Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biosalud
Print version ISSN 1657-9550
Biosalud vol.9 no.2 Manizales July/Dec. 2010
EFFECT OF ADDING THE INTERNAL STANDARD TO BLOOD SAMPLES, PRIOR TO THE PREPARATION OF BLOOD SPOTS FOR ACYLCARNITINE ANALYSIS
EFECTO DE LA ADICIÓN DEL ESTANDAR INTERNO A MUESTRAS DE SANGRE ANTES DE LA PREPARACIÓN DE MANCHAS DE SANGRE PARA EL ANALISIS DE ACYLCARNITINAS
José Henry Osorio1 y Morteza Pourfarzam2
1 Universidad de Caldas, Laboratorio de Patología Molecular, Departamento de Ciencias Básicas de la Salud, Manizales, Colombia. E-mail: jose.osorio_o@ucaldas.edu.co.
2 Spence Biochemical Genetics Unit, Royal Victoria Infirmary. Newcastle Upon Tyne, England.
Recibido: septiembre 21 de 2010 - Aceptado: diciembre 26 de 2010
ABSTRACT
Background: some general factors can influence when determining acylcarnitines through tandem mass spectrometry.
Objective: to study the effect of adding the internal standard to blood samples before the preparation of filter paper cards compared with the addition of internal standard after having the filter paper cards prepared for determining acylcarnitines in blood for tandem mass spectrometry.
Methodology: two groups of blood samples were prepared: group one without adding internal standard before the preparation of filter paper cards, and group two adding internal standard prior to the preparation of filter paper cards. Subsequently the acylcarnitines profile was measured using tandem mass spectrometry.
Results: the recovery of acylcarnitines during extraction for the samples which were not added the internal standard before the preparation of the filter paper cards was 48% while for the samples that were added the internal standard the recovery percentage was 96% which shows a significant difference between the two groups.
Conclusion: the ideal extraction process showing the highest acylcarnitines recovery level happens by adding the internal standard to the sample prior to the preparation of filter paper cards. However, for diagnostic purposes, filter paper cards containing samples without internal standard are normally received for the acylcarnitines analysis and, therefore, the method used to add the internal standard after preparing the samples on filter paper without internal standard is appropriate for determining acylcarnitines in blood.
Abbreviations: ESI-MS/MS, Electrospray and tandem mass spectrometry.
KEY WORDS: carnitine, acylcarnitines, tandem mass spectrometry.
RESUMEN
Antecedentes: algunos factores generales pueden influir en la determinación de acilcarnitinas por espectrometría de masas en tándem.
Objetivo: estudiar el efecto de agregar el estándar interno a las muestras de sangre antes de la preparación de las tarjetas en papel de filtro, comparado con la adición del estándar interno luego de preparar las tarjetas en papel de filtro sobre la determinación de acilcarnitinas en sangre por espectrometría de masas en tándem.
Metodología: dos grupos de muestras de sangre fueron preparados: al grupo uno no le fue adicionado el estándar interno antes de la preparación de las tarjetas en papel de filtro, mientras que al grupo dos sí, y posteriormente se midió el perfil de acilcarnitinas por espectrometría de masas en tándem.
Resultados: la recuperación de acilcarnitinas durante la extracción para las muestras a las cuales no se les agregó el estándar interno antes de la preparación de las tarjetas en papel de filtro fue del 48%, mientras que con las muestras a las cuales se les adicionó el estándar interno se obtuvo un porcentaje de recuperación del 96%, observándose una diferencia significativa entre los dos grupos.
Conclusión: el proceso de extracción ideal que presenta el máximo nivel de recuperación de acilcarnitinas se da agregando el estándar interno a las muestras de sangre antes de preparar las tarjetas en papel de filtro. Sin embargo, con fines diagnósticos, las tarjetas de papel de filtro que contienen muestras de sangre sin estándar interno son recibidas para el análisis de acilcarnitinas y, por lo tanto, el método mediante el cual se agrega el estándar interno luego de preparar las muestras en papel de filtro sin estándar interno es adecuado para la determinación de acilcarnitinas en sangre.
Abbreviations: ESI-MS/MS, Electrospray tandem mass spectrometry.
PALABRAS CLAVE: carnitina, acilcarnitinas, espectrometría de masas en tándem.
