |
| eGFR <50 ml/min/1.73 m² | 11 (22.9)a |
| Involved light chain | |
| Lambda | 45 (71) |
| Kappa | 13 (21) |
| No data | 5 (8.0) |
| Involved heavy chain | |
| IgG | 19 (30.0) |
| IgA | 9 (14.0) |
| IgM | 2 (3.0) |
| IgD | 1 (1.5) |
| No heavy chain involvement | 27 (42.0) |
| No data | 6 (9.5) |
| Associated neoplasm | |
| Multiple mieloma | 37 (58.0) |
| Chronic lymphocytic leukemia | 1 (1.5) |
| None | 25 (40.0) |
| Mean plasma cell infiltration in bone marrow | 19% (SD: 22.3) |
| Plasma cell infiltration in bone marrow ≥10% | 33 (52) |
| Abnormal kappa/lambda ratio (<0.26 or >1.65) | 29 (87)b |
| Mean dFLC at admission (mg/L) | 9,553 (SD: 17,519) |
| Mean proteinuria at admission (mg/24h) | 5,444 (SD: 6,334)c |
| Nephrotic range proteinuria (≥3,500 mg/24 hours) | 22 (49) |
| Mean BNP at admission (ng/L) | 663 (SD: 619)d |
| BNP >81 ng/L | 20 (86) |
| BNP >700 ng/L | 10 (43) |
| Organ involvement | |
| Renal | 42 (66.0) |
| Cardiac | 39 (61.0) |
| Peripheral nervous system | 22 (34.0) |
| Soft tissues | 17 (26.0) |
| Hepatic | 16 (25.0) |
| Pulmonary | 8 (12.6) |
| Gastrointestinal | 6 (9.5) |
| Two or more organs | 39 (61) |
GFR = glomerular filtration rate. dFLC = involved-uninvolved light chain difference. SD= standard deviation. aInformation available in 48 patients. bInformation available in 33 patients. cInformation available in 45 patients. dInformation available in 23 patients.
The mean age of the patients was 60 years (SD: 14.2), ranging from 34 to 87 years. Thirty-six (57%) patients were male. Forty-three (68%) patients had an ECOG performance status of ≤2. According to the criteria of the International Symposium on Amyloid and Amyloidosis (26), 42 (66%) patients had renal involvement, followed by cardiac involvement (61%), peripheral nervous system involvement (34%), soft tissue involvement (26%), hepatic involvement (25%), pulmonary involvement (12.6%), and gastrointestinal involvement (9.5%). The majority of patients (61%) had involvement of two or more organs. Among the patients with renal involvement, 22 (52%) had nephrotic-range proteinuria (≥3500 mg/24 hours), representing 34% of all evaluated patients. Ten (43%) cardiac-involved patients had brain natriuretic peptide (BNP) levels ≥700 ng/L.
Histological and laboratory findings
Information on histological results was obtained for 57 (90%) patients, including 63 biopsies taken from different tissues. Among the analyzed patients with available histological results, amyloid deposition was most frequently detected in renal biopsies (40%), followed by abdominal fat biopsy (17%), bone marrow biopsy (12%), myocardium (8%), liver (8%), lymph node (5%), gastrointestinal tract (5%), skin (5%), minor salivary gland (3%), and lung (3%).
Regarding laboratory results during the initial diagnostic approach, 16 (25%) patients had anemia, 6 (9.5%) had thrombocytosis, nine patients (14%) presented with creatinine greater than 1.5 mg/dL, and 11 patients (22.9%) had a GFR < 50 ml/min/1.73 m² at the time of diagnosis. In most cases, the involved immunoglobulin light chain was the lambda chain (71%), while the kappa isotype was present in 13 patients (20%). Out of 33 patients with information on serum free light chain concentration was obtained, 29 (87%) had an abnormal kappa/lambda ratio. The distribution of immunoglobulin heavy chains was as follows: serum immunofixation by electrophoresis was positive in 58 patients (90%), including 19 (30%) patients with IgG, 9 (14%) with IgA, 2 (3%) with IgM, 1 (1.5%) with IgD, and 27 (42%) patients with isolated immunoglobulin light chain involvement.
Thirty-eight patients (60%) had another neoplasm associated with AL amyloidosis, 37 (58.5%) had multiple myeloma, and 1 (1.5%) had chronic lymphocytic leukemia. The average percentage of plasma cell infiltration in the bone marrow was 19% (SD: 22.3), and 33 (52%) patients had 10% or more infiltration of the bone marrow by plasma cells.
