Remark
| 1) Why was this study conducted? |
| This study was conducted due to the lack of molecular information related to the driver mutations in the genes JAK2, MPL and CALR in Colombian patients. These mutations are known to be associated with the diagnosis and prognosis of pathologies such as polycythemia vera, primary myelofibrosis, and essential thrombocythemia |
| 2) What were the most relevant results of the study? |
| The most relevant results of this study can be summarized as follows: Despite the relatively small size of the population cohort, we were able to find a patient with mutations in both JAK2 and CALR genes. Furthermore, our investigation shows a mutation in the CALR gene that has not been reported before. Notably, our study stands as a pioneering effort to describe the mutational frequency of these three gene mutations in Colombian patients with myeloproliferative neoplasm |
| 3) What do these results contribute? |
| To date, this study is the first to describe the mutational profile of these three genes in BCR-ABL1-negative chronic myeloproliferative neoplasms in Colombia which allow clinician an researchers to understand the importance of molecular profiling in the diagnosis and prognosis of patients with these pathologies. |
Introduction
Chronic myeloproliferative neoplasms (MPNs) not associated with negative BCR-ABL or Philadelphia mutations are a cluster of diseases whose main characteristic is the clonal proliferation of myeloid cells with variable morphological maturity and hematopoietic efficiency. The most common neoplasms in this group are polycythemia vera, primary myelofibrosis, and essential thrombocythemia 1. According to Shallis et al. 2, the incidence of negative BCR-ABL chronic myeloproliferative neoplasms is 0.44-5.87 cases per 100,000 inhabitants, with rates of 0.84, 1.03, and 0.47 cases per 100,000 inhabitants for polycythemia vera, essential thrombocythemia, and primary myelofibrosis, respectively 2.
Studies on BCR-ABL-negative neoplasms in Colombia are scarce. The only study to have investigated the clinical characteristics of these neoplasms was presented in the first report of the Colombian myeloproliferative neoplasms registry performed by the Colombian Association of Hematology and Oncology (ACHO), comprising 79 patients from different hospitals in the country from 2013 to 2017. In this group, essential thrombocythemia was the most frequent disease of the three pathologies with 93 patients (51.9%), followed by polycythemia vera with 55 patients (30.7%) and primary myelofibrosis with 31 patients (17.3%) 3. However, no molecular studies of these neoplasms in the Colombian population have been published to date.
Mutations in genes regulating the JAK-STAT signaling pathway, like JAK2, MPL, and CALR genes, cause negative BCR-ABL chronic myeloproliferative neoplasms. For example, the tyrosine kinase JAK2 V617F missense mutation leads to an exchange of valine for phenylalanine at position 617, producing constitutive phosphorylation of JAK2 and Ba/F3 or Ba/F3-EpoR, allowing several cell lines to survive and proliferate independently of cytokines 4. Likewise, mutations in the MPL gene, such as the W > L or W > K change in codon 515, cause spontaneous activation of the JAK-STAT pathway 5. Moreover, somatic mutations have been informed by the CALR gene involving changes in the reading frame caused by deletions or insertions in exon 9 6.
The main clinical characteristic of polycythemia vera is increased red blood cell production independent of the mechanisms that normally regulate erythropoiesis 7. Ninety-five percent of patients with this pathology have the V617F mutation or another mutation in the JAK2 gene, resulting in the rapid increase of the erythroid lineage and other myeloid lines (panmyelosis) 1. However, essential thrombocythemia primarily compromises the megakaryocytic lineage and is characterized by sustained thrombocytosis (platelet count >450 x 109/L) in the peripheral blood and a rising number of large mature megakaryocytes, which form clusters 8. Patients with essential thrombocythemia have a JAK2 mutation in 50-60% of cases, CALR mutations in 30%, and MPL mutations in 3%; approximately 12% are triple-negative. None of these mutations is specific to TE, but their presence excludes reactive thrombocytosis (the main differential diagnosis of TE) 4. Finally, primary myelofibrosis is characterized by the rapid increase of granulocytes and megakaryocytes in the bone marrow related to the deposition of fibrous connective tissue and extramedullary hematopoiesis in the late stages of the disease 9. The JAK2 V617F mutation is observed in 50-60% of cases regardless of the disease stage, the CALR mutation in 24% of cases, and MPL mutations in 8%; approximately 12% of cases are triple negative 5.