INTRODUCTION
Carnitine is an endogenous compound with well- established roles in intermediary metabolism. It is important for the mitochondrial oxidation of fatty acids, it is a critical source of energy and also protects the cell from acyl-CoA excretion through the generation of acylcarnitines (1). Methods used to measure carnitine, acetylcarnitine (C2), and total carnitine often involved enzymatic and radioenzymatic assays, whereas measurement of acylcarnitines usually involved HPLC or tandem mass spectrometry (2).
The establishment of new methods for carnitine and acylcarnitine measurement became important as carnitine and acylcarnitines contain a quaternary ammonium functional group making them perform positive ions (cations) that are polar and non-volatile (3, 4, 5). The butylester derivatives and underivatized carnitine and acylcarnitines share a common product ion, which is singly charged with a mass of 85 Da, and thus allows the use of tandem mass spectrometry (MS/MS) for the analysis of these compounds (6), however the methods used for measuring acylcarnitines can be influenced by several factors when samples are obtained or even during the laboratory procedure when samples are analyzed by MS/MS. The present work studies the convenience of the addition of internal standard or not prior to preparation of the samples for MS/MS analysis.
MATERIALS AND METHODS
All the chemicals used were of analytical grade. Unlabelled acylcarnitine, and deuterated carnitine and acylcarnitines ([d3]C2cn, [d9]C2cn, [d3]C3cn, [d3]C8cn, [d9]C8cn, [d3]C16cn, [d3]C16cn) were obtained from Cambridge isotopes laboratories, (Andover, MA, USA). Butanolic HCL was prepared by passing HCL gas through anhydrous n-butanol (Sigma-aldrich Company, Ltd., Poole, UK) for 30 min. The concentration of the acid was determined by back tritiation and adjusted.
Blood specimens and card preparation. Blood samples used in this study were from 10 healthy volunteers. Blood was collected into tube containing EDTA (23.5µmol/tube). Aliquots of 20µl were spotted on filter paper cards (No. 903, 1.88 mm thick; Schleicher & Schuell, Dassel, Germany), dried overnight at room temperature, vacuum sealed and kept in the freezer (-80°C) until analysis.
Extraction of blood acylcarnitines using microtitre plates (7). Blood spots were punched from the card, (6.35 mm diameter, corresponding to 12µl of whole blood) and placed into microtitre plates (96 samples each plate). 100µl of the internal standard (containing the following labeled acylcarnitines in 100µl methanol: [d3]cn, 360pmol; [d3]C2cn, 120pmol; [d3]C3cn, 24pmol; [d9]C8cn, 12pmol; [d9]C16cn, 24pmol) were added, plus 500ml of methanol to each sample. The plates were placed on an orbital shaker (setting 750 rpm) for 30min and then sonicated for 15min (sonic bath. 175SR). The plates were returned to the shaker for a further 2 h and sonicated again for another 30 min. The filter discs from the card punch were removed and the resulting eluate was evaporated under air at 45°C until dry.
Derivatization process and tandem mass spectrometry analysis (8). 50ml of 1 M Butanolic HCl was added to each sample and incubated at 60°C for 15 min. Samples were immediately returned to the fume cupboard and evaporated under air at 45°C until dry and re-dissolved in 100ml of 70% (v/v) acetonitrile in water prior to analysis by ESI -MS/MS. The MS/MS blood analysis for acylcarnitines was performed using the following scan function: parents of m/z 85, scan range 200-500 (m/z), collision energy 25 eV, cone voltage 30V, scan time 2.0 sec., interscan time 0.1 sec, collision gas Argon, collision gas pressure 1.6-2 x 10-3 mBar. All analyses were performed using a Quattro II, triple quadrupole tandem mass spectrometer (Micromass, Manchester, UK) equipped with an ion spray source (ESI) and a micromass MassLynx data system. The samples were introduced into the mass spectrometer source using a Jasco AS980 autosampler and a Jasco PU980 HPLC pump. For this kind of works the use of selected reaction monitoring for each analyte could provide better quantitiation data; however, analysis for acylcarnitines using parents of m/z 85 is the routine method when analyzing samples from patients, then we adopted this scan function.