Treatment, response and, survival
The study population was treated at different healthcare centers over ten years, using various treatment regimens, detailed in Table 2. Bortezomib-based therapies (VCD, VD, and Dara-VCD) were the most commonly used (68%), followed by combinations with immunomodulatory drugs such as thalidomide or lenalidomide (14%), and therapies based on alkylating agents such as melphalan or cyclophosphamide (9%); five (8%) patients did not receive induction therapy, and 15 (23.8%) patients received high-dose chemotherapy with autologous stem cell transplantation (HDCT-AHCT) after induction therapy.
Table 2 First-line Treatment Regimens and consolidation with HDCT-AHCT for Patients with AL Amyloidosis
| Regimen | Number of patients (%) |
|---|---|
| VCD, bortezomib, cyclophosphamide, and dexamethasone | 37 (59) |
| VD, bortezomib and dexamethasone | 5 (8.0) |
| Daratumumab-VCD, daratumumab + bortezomib, cyclophosphamide, and dexamethasone | 1 (1.6) |
| DRD, daratumumab, lenalidomide, and dexamethasone | 1 (1.6) |
| VRD, bortezomib, lenalidomide, and dexamethasone | 2 (3.1) |
| VTD, bortezomib, thalidomide, and dexamethasone | 1 (1.6) |
| MDT, melphalan, dexamethasone, and thalidomide | 3 (4.7) |
| CTD, cyclophosphamide, thalidomide, and dexamethasone | 2 (3.1) |
| Mdex, melphalan and dexamethasone | 2 (3.1) |
| MDV, melphalan, dexamethasone, and vincristine | 1 (1.6) |
| CD, cyclophosphamide, and dexamethasone | 3 (4.7) |
| None | 5 (8.0) |
| HDCT-AHCT, high-dose chemotherapy followed by autologous hematopoietic cell transplantation. | 15 (23.8) |
Hematologic response information was obtained for 21 patients. Hematologic response was achieved in 20 patients with first-line treatment, of which 10 achieved a complete hematologic response, 3 achieved very good partial response, 7 achieved a partial response, and 1 patient experienced progression during treatment. Information regarding hematologic response was unavailable for 42 (66%) patients. Organ response was observed in 6 (15%) of 39 patients with cardiac involvement, 6 (14%) of 42 patients with renal involvement, and 1 (6%) of 16 patients with hepatic involvement.
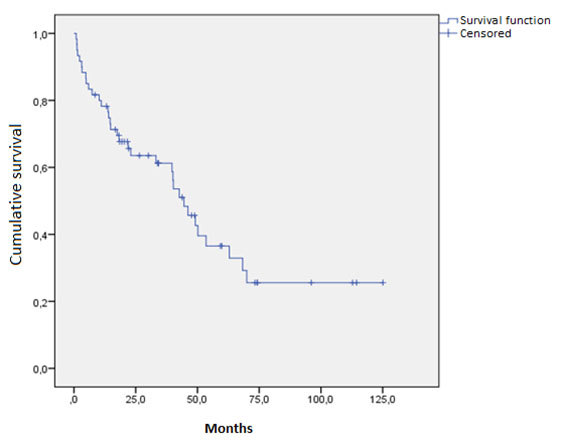
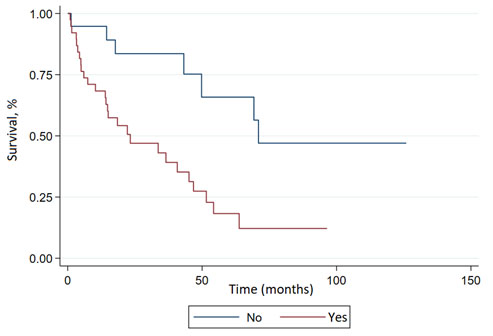
The median overall survival of the studied population was 45.1 months (95% CI: 22.2-63.8) (Figure 1), with an average follow-up of 24.7 months at the last follow-up on March 9, 2023. The effect of various analyzed factors on survival is shown in Table 3. Cardiac involvement was significantly associated with inferior overall survival (HR, 3.27; 95% CI: 1.23-8.73; p= 0.018) (Figure 2), while renal involvement (HR: 1.84; 95% CI: 0.65-5.19; p= 0.248) or the presence of another associated neoplasm (HR: 0.98; 95% CI: 0.49-1.97; p= 0.956) did not have a significant impact on the prognosis of the evaluated population. Patients who received HDCT-AHCT showed a non-significant trend (HR: 0.25; 95% CI: 0.06-1.09; p= 0.065) towards improved overall survival.
