Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biosalud
Print version ISSN 1657-9550
Biosalud vol.9 no.1 Manizales Jan./June 2010
EFFECT OF ACETONITRILE CONCENTRATION ON ACYLCARNITINES MEASUREMENT BY TANDEM MASS SPECTROMETRY
EFECTO DE LAS CONCENTRACIONES DE ACETONITRILO EN LA DETERMINACIÓN DE ACILCARNITINAS MEDIANTE ESPECTROMETRÍA DE MASAS EN TÁNDEM
José Henry Osorio1
1 Departamento de Ciencias Básicas de la Salud, Laboratorio de Investigación en Bioquímica Clínica y Patología Molecular, Universidad de Caldas. E-mail: jose.osorio_o@ucaldas.edu.co.
Recibido: mayo 6 del 2010 - Aceptado: junio 10 del 2010
ABSTRACT
Background: the several steps for acylcarnitine analysis by tandem mass spectrometry such as extraction, derivatisation, injection and others, can be influenced by some technical factors improving or impairing the levels of detection.
Objective: the present study evaluated the effect of different concentrations of the acetonitrile used during blood acylcarnitines measurement on the sensitivity obtained during the analysis.
Methodology: prior to acylcarnitine analysis by tandem mass spectrometry, samples were re-dissolved in different acetonitrile in water dilutions [50, 60, 70, 80, 90, and 100% (v/v)] and each single blood specimen was processed five times for each dilution.
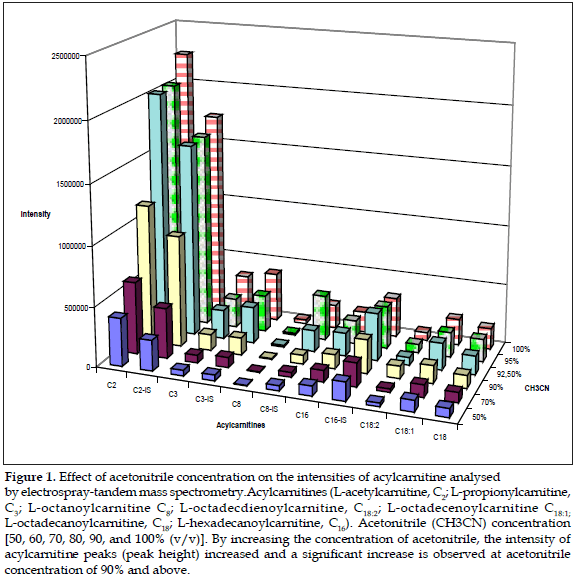
Results: it was observed that by increasing the concentration of acetonitrile the intensity of acylcarnitine peaks significantly increased. However at the highest concentrations of acetonitrile, this reacted with the polystyrene material of the microtitre plates, and resulted in peaks appearing in acylcarnitine profiles, specifically at m/z 302.
Conclusion: a mixture of 70% v/v acetonitrile/water is recommended to use as optimum solvent to dissolve the extracts prior to blood acylcarnitine analysis by tandem mass spectrometry.
KEY WORDS: MS/MS, tandem mass spectrometry.
RESUMEN
Antecedentes: las diferentes etapas que conforman el análisis de acilcarnitinas por espectrometría de masas en tándem, tales como extracción, derivatización, inyección y otras, pueden ser influenciadas por algunos factores técnicos incrementando o disminuyendo los niveles de detección.
Objetivo: el presente estudio evaluó el efecto de la concentración del acetonitrilo utilizado durante la determinación de acilcarnitinas en sangre, sobre la sensibilidad obtenida durante el análisis.
Metodología: antes del análisis de acilcarnitinas por espectrometría de masas en tándem, las muestras fueron disueltas en diferentes disoluciones de acetonitrilo en agua [50, 60, 70, 80, 90, and 100% (v/v)] y cada espécimen de sangre fue procesado 5 veces para cada dilución.
Resultados: se observó que a medida que se incrementan los niveles de acetonitrilo, la intensidad de los picos de acilcarnitinas se incrementan significativamente. Sin embargo, a las concentraciones más altas de acetonitrilo, éste reacciona con el poliestireno de las bandejas, apareciendo picos en el perfil de acilcarnitinas, específicamente a m/z 302.