Mutations in the JAK2, MPL, and CALR genes are fundamental for diagnosing, prognoses, and monitoring patients with chronic myeloproliferative neoplasms and h were proposed and reviewed by the WHO in 2008-2016 10. No publication has identified the frequency and type of these mutations in these three genes in patients with BCR-ABL-negative chronic myeloproliferative neoplasms in Colombia. This study aimed to establish the frequencies of mutations in the JAK2, MPL, and CALR genes in Colombian patients with a negative clinical diagnosis of BCR-ABL chronic myeloproliferative neoplasms. The genetic analysis enables the determination of a more precise molecular diagnosis in these Colombian patients to establish the most appropriate prognosis and treatment that impact their survival.
Materials and Methods
A descriptive, cross-sectional study was performed. The study population comprised a convenience sample of patients with a clinical and histopathological diagnosis of negative chronic myeloproliferative neoplasms BCR-ABL according to the criteria of the World Health Organization (WHO) 10 and older than 16 years old. Exclusion criteria were: diagnosis <15 year old, there were no molecular studies from whole blood samples and patients were not willing to participate in the study. Samples were obtained from patients at diagnosis and patients who relapsed in any of the three diseases considered in this study Clinical and laboratory parameters were obtained from the time of diagnosis or relapse by reviewing their clinical records. Samples were referred to the Medical Genetics Unit of the Universidad de Antioquia from three hospitals in Medellín-Colombia (Hospital San Vicente Fundación, Clínica León XIII, IPS Universitaria, and Hospital Pablo Tobón Uribe) between 2017 and 2021. After the patients provided signed informed consent, their peripheral blood samples were used for molecular biology analysis of the JAK2, MPL, and CALR genes. The study protocol and informed consent were approved by the Bioethics Committee for experimentation in humans of the Faculty of Medicine of Universidad de Antioquia.
Molecular analysis of the JAK2, MPL, and CALR genes
DNA extraction was performed using whole blood samples and the commercial QIAamp DNA Mini and Blood Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's recommendations.
The p.V617F mutation located in exon 14 of the JAK2 gene was detected using real-time PCR (qPCR) and the commercial JAK2 Mutation Detection Kit from Amoydx (Haicang District, Xiamen- China). The MPL W515L and MPL W515K mutations were detected using the commercial MPL W515L / K MutaScreen Kit from ipsogen, following the manufacturer's instructions. Samples with mutations were confirmed by direct, bidirectional Sanger sequencing using an ABI 3500 Applied Biosystems genetic analyzer and the primers MPL-F: TGGGCCGAAGTCTGACCCTTT and MPL-R: ACAGAGCGAACCAAGAATGCCTGT, as previously reported 11.
For the CALR gene, the insertions/deletions (indels) in exon 9 were evaluated using by analyzing the size of the amplified fragments. The following primers were used for amplification: CALR-F: 5'GAGGTGTGTGCTCTGCCTG3' and CALR-R: 5'AGAGACATTATTTGGCGCGG3'. The forward allele was labeled with 6-fluorescein (6-FAM), and the normal allele had a size of 298 bp, according to Jones et al 12. Fragment size analysis was performed using bidirectional Sanger sequencing and an ABI 3500 Applied Biosystems genetic analyzer using the Gene Mapper program. The samples positive for indels were sequenced by the Sanger method to confirm the results obtained by fragment analysis. Sequencing was performed using the following primers: CALR.SF: 5'ACAACTTCCTCATCACCAACG3' and CALR.SR: 5'GGCCTCAGTCCAGCCCTG3' following the conditions of Klampfl et al13. In their approach, amplification, subsequent purification, and sequencing of the products of PCR are required. The chromatograms obtained were analyzed using the Chromas Pro program, and the sequences were aligned with the reference sequence reported in GenBank using the Clustal Omega program.