Table 3 Effect of clinical characteristics on overall survival
| Characteristics | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | p-value | IC95% | HR | p-value | IC95% | |||
| Cardiac involvement | ||||||||
| Yes | 3.47 | 0.004 | 1.48 | 8.14 | 3.27 | 0.018 | 1.23 | 8.73 |
| No | 1.00 | 1 | ||||||
| Renal involvement | ||||||||
| Yes | 1.05 | 0.927 | 0.40 | 2.77 | 1.84 | 0.248 | 0.65 | 5.19 |
| No | 1.00 | 1 | ||||||
| Associated neoplasm | ||||||||
| Yes | 0.98 | 0.956 | 0.49 | 1.97 | ||||
| No | 1 | |||||||
| Sex | ||||||||
| Male | 1.04 | 0.906 | 0.53 | 2.05 | ||||
| Female | 1.00 | |||||||
| Age at diagnosis | 1.01 | 0.685 | 0.98 | 1.04 | ||||
| Intervention | ||||||||
| HDCT-AHCT | ||||||||
| Yes | 0.15 | 0.0114 | 0.04 | 0.65 | 0.25 | 0.065 | 0.06 | 1.09 |
| No | 1.00 | 1 | ||||||
Median overall survival: 45.1 months (95% CI: 22.2-63.8) Abbreviations: HDCT-AHCT = high-dose chemotherapy followed by autologous hematopoietic cell transplantation.
Discussion
A retrospective analysis was conducted on patients with AL amyloidosis treated at different centers in Medellin, Colombia, to define their characteristics and outcomes, aiming to enrich the available information on the disease in our region. The average age at the time of diagnosis in the studied population was 60 years, like what has been observed in Latin American cohorts 28,29, North American population 30, and recent data from European countries 14,17, among other regions of the world 30,31.
AL amyloidosis can be associated with other neoplasms derived from plasma cells or B lymphocytes 2; in our study, most patients (58%) had multiple myeloma associated with AL amyloidosis, a higher proportion compared to the results reported by Kourelis et al. 24, who reported a multiple myeloma frequency of 46% in their cohort of patients with AL amyloidosis. In the mentioned study, patients with AL amyloidosis and multiple myeloma had lower overall survival than patients with AL amyloidosis without associated neoplasm, but this effect on prognosis was not observed in the univariate analysis of our study.
Organ involvement, particularly cardiac involvement, and its severity, is the main determinant of prognosis in patients with AL amyloidosis 2,23. The frequency of multiorgan involvement in the analyzed patients was 61%, like the recently published data from a European cohort by Palladini et al. 17, where 63% of patients diagnosed with AL amyloidosis after 2010 had involvement of 2 or more organs. The frequency of multiorgan involvement in our study was higher than the published data by Muchtar et al. 32, in North American patients, as their study considered multiorgan involvement from three or more organs.
Regarding the individual frequency of organ involvement, most of our patients (66%) had renal involvement, followed closely by cardiac involvement (61%); the published information on which of the two organs is most frequently affected in AL amyloidosis is heterogeneous. In a study published by Michael et al. 30, conducted in multiple hospitals in Greece, 71% of patients had renal involvement, followed by 59% of patients with cardiac involvement. Recent studies in the Chinese population also show a higher frequency of renal involvement than cardiac involvement 31. On the other hand, the aforementioned European cohort 17, and the results of the study by Muchatr et al. 32, reported contrary findings where cardiac involvement was more frequent than renal involvement. The recently published study by Posadas-Martinez et al. 28, in Argentinean patients also showed a higher frequency of cardiac involvement. While these differences are likely due to racial differences 33, it is important to note that in our setting, cardiac damage biomarkers such as BNP and N-terminal pro-B-type natriuretic peptide (NT-proBNP) have been available for a short time and in few healthcare centers; therefore, the differences in the frequency of cardiac and renal involvement compared to other cohorts may also be explained by the underdiagnosis of cardiac involvement, which includes criteria such as elevated BNP or NT-proBNP.