Study of the effect of adding the internal standard to blood samples. In order to remove the endogenous free carnitine and acylcarnitines from blood specimens, aliquots of blood specimen (1 ml) were placed in the dialysis membrane bags and were placed in PBS solution, pH 7.4 (136.9nM NaCl, 2.8mM KCl, 1.47mM KH2PO4, 8.1mM Na2PO4), (1ml blood/250ml PBS) at 4°C with gently stirring for up to 48 h. Aliquots were removed for analysis at 0, 6, 12, 24, and 48 h, and free carnitine and acylcarnitines were analysed in each specimen. To estimate the effect of adding the internal standard to blood samples, prior to preparation of blood spots or not, ten aliquots of whole blood sample were enriched with acetyl-L-carnitine, octanoyl-L-carnitine, palmitoyl-L-carnitine (C2cn, C8cn, C16cn) (Cambridge isotopes laboratories, Andover, MA, USA), with final expected concentrations of 0, 0.1, 0.2, 1, 2, 5, and 10mM of each acylcarnitine, and cards from these sets were made (group 1). A second group of samples were prepared using the same concentrations of C2cn, C8cn, and C16cn, but also [d9]C2cn, [d3]C8cn and [d3]C16cn with final concentration of 2µM, were added respectively to each one, prior to preparation of dried blood cards.
Cards from these aliquots were prepared. For blood enriched with C2cn, C8cn and C16cn (group 1) the extraction method and the calculations were performed using internal standards added in methanol. For cards containing the acylcarnitines plus [d9]C2cn, [d3]C8cn, and [d3]C16cn (group 2) the extraction was performed using methanol only, and the ratios of C2cn / [d9]C2cn, C8cn / [d3]C8cn, and C16cn / [d3]C16cn were used to calculate the final concentration.
The samples were extracted and analyzed in triplicate and the experiment was repeated on three separate occasions.
Statistical analysis. Statistical comparisons were performed using one-way ANOVA (SigmaStat version 3.1 statistical software), followed by Dunnett's test. P<0.05 was considered significant. According to article 11 on its literal a. from resolution number 8430 promulgated by the health ministry for scientific, technical and administrative guidelines for health research, the present study is considered without risk. The study was approved by the corresponding ethical committee.
RESULTS
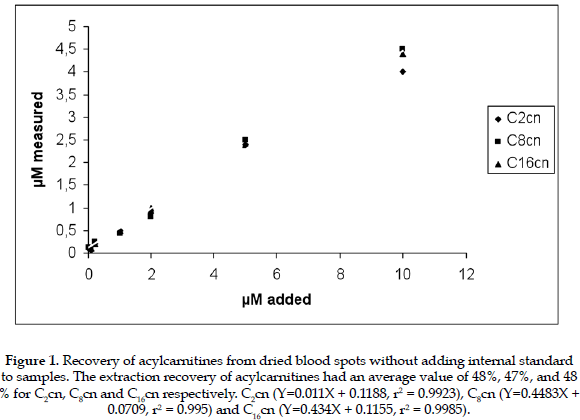
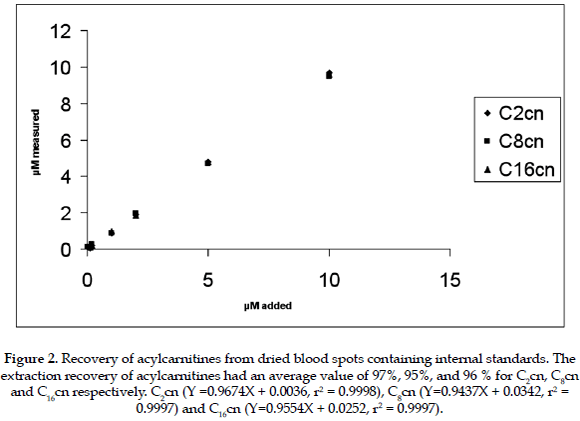
After dialysis of blood samples, it was observed that the longer the dialysis time, the lower the concentration of acylcarnitines in dialyzed blood. From these experiments it appears that it is not possible to prepare blood samples that are completely free from endogenous acylcarnitines (Table 1). The samples prepared however have sufficiently low values to be used for the construction of calibration curves, and for the objective of the present study. It was found a significant difference between the two studied procedures, P value for the F test < 0,05 (0,0174). The extraction recovery of acylcarnitines from dried blood spots using the internal standard had an average value of 48%, 47%, and 48 % for C2cn C8cn and C16cn respectively. For samples in which the internal standard was added to the blood (group 2), prior to the preparations of blood spots, the extraction recovery of acylcarnitines averaged 97%, 95% and 96% for C2cn, C8cn, and C16cn respectively. These results show that the recovery of acylcarnitines from dried blood spots is linear within the concentration range investigated (Figures 1 and 2). In addition the recovery of acylcarnitines is chain-length independent. Although the average recovery was about 48% this was reproducible in a 99% with a coefficient o variance of 2.3.