Statistical analysis
The results are shown as absolute frequencies and percentages for categorical variables (sex, JAK2 mutation, CALR mutation, MPL mutation, and triple-negative mutation) and medians and interquartile ranges (25th percentile and 75th percentile) for quantitative variables (hemoglobin level, hematocrit level, platelet count, leukocyte count, lymphocyte count, neutrophil count). Comparisons among the JAK2, CALR, and triple-negative mutation groups and different types of neoplasms concerning hematological results were made using the Kruskal-Wallis test. Paired group comparisons were made using the Mann-Whitney U test. For all the analyses, the R environment (R Core team 2021) version 4.0.5 (2021-03-31) and Rstudio Version 1.4.1106 were used 14.
Results
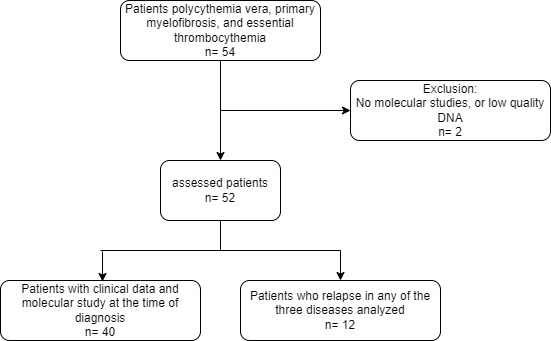
Fifty-four people with this pathology were included in the study, of whom two were excluded because there were no molecular studies because low quality of samples (Figure 1). Twenty-seven patients (51.9%) were men and 25 were women (48.1%). The average age was 58 years (SD: 15.8), with a minimum of 16 and a maximum of 87 years, where primary myelofibrosis presented at a later age of diagnosis (age value = 68.25 years) (Table 1). The most frequent diagnosis was essential thrombocythemia with 20 patients (38.46%), followed by primary myelofibrosis with 16 patients (30.76%) and polycythemia vera with 13 patients (25%). However, 3 (5.76%) patients could not be classified into one of the diagnoses and were classified as having non classifiable BCR-ABL1-negative chronic myeloproliferative neoplasms. Significant differences were found in the hemoglobin level, hematocrit level, hematocrit, and platelet count among the three neoplasms (p= <0.001). Patients diagnosed with polycythemia vera had the highest hemoglobin and hematocrit levels, essential thrombocythemia patients had the highest platelet count, and primary myelofibrosis patients had the lowest hemoglobin levels (Table 1).
Table 1 Demographic characteristics and hematological results of patients with BCR-ABL1-negative chronic myeloproliferative neoplasms according to diagnosis, sex, and age
| Polycythemia vera n= 13 | Essential thrombocythemia n= 20 | primary myelofibrosis n= 16 | nonclassifiable BCR-ABL1-negative chronic myeloproliferative neoplasms n= 3 | |
|---|---|---|---|---|
| N | 13 | 20 | 16 | 3 |
| Age (mean (SD)) | 52.92 (15.98) | 55.45 (16.12) | 68.25 (9.90) | 53.00 (21.38) |
| Female (%) | 5 (38.5) | 13 (65.0) | 6 (37.5) | 1 (33.3) |
| Male (%) | 8 (30.0) | 7 (26.0) | 10 (37.0) | 2 (7.0) |
| Hemoglobin level (g/dL), (median [QR]) | 17.80 [16.00, 20.00] | 12.90 [11.23, 13.70] | 11.50 [9.55, 13.45] | 14.50 [14.25, 14.75] |
| Hematocrit level (%) (median [QR]) | 54.00 [50.00, 60.00] | 38.20 [33.45, 43.20] | 36.15 [28.50, 40.62] | 45.00 [45.00, 45.50] |
| Platelet count (number of platelets/µL) (median [QR]) | 376000.00 [288250.00, 534750.00] | 792500.00 [503750.00, 1278000.00] | 159000.00 [71250.00, 255000.00] | 236000.00 [158500.00, 286000.00] |
| Leukocyte count (number of leukocytes/µL) (median [QR]) | 8900.00 [7700.00, 10760.00] | 9300.00 [6730.00, 12435.00] | 7820.00 [4210.00, 10700.00] | 6327.00 [5848.50, 51963.50] |
| Lymphocyte count (number of lymphocytes /µL) (median [QR]) | 2090.00 [1733.75, 2700.00] | 2231.00 [1504.70, 3050.00] | 1120.00 [876.50, 1852.00] | 2505.00 [2397.50, 2612.50] |
| Neutrophil count (number of neutrophils /µL) (median [QR]) | 6130.00 [3846.25, 7360.00] | 5250.00 [3580.00, 7815.00] | 6710.00 [3752.50, 8612.00] | 3830.00 [3344.50, 11876.50] |
Of the 52 patients analyzed, 51.9% had the JAK2 V617F mutation, 85% had polycythemia vera, 35% had essential thrombocythemia, 43.7% had primary myelofibrosis and 67% had non-classifiable BCR-ABL1-negative chronic myeloproliferative neoplasms. In addition, mutations in CALR exon 9 were found in 35% of essential thrombocythemia patients and 31% of primary myelofibrosis patients. Finally, the mutation frequency of the MPL was 12.5% of primary myelofibrosis. Furthermore, in the group of patients diagnosed with essential thrombocythemia, a double-positive case was found for JAK2 V617F and CALR, contributing doubly to the frequencies of these genes (Table 2).