Although staging systems for AL amyloidosis have been available since 2004, they are mainly based on cardiac involvement 2,23,34-36, using markers of myocardial damage such as BNP, NT-proBNP, cardiac troponin T (cTNT), high-sensitivity cardiac troponin T (hs-cTNT), or cardiac troponin I (cTnI). None of the analyzed patients had validated troponin studies according to AL amyloidosis staging systems; only information on high-sensitivity cardiac troponin I (hs-cTnI) levels was available, and since there are currently no published staging scales incorporating hs-cTnI, it was not possible to stage the disease in our population. Among staging systems for AL amyloidosis, the Boston scale 34 includes serum BNP levels >700 ng/L as a marker of poor prognosis. Of the 23 (36%) patients in our study who had serum BNP levels at diagnosis, 43% had BNP levels >700 ng/L. Future studies should explore the impact of this marker on outcomes in patients with AL amyloidosis in Colombia.
The diagnosis of AL amyloidosis is histological and, therefore, always requires the demonstration of amyloid deposition through histopathological analysis of tissue 2,37. While abdominal fat and minor salivary gland biopsies are minimally invasive alternatives with adequate sensitivity 37,38, in the analyzed patients, only 20% had positive Congo red staining in one of these tissues. In comparison, amyloid deposition was demonstrated in renal biopsies in 40% of cases. This emphasizes that negative results in abdominal fat or minor salivary gland biopsies do not exclude the diagnosis of AL amyloidosis, and it is still necessary to perform biopsies of the affected organ as part of the diagnostic approach in patients suspected of having this disease.
Since the 2010s, treatment protocols based on bortezomib have become the most frequently used regimens in patients with AL amyloidosis 2,17. This trend was also observed in our study population, where bortezomib-based regimens were the preferred therapy in 68% of patients, achieving a partial or better hematologic response in 20 (98%) out of 21 patients with available hematologic response information. Consolidating response with HDCT-AHCT has been reported as a superior strategy to induction chemotherapy alone 20. Of the evaluated patients, 23.8% were consolidated with HDCT-AHCT, a higher proportion than in similar studies 17,31,32 where 4.5% to 11.4% of patients received HDCT-AHCT following induction therapy.
The evaluated population had a median survival of 45.1 months (95% CI: 22.2-63.8), similar to the data reported in previous studies conducted in other regions, where the median survival ranged from 34 to 48 months 17,30-32. In the multivariate analysis, patients with cardiac involvement had lower survival, with no differences in the survival of patients with renal involvement, a finding similar to previous studies such as the work published by Sidana et al. 22, where the median survival of patients with renal involvement was higher compared to patients with cardiac involvement or involvement of both organs. The multivariate analysis also showed a non-significant trend towards higher survival in patients consolidated with HDCT-AHCT, similar to the results reported by Gerts et al. 20, who reported higher 3-year survival in patients treated with HDCT-AHCT following induction therapy compared to patients treated with melphalan and dexamethasone alone. However, recent studies suggest that sequential therapy with HDCT-AHCT reserved only for patients with poor response to bortezomib-based regimens could achieve similar outcomes 39. Table 6 summarizes the characteristics of the present study and other similar studies previously conducted.