Conclusión: una mezcla al 70% v/v acetonitrilo/agua, se recomienda para usar como solvente óptimo, para la disolución de los extractos antes del análisis de acilcarnitinas en sangre mediante espectrometría de masas en tándem.
PALABRAS CLAVE: carnitina, acilcarnitinas, espectrometría de masas en tándem.
INTRODUCTION
Mass spectrometry (MS) is basically a sophisticated method for weighing molecules (1). The essential requirement to obtain a mass spectrum is to produce ions in a gas phase, accelerate them to a specific velocity using electric fields, project them into a suitable mass analyser that separates the entities by masses, and finally to detect each charged entity of particular masses sequentially in time as mass spectrometers require charged, gaseous molecules for analysis, and biomolecules are large and polar, they are not easily transferred into the gas phase and ionised (2).
The three main sub-systems in the mass spectrometer device are: the ion source in which the ionisation of the organic molecules takes place; the mass analyser which separates the ions according to their mass/charge (m/z) values; and the detector where relative intensities (abundance) of the separated ions are determined. Very low pressures, i.e. high vacuum, in the region of 10-5 torr are used. This permits the ions to travel from the ion source to the detector virtually unimpeded with the minimal interaction with other gas phase molecules which might otherwise scatter or fragment the ions and cause a reduction in sensitivity. During the ionisation step considerable amounts of energy are imparted to the initially formed molecular ions. The excess of energy causes some of the molecular ions to fragment. The mass spectrometer also measures the masses of all of the charged products of this fragmentation (the so-called fragment ions). A mass spectrum is a snapshot of the abundances of the molecular and fragment ions plotted against their masses. Such a mass spectrum acts as a characteristic molecular fingerprint for individual substances (3).
Electrospray ionisation (ESI) can also be named as soft ionisation technique. The essential principle in this method is that some form of atomisation, or nebulisation, produces a spray of charged liquid droplets. The species to be investigated are solvated on a charged droplet. As the solvent evaporates in the high vacuum region, the droplet size decreases and the charge eventually resides on the entity under study. Liquid containing the analyte (large, highly polar biomolecules) is pumped at low microliterper- minute flow rates (0.1-10 µl/min) through a capillary. Depending on the analytes a high positive or negative voltage (2-5 kV) is applied to the capillary. These droplets move through the atmosphere towards the entrance to the mass spectrometer and generate a cloud of charged analyte molecules (ions) (4), to be analysed.
When tandem mass spectrometry is used, the ions formed can be induced to fragment further by the addition of more internal energy after they leave the source, then a particular peak can be selected for further investigation. The ions comprising this peak are made to undergo further fragmentation, usually by the method known as collisionally induced dissociation (CID) The ions are allowed to interact, and collide with atoms or molecules of an inert gas (helium, neon, argon, or nitrogen). Energy transferred to the ions under investigation can be distributed in a variety of ways (5).
Some of the transferred energy will remain as translational (involved in direction changes, scattering etc.) whilst some will be distributed into vibrational modes of the chemical bonds of the ion It is the latter energy that, if sufficient, can cause further degradation, the products of which can be analysed in another mass analyser. If the gas pressure is too high, all ions will be scattered by collision and none will get through the cell, hence successful CID depends on having a high enough gas pressure so that multiple collisions produce sufficient fragment ions for the following analyser to detect (6).
Carnitine and acylcarnitines contain a quaternary ammonium functional group, making them preformed positive ions (cations) that are polar and non-volatile. Ions produced in the source are selected by MS1 for transmission to the collision cell. The fragments produced after CID are transmitted to MS2 where they are again selected for transmission to the detector. Ions transmitted by MS1 to the collision cell are called precursor ions (commonly referred as "parent" ions), and the fragments produced from CID are product ions (known as "daughter" ions). During the derivatisation process butyl esters of acylcarnitines are formed. These butyl esters are well suited for analysis by MS/MS since they already carry a positive charge and accordingly no additives are needed in the mobile phase. Both butyl esters derivatives and underivatised carnitine and acylcarnitines share a common product ion upon CID, which is singly charged with a mass of 85 Da, corresponding to +CH2-CH=CH-COOH. This fragment results from the loss of elements of both (CH3)3N and C4H8 and the side chain acyl group as RCOOH (7).