Table 2 Frequencies of mutations in the three genes analyzed in patients with BCR-ABL1-negative chronic myeloproliferative neoplasms
| Total patients n=52 (%) | JAK2 | CALR | MPL | Triple Negative |
|---|---|---|---|---|
| Total frequency n= 52 (%) positive n (%) | 27 (51.9) | 12 (23.0) | 2 (3.8) | 12 (23.0) |
| Frequency by disease | ||||
| Polycythemia vera n=13 (%) | 11 (21.1) | 0 | 0 | 2 (3.8) |
| Essential thrombocythemia n= 20 (%) | 7 (13.5) | 7 (13.5) | 0 | 7 (13.5) |
| Primary myelofibrosis n= 16 (%) | 7 (13.5) | 5 (9.6) | 2 (3.8) | 2 (3.8) |
| nonclassifiable BCR-ABL1-negative chronic myeloproliferative neoplasms n= 3 (%) | 2 (3.8) | 0 | 0 | 1 (1.9) |
Note: There is a patient who is double positive for CARL and JAK2 and therefore the number of mutations is 53.
Regarding the mutation in exon 10 of MPL, 2 two patients had the W515K mutation. Of the 12 patients with mutations in CALR exon 9, seven presented a 52 bp deletion (type I mutation- c.1092_1143del; L367fs*46), two presented a 5 five bp insertion (type II mutation - c1154_1155ins- TTGTC; K385fs*47) and two had type I "alike" mutations (del 34 bp and 46 bp). Notably, one patient had a triple mutation in the CALR gene: a deletion of 10p (NM_004343.4):c.1130_1139delAAGAGGAGGA); this location also had an insertion of 9 bp (NM_004343.4):c.1130_1131insGCCTCTGTC) and another 9 bp deletion ((NM_004343.4) c.1177_1185delGAGGAUGAG)-(CALR:p.E398_D400del)).
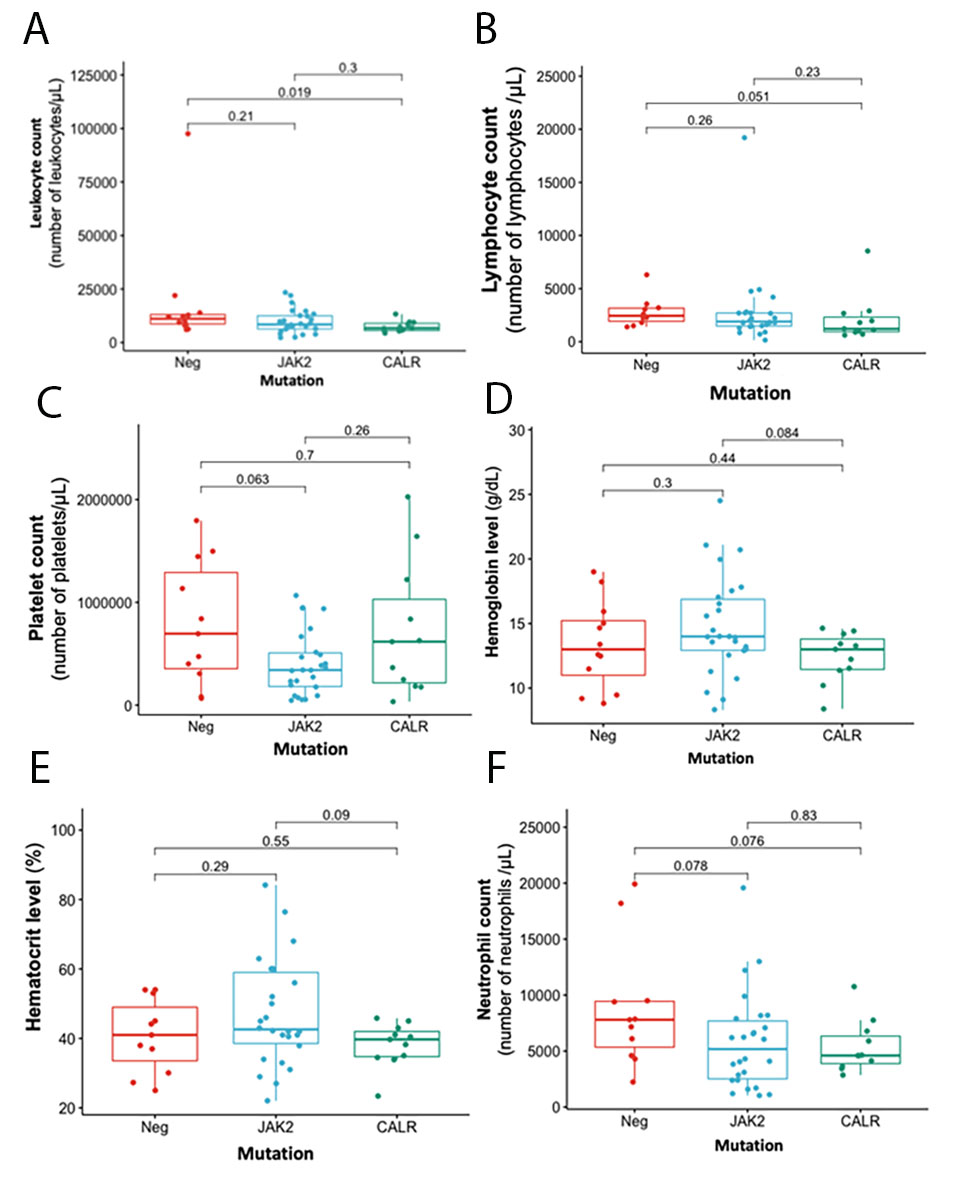
Figure 2 describes the main clinical and hematological characteristics of patients main clinical and hematological characteristics with BCR-ABL1-negative chronic myeloproliferative neoplasms according to JAK2, CALR and triple-negative mutations in these genes. Patients with a mutation in MPL were excluded from the statistical analysis because of the small sample size (two patients). Regarding the hematological results, a significant difference was found between triple-negative patients and those with mutations in CALR in the counts of leukocytes (p= 0.019) and lymphocytes (p= 0.051). Patients positive for CALR mutations had the lowest counts for these two hematologic variables (Figure 2A and B.). Although patients with the JAK2 mutation had a lower platelet count than those with the CALR and triple negative mutations (Figure 2C ), they had higher hemoglobin and hematocrit values (Figure 2D and E ).
Discussion
The present study evaluated the frequencies of mutations in the JAK2, MPL and CALR genes in 52 Colombian patients. To date, this study is the first to describe the mutational profile of these three genes in BCR-ABL1-negative chronic myeloproliferative neoplasms in Colombia. Although the sample size was small, frequencies similar to those reported in other studies of Latin American populations, such as those in Argentina and Mexico were obtained (Table 3) 15, 16. These similarities were likely because the populations share a common genetic background with a triethnic origin: European, Amerindian and African.