Table 4 Characteristics of compared studies
| Region | Colombia | Greece | China | United States | Argentina | Europe (post-2010) |
|---|---|---|---|---|---|---|
| Publication year | 2024 | 2010 | 2016 | 2019 | 2022 | 2023 |
| Diagnostic Methods | Congo Red + Monoclonal gammopathy | Congo Red + Monoclonal gammopathy | Congo Red + Monoclonal gammopathy | Mass spectrometry | ||
| Number of patients | 63 | 112 | 123 | 592 | 90 | 3,065 |
| Age (range) | 60 (34-87) | 62 (38-84) | 54 (34-82) | 63 (56-71) | 63 (50-76) | 64 (29-91) |
| Sex | Male (57%) | Female (53%) | Male (67%) | Male (64%) | Male (54%) | Male (58%) |
| Anemia/Thrombocytosis | 34.5% | |||||
| Renal function | ||||||
| Creatinine ≥1.5 | 14% | 25% | 28% (>1.2) | |||
| Mean glomerular filtration rate | 73 (DS: 29.5) | 60 +/- 51 | ||||
| eGFR <50 ml/min/1.73 m² | 23% | 27% | ||||
| Proteinuria ≥ 3.5 g/24h | 49% | 39% | 65% | 35% (>2 g/24h) | ||
| Monoclonal gammopathy | ||||||
| Involved light chain | Lambda (71%) | Lambda (86%) | Lambda (75%) | Lambda (71%) | ||
| Involved heavy chain | IgG (30%) | IgG (41%) | IgG (33%) | |||
| Associated neoplasms | MM (58%) | Excluded symptomatic MM | Excluded MM | Included MM (NR) | ||
| Infiltration by PC >10% | 52% | 23% (>30) | 13.8% (>5) | 54% | 54% | |
| Organ involvement | ||||||
| BNP >81 ng/L | 86% | |||||
| BNP >700 ng/L | 43% | |||||
| Renal | 66% | 71% | 98% | 53% | 68% | 66% |
| Cardiac | 61% | 59% | 55% | 76% | 72% | 69% |
| PNS | 34% | 38% | 9.8% | 24% | 35% | 14% |
| Soft tissues | 26% | 31% | 18% | 12% | 20% | |
| Hepatic | 25% | 18% | 13% | 18% | 16% | 13% |
| Pulmonary | 12.6% | 3.6% | 0.9% | |||
| GI | 9.5% | 16% | 72% | 17% | 36% | 7% |
| 2 or more | 61% | 91% | 25% (≥3) | 63% | ||
| Biopsy site | Renal (40%) | Renal (86%) | ||||
| Treatment | ||||||
| Firs-line treatment | bortezomib-based (68%) | VAD or high dose steroid-based (43%) | high dose steroid-based (34%) | bortezomib-based (30%) | bortezomib-based (75%) | bortezomib-based (74%) |
| HDCT-AHCT | 23.8% | 4.5% | 12% | 31% | 11.4% | 9.8% |
| Response | ||||||
| Cardiac | 15% | 6% | 4% | |||
| Renal | 14% | 26% | 84% | |||
| Hepatic | 6% | 33% | 8% | |||
| Hematologic | 95% (HRC 47%) | 50% (HRC 14%) | 100% (HRC 44%) | 84% (HRC 34%) | 61% (HRC 47%) | 63% (HRC 23%) |
| Survival analysis | ||||||
| Median survival (months) | 45 | 34 | 38 | 43 | 60 | 46 |
| Multivariate analysis | Cardiac involvement. | Cardiac involvement. PC >30%. Age >65. Cr >1.5. | Cardiac involvement. Renal involvement. | Cardiac involvement. Proteinuria >5 g/24h. Age >65. | Complete hematologic response. | |
Cr= creatinine. GI= gastrointestinal. HCR= complete response. IMiD= immunomodulators. MM= multiple myeloma. NR= not reported. PC= plasma cells. PNS= peripheral nervous system. VAD= vincristine, doxorubicin, dexamethasone
This study has several limitations. Firstly, due to its retrospective design, complete information on some variables, such as levels of cardiac damage biomarkers, hematologic response evaluation, and organ response, was not obtained. Although AL amyloidosis is a rare and difficult-to-diagnose disease, the small sample size of our study is an additional limitation that may have influenced the absence of statistical associations for some of the variables evaluated in the survival analysis. This also prevents estimating multivariate models with multiple factors simultaneously. Consequently, the associations presented in this study are exploratory and necessitate confirmation through further research. The methods used for the diagnosis of AL amyloidosis in our study represent another limitation, considering the false positive and false negative rates of Congo red staining, as well as the concurrent presence of monoclonal gammopathy in patients with transthyretin amyloidosis or hereditary amyloidosis. At the same time, mass spectrometry is the gold standard for characterizing amyloid protein; it is not available in our country. However, to our knowledge, this is the first study reporting the characteristics and outcomes of Colombian patients with AL amyloidosis, providing a basis for designing future studies with a broader scope.
Conclusion
We present the first study reporting the characteristics and outcomes of Colombian patients with AL amyloidosis. The analyzed group of patients had a similar age distribution as reported in previous studies, with a higher frequency of renal involvement compared to cardiac involvement and a higher frequency of multiorgan involvement compared to the US population, although similar to other regions of the world, such as Europe and Asia. The proportion of patients consolidated with HDCT-AHCT was higher than reported in other studies, with a similar overall survival rate to that observed in other regions worldwide. The multivariate analysis conducted in the study identified cardiac involvement and consolidation with HDCT-AHCT as potential prognostic factors.











 text in
text in