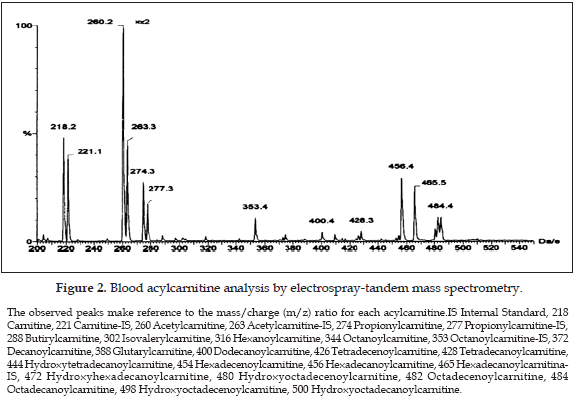
The measurement of acylcarnitines using MS/MS has been reported in whole blood (8), plasma (9), urine (10), amniotic fluid (11), and bile (12). There are several technical factors during samples manipulation which can influence the concentrations obtained, as the obtained chart shows the acylcarnitines peaks, related to an internal standard. During initial experiments it appeared that the choice of solvent for samples injection to the equipment had some effect on the intensities of acylcarnitine measured, showing that acetonitrile was overall a better solvent than methanol, as judged by measuring the mass spectrometry peak intensities (13).
The objective of the present study was to evaluate the effect of the concentration of acetonitrile on acylcarnitine measurement by tandem mass spectrometry.
MATERIAL AND METHODS
All the chemicals used were of analytical grade. Unlabelled acylcarnitine, and deuterated carnitine and acylcarnitines ([d3]C2cn, [d9]C2cn, [d3]C3cn, [d3]C8cn, [d9]C8cn, [d33]C16cn, [d3]C16cn) were obtained from Cambridge isotopes laboratories (Andover, MA, USA). Butanolic HCL was prepared by passing HCL gas through anhydrous n-butanol (Sigma-aldrich Company, Ltd., Poole, UK) for 30 min. The concentration of the acid was determined by back tritiation and adjusted, methanol was obtained from BDH Merck (Merck Eurolab, VWR International, Ltd., Poole UK) and acetonitrile was purchased from Sigma-aldrich Company, Ltd. (Poole, UK).
Blood specimens and card preparation. Blood samples used in this study were from healthy volunteers. Blood was collected into tube containing EDTA (23.5 µmol/tube). Aliquots of 20 µl were spotted on specimen collection filter paper cards (No. 903, 1.88 mm thick; Schleicher and Schuell, Dassel, Germany), dried overnight at room temperature, vacuum sealed and kept in the freezer (-80 °C) until analysis.
Extraction of blood acylcarnitines using microtitre plates (14). Blood spots were punched from the card, (6.35 mm diameter corresponding to 12 µl of whole blood, as described and placed into microtitre plates (96 samples each plate). 100 µl of the internal standard (containing the following labeled acylcarnitines in 100 µl methanol: [d3] cn, 360 pmol; [d3]C2cn, 120 pmol; [d3]C3cn, 24 pmol; [d9]C8cn, 12 pmol; [d9]C16cn, 24 pmol) were added, plus 500 µl of methanol to each sample. The plates were placed on an orbital shaker (setting 750 rpm) for 30 min and then sonicated for 15 min (sonic bath, 175SR). The plates were returned to the shaker for a further 2 hours and sonicated again for another 30 min. The filter discs from the card punch were removed and the resulting eluate was evaporated under air at 45 °C until dry.
Derivatization process and injection of samples to the tandem mass spectrometer. 50 µl of 1 M Butanolic HCl was added to each sample and incubated at 60 °C for 15 min. Samples were immediately returned to the fume cupboard and evaporated under air at 45 °C until dry. To inject samples to the tandem mass spectrometer for acylcarnitine analysis, samples were re-dissolved in 100 µl of the following acetonitrile in water dilutions: 50, 60, 70, 80, 90, and 100% (v/v) (Sigma-Aldrich Company, Ltd., Poole, UK). Each single blood specimen was processed five times.