Table 3 Comparison of the frequencies of mutations in the JAK2, CALR and MPL genes in different populations
| JAK2 | CALR | MPL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | N | PV (%) | ET (%) | PMF (%) | PV (%) | ET (%) | PMF (%) | PV (%) | ET (%) | PMF (%) | |
| 1 | OMS 10 | >95 | 50-60 | 50-60.0 | 0.0 | 30.0 | 24.0 | 0 | 3.0 | 8.0 | |
| 2 | Argentina 15 | 439 | 94.9 | 61.2 | 62.0 | 0.0 | 21.5 | 16.3 | 0 | 2.8 | 6.1 |
| 3 | Mexico 16 | 27 | 62.0 | 36.0 | 25.0 | 0.0 | 29.0 | 25.0 | 0 | 7.0 | 0 |
| 4 | South Korea 17 | 199 | 91.4 | 63.3 | 57.4 | 0.0 | 17.7 | 14.8 | 0 | 2.5 | 9.3 |
| 5 | USA 18 | 3023 | 100 | 61.0 | 67.0 | 0.0 | 15.0 | 3.0 | 0 | 13.3 | 6.0 |
| 6 | Japan 19 | 143 | 97.0 | 59.9 | 60.2 | 0.0 | 26.9 | 21.6 | 0 | 4.7 | 1.1 |
| 7 | Italy 20 | 1282 | NA | 62.0 | 62.0 | NA | 24.0 | 26.0 | NA | 4.0 | 5.0 |
| 8 | Brazil 21 | 73 | 100 | 37.0 | 62.0 | 0.0 | 41.0 | 33.0 | NA | NA | NA |
| 9 | Thailand 22 | 100 | 94.7 | 74.5 | 25.0 | NA | 35.7 | 33.3 | NA | 0 | 0 |
| 10 | China 23 | 492 | 61.5 | 40.4 | 44.2 | 0.0 | 27.9 | 15.4 | 0 | 1.3 | 0 |
| 11 | India 24 | 130 | 100 | 61.7 | 57.6 | 0.0 | 15.1 | 23.7 | 0 | 9.1 | 3.4 |
| 12 | Brazil 25 | 65 | NA | 52.0 | 52.0 | NA | 13.0 | 38.0 | NA | 4.0 | 0 |
| 13 | Egypt 26 | 200 | 48.9 | 44.1 | 32.5 | 0.0 | 19.1 | 17.5 | 0 | 0 | 0 |
| 14 | This project (Colombia) | 52 | 85.0 | 35.0 | 44.0 | 0.0 | 35.0 | 31.0 | 0 | 0 | 12.5 |
The genetic characterization of Colombian patients with BCR-ABL1-negative chronic myeloproliferative neoplasms whose genetic context is different from other populations is essential for an accurate diagnosis, good prognosis and adequate management and survival of the patient. The JAK2 V617F mutation is associated with an increased risk of arterial thrombosis and a decreased risk of post-arterial thrombocythemia myelofibrosis. In polycythemia vera, a higher frequency of the JAK2V617F mutant allele has been associated with pruritus and fibrotic transformation. In general, the JAK2V617F mutation is associated with older age, higher hemoglobin level, leukocytosis, and lower platelet level. In addition, JAK2 exon 12 mutation-positive patients generally have predominantly erythroid myelopoiesis, subnormal serum erythropoietin, and younger age at diagnosis, but their prognosis is similar to exon 14 JAK2V617F 5. In essential thrombocythemia, the CALR mutation is associated with lower hemoglobin level, higher platelet count, lower white blood cell count, and a younger age compared to the JAK2V617F mutation. Similar features have been observed in patients with primary myelofibrosis. The CALR mutation is also associated with the male gender, slower risk of thrombosis, and better overall survival 8.
The JAK2 V617F mutation was the most common among the three neoplasms evaluated in our cohort, with frequencies similar to those reported in other populations 15,16,19. However, few differences were observed in mutational frequencies given that the most common mutation associated with this pathology was the JAK2 V617F mutation studied herein 13. This mutation activates three main receptors: the erythropoietin receptor, granulocyte colony-stimulating factor receptor and MPL. For this reason, JAK2V617F is associated with polycythemia vera, essential thrombocythemia and primary myelofibrosis 27. However, the MPL W515K mutation was reported only in two patients with primary myelofibrosis. This mutation occurs at a low frequency, primarily in patients with ET or primary myelofibrosis, implying that it may favor determining the megakaryocytic lineage instead of the erythroid line 28.