Tandem mass spectrometry analysis (15). The MS/MS blood analysis for acylcarnitines was performed using the following scan function: parents of m/z 85, scan range 200-500 (m/z), collision energy 25 eV, cone voltage 30 V, scan time 2.0 sec, interscan time 0.1 sec, collision gas Argon, collision gas pressure 1.6-2 x 10-3 mBar. All analyses were performed using a Quattro II, triple quadrupole tandem mass spectrometer (Micromass, Manchester, UK) equipped with an ion spray source (ESI) and a micromass MassLynx data system. The samples were introduced into the mass spectrometer source using a Jasco AS980 autosampler and a Jasco PU980 HPLC pump. For this kind of works the use of selected reaction monitoring for each analyte could provide better quantitiation data, however, analysis for acylcarnitines using parents of m/z 85 is the routine method when analyzing samples from patients, then we adopted this scan function.
Statistical analysis. Statistical comparisons were performed using one-way ANOVA (SigmaStat version 3.1 statistical software), followed by Dunnett's test. P<0.05 was considered significant. According to article 11 on its literal a from resolution number 8430 promulgated by the Colombian health ministry for scientific, technical and administrative rules for research in health, the present study is considered without risk. The study was approved by the correspondent ethical committee.
RESULTS
Results showed that by increasing the concentration of acetonitrile the intensity of acylcarnitine peaks significantly increased; this was observed at acetonitrile concentration of 90% and above, then solutions of 90, 92.5, 95, 97.5, and 100% were prepared and the procedure repeated five times (Figure 1).
It was also observed that at higher concentrations of acetonitrile, this reacted with the polystyrene material of the microtitre plates. The use of microtiter plate made from polypropylene was also investigated. However this resulted in peaks appearing in acylcarnitine profiles, specifically at m/z 302. At the time of this investigation, deep-well microtitre plates were only available from two manufacturers and both gave similar results.
DISCUSSION
The butylester derivatives and underivatized carnitine and acylcarnitines share a common product ion, which is singly charged with a mass of 85 Da, and thus allows the use of MS/ MS for the analysis of these compounds (16), however the methods used for measuring acylcarnitines can be influenced by several factors when samples are obtained or even during the laboratory procedure when samples are analyzed. Recent studies shown that the temperature used during the procedure can affect the carnitine/acylcarnitine ratio (17), but there is no any report in the scientific literature related to the use of different concentrations of acetonitrile prior to injection of samples. It was found that the intensity of acylcarnitine peaks significantly increased at acetonitrile concentration of 90% and above, but this resulted in peaks appearing in acylcarnitine profiles, specifically at m/z 302, this can result in a misinterpretation of the blood acylcarnitine profile as the same ion with an m/z of 302 appears as isovaleryl-/valerylcarnitine parent ion, which is increased in patients suffering of isovaleric acidemia (OMIM 243500) an autosomal recessive disorder of leucine catabolism caused by reduced activity of isovaleryl-CoA dehydrogenase (18). This enzyme catalyzes the conversion of isovalery-CoA to 3-methylcrotonyl-CoA, and a deficiency of this enzyme results in toxic accumulation of isovaleric acid and derivative organic acids. Patients with this condition may have severe overwhelming illness as neonates or, in the chronic intermittent form, exhibit episodic vomiting, ketosis, dehydration, and altered mental status as young children, often associated with the "sweaty feet" odor of isovaleric acid. The main isovaleric metabolites, excreted by these patients are principally isovalerylglycine and isovalerylcamitine (19). Then, in order to avoid potential contamination of the ion source and the transfer line as a result of the adverse reaction between acetonitrile and polystyrene at higher concentrations of acetonitrile, a mixture of 70% v/v acetonitrile/water is recommended to use as optimum solvent to dissolve the extracts prior to analysis by MS/MS.
REFERENCES
1. Rose ME, Johnstone RAW. Mass spectrometry for chemists and biochemists. Cambridge: Cambridge University Press; 1982. p. 1-148. [ Links ]
2. Wilson K. Mass spectrometric techniques. In: Wilson K, Walker J, eds. Practical Biochemistry. 4 ed. Cambridge: Cambridge University Press. 1994. p. 381-424. [ Links ]
3. Harrata AK, Domelsmith LN, Cole RB. Electrospray mass spectrometry for characterization of lipid A from Enterobacter agglomerans. Biol Mass Spectrom 1993;22:59-67. [ Links ]
4. Niwa T. Basic theory of mass spectrometry. Clin Chim Acta 1995; 241-242:15-71. [ Links ]
5. McClellan JE, Murphy JP, Mulholland J, Yost RA. Effects of fragile ions on mass resolution and on isolation for tandem mass spectrometry in the quadrupole ion trap mass spectrometer. Anal Chem 2002;74:402-412. [ Links ]
6. Johnstone RAW, Rose ME. Mass spectrometry for chemist and biochemist. 2 ed. Cambridge: Cambridge University Press; 1994. [ Links ]
7. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem Mass Spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inher Metab Dis 1990;13:321-324. [ Links ]
8. Johnson JV, Lee MS, Lee MR, Yost T. Triple Quadrupole MS/MS in Biomedical Research. Mass Spectrometry in Biomedical Research. 2 ed. New York: Plenum Press; 1986. Chapter 25:459-457. [ Links ]
9. Millington DS, Chace DH. Carnitine and acylcarnitines in metabolic disease diagnosis and management. In: Desiderio DM, ed. Mass spectrometry: clinical and biomedical applications. Vol. I. New York: Plenum Press; 1992. p. 299-316. [ Links ]
10. Libert R, Van Hoof F, Thillaye M, Vincent MF, Nassogne MC, Stroobant V, et al. Identification of new medium-chain acylcarnitines present in urine of a patient with medium-chain acyl-CoA dehydrogenase deficiency. J Inher Metab Dis 1999;22:9-18. [ Links ]
11. Shigmatsu Y, Hata I, Nakai A, Kikawa Y, Sudo M, Tanaka Y, et al. Prenatal Diagnosis of Organic Acidemias Based on Amniotic Fluid Levels of Acylcarnitines. Pediatr Res 1996;39:680-684. [ Links ]
12. Rashed MS, Ozand PT, Bennet MJ, Barnard JJ, Govindaraza DR, Rinaldo P. Inborn errors of metabolism diagnosed in sudden death cases by acylcarnitine analysis of postmorten bile. Clin Chem 1995;41:1109-1114. [ Links ]
13. Millington DS, Norwood DL, Kodo N, Roe CR, Inoue F. Application of fast atom bombardment with tandem mass spectrometry and liquid chromatography/mass spectrometry to the analysis of acylcarnitines in human urine, blood, and tissue. Anal Biochem 1989;180(2):331-9. [ Links ]
14. Stevens RD, Hillman SL, Worthy S, Sanders D, Millington DS. Assay for free and total carnitine in human plasma using tandem mass spectrometry. Clin Chem 2000;46(5):727-9. [ Links ]
15. Kodo N, Millington DS, Norwood DL, Roe CR. Quantitative assay of free and total carnitine using tandem mass spectrometry. Clin Chim Acta 1990;186(3):383-90. [ Links ]
16. Lepage N, Aucoin S. Measurement of plasma/serum acylcarnitines using tandem mass spectrometry. Methods Mol Biol 2010;603:9-25. [ Links ]
17. Osorio JH, Pourfarzam M. Hydrolysis of acylcarnitines during measurement in blood and plasma by tandem mass spectrometry. Acta Bioquim Clin Latinoam 2010;44(2):15-20. [ Links ]
18. Budd MA, Tanaka K, Holmes LM, Efron ML, Crawford JD, Isselbacher KJ. Isovalefic acidemia: clinical features of a new genetic defect of leucine metabolism. N Engl J Med 1967;277:321-4. [ Links ]
19. Osorio JH, Pourfarzam M. Early diagnosis of neurometabolic diseases by tandem mass spectrometry. Cord blood acylcarnitine profile. Rev Neurol 2004;38:11-16. [ Links ]