In this study, the frequency of mutations in CALR exon 9 was 35% for both essential thrombocythemia and primary myelofibrosis. Similar frequencies were in different studies, as reported in Table 3. Notably, in patients diagnosed with essential thrombocythemia, a double-positive case was found for JAK2 V617F and CALR. Recently, the cooccurrence of CALR and JAK2V617F mutations was reported in some cases of chronic BCR-ABL1-negative chronic myeloproliferative neoplasms 29-35, as well as JAK2 V617F and MPL W515L/S mutations 36. Cooccurrence among these genes occurred in 5-15% of MPL cases. These findings contrast the hypothesis that mutations in JAK2, CALR and MPL are mutually exclusive(10). Furthermore, in this study, six types of mutations were found in exon 9 of CALR, primarily deletions; the most prevalent was the type I mutation, followed by type II 33-35.
Notably, a patient presented three mutations in the CALR gene: a deletion of 10 bp (NM_004343.4):c.1130_1139delAAGAGGAGGA); this location also had an insertion of 9 bp (NM_004343.4):c.1130_1131insGCCTCTGTC) and another downstream nine bp deletion ((NM_004343.4) c.1177_1185delGAGGAUGAG)- (CALR:p.E398_D400del)). The ten bp deletion was reported in refractory anemia as a pathogenic variant (COSV57120310), changing the reading frame to that of the more frequent CALR mutations 37. The insertion of 9 bp at this point has not been reported to date and represents a striking finding in this patient. Finally, the third mutation is a nine bp deletion reported in a study of myeloproliferative neoplasms. This variant is annotated as a 9 bp polymorphism (rs550353351; NM_004343.4: c.1177_1185del- GAGGATGAG) that eliminates three amino acids (NP_004334.1: p. Glu398_Asp400del) that shifts the +1 bp reading frame, resulting in replacement of the normal C-terminal end by a new amino acid sequence 36. The severity of these mutations could explain the patient's lack of a treatment response and subsequent death.
We could not compare the clinical and hematological characteristics stratified by disease and mutation, given the small sample size. However, an analysis of the clinical and hematological characteristics by mutation was performed in the total study population. Patients with JAK2 mutations had lower platelet counts than those with CALR and triple-negative mutations, findings that were similar to those published in different populations, such as those in Brazil and Thailand 22,25. Patients with JAK2 mutations also presented high hemoglobin and hematocrit levels, are ported in the 2017 WHO guidelines and other studies 10,27,38. However, the leukocyte and lymphocyte counts were lower in patients with CALR mutations than in triple-negative patients. This result is consistent with several populations in Latin America, Korea, and Italy and WHO reports 10,15,17,33,34. Notably, the two patients with rare mutations (double positive for JAK2 and CALR and triple mutation in the CALR) had a diagnosis of essential thrombocythemia and presented low hemoglobin and hematocrit values compared with normal reference values in this patient type. The double-positive patient for JAK2 and CALR also had a low white blood cell count. The diagnosis of essential thrombocythemia in patients with rare mutations has also been reported in other studies of different populations 31,35,39.
Regarding the relationship between hematological characteristics and neoplasms, patients with polycythemia vera presented high hemoglobin and hematocrit values, a finding that is expected in this pathology associated with a hematological phenotype of isolated erythrocytosis because of a predominant alteration of the erythroid lineage. Furthermore, variable hyperplasia of the megakaryocytic/granulocytic lineages was observed 9,10,27,40. Patients with essential thrombocythemia presented a high platelet count independent of their mutational profile, a finding that is consistent with what is reported in WHO guidelines 10,17,22,33,34. Another characteristic of essential thrombocythemia was a higher frequency of female diagnosed patients (13 women vs. 7 men), a finding that has also been reported in other studies 41-43. Finally, patients with primary myelofibrosis presented low hemoglobin levels, coinciding with other findings reported in the literature 4,18 and considered a minor diagnostic criterion in the WHO guidelines 10.
This study has a limitation in the sample size, decreasing the power to detect significant associations and increasing the risk of reporting false positives. However, the variants analyzed have been previously reported, substantially reducing the risk and increasing the reliability of the results. Therefore, future studies with a larger sample size are suggested, such as a prospective multicenter study to perform complete genetic analysis and validate our study results and the usefulness of prognosis in Colombian patients with BCR-ABL1-negative chronic myeloproliferative neoplasms.











 texto en
texto en