DISCUSSION
The importance of ESI/MS/MS for the diagnosis of inherited metabolic diseases, and its use for the confirmation of diagnostic errors have been demonstrated (9). A wide range of compounds, i.g. proteins, oligonucleotides, sugars, and polar lipids, can be analysed by ESI-MS/MS; the only requirement is that the molecule be sufficiently polar to allow attachment of a charge (10). For a given compound, the signal strength (peak height in the spectrum) increases linearly with the analyte concentration over a wide range until saturation of the detectors occurs. However, the signal is to a first approximation independent of liquid flow rate, which makes it desirable to operate at the lowest flow rate possible (11).
The linearity and recovery obtained in the present work for acylcarnitines confirm the reliability and reproducibility of the method used. According to the results the ideal extraction process, giving the highest percentage of recovery for acylcarnitines, is by adding the internal standard to the liquid sample. This means that due to the extraction process, approximately only a half of the acylcarnitines can be obtained from the filter paper cards for sample analysis, then the relationship for acylcarnitines from the sample/internal standard when adding the internal standard after sample preparation is approximatel 1/2, compared to a relationship of acylcarnitines from the sample/internal standard, when adding the internal standard during sample preparation of 1/1. There are no reports in the scientific literature related to the use of internal standard added to the samples before preparing the filter paper cards for blood acylcarnitine analysis, and probably it can be ideal to use this method for experimental purposes.
However, other factors like hematocrit and localization of punch in dried blood spots can modify the expected results. It has been reported that total acylcarnitines, free carnitine, some long, medium and short chain acylcarnitines correlate positively with hematocrit levels and in samples with low hematocrit, levels of free carnitine were higher in the peripheral than in the central disk of filter paper (12). The conservation of samples also can influence the metabolite measurements, it is generally accepted that the correct quantification of free carnitine and acylcarnitines in plasma relies on rapid centrifugation of the blood after collection or storage on ice and centrifugation within 1 h (13). Also, it has been reported that plasma samples from whole blood left for 3 h at 37°C and 50°C showed moderate haemolysis compared with those in the "soon", 0°C and 25°C groups. A recent study reports that factors like temperature can influence the measurement of carnitine and acylcarnitine in blood and plasma by tandem mass spectrometry (14).
It can be concluded that the ideal extraction process giving the highest recovery of the acylcarnitines is by adding the internal standards to the sample prior to preparation of dried blood spots cards. However, in the majority of cases this is not practical and for diagnostic and screening purposes it is useful to collect the sample directly on the card before submission to the laboratory.
BIBLIOGRAPHY
1. Strijbis K, Vaz FM, Distel B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life. 2010;62(5):357-62. [ Links ]
2. Osorio JH, Pourfarzam M. Determination of normal acylcarnitine levels in a healthy pediatric population as a diagnostic tool in inherited errors of mitochondrial fatty acid beta-oxidation. An Pediatr (Barc) 2007;67(6):548-52. [ Links ]
3. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem Mass Spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inher Metab Dis 1990;13:321-4. [ Links ]
4. Van Hove JL, Zhang W, Kahler SG, Roe CR, Chen YT, Terada N, et al. Medium chain acyl-CoA dehydrogenase (MCAD) deficiency: diagnosis by acylcarnitine analysis in blood. Am J Hum Gen 1993;52:958-66. [ Links ]
5. Nada MA, Chace DH, Sprecher H, Roe CR. Investigation of beta-oxidation intermediates in normal and MCAD-deficient human fibroblasts using tandem mass spectrometry. Biochem Mol Med 1995;54:59-66. [ Links ]
6. Osorio JH, Pourfarzam M. Early diagnosis of neurometabolic diseases by tandem mass spectrometry. Acylcarnitine profile from cord blood. Rev Neurol 2004;38(1):11-6. [ Links ]
7. Stevens RD, Hillman SL, Worthy S, Sanders D, Millington DS. Assay for free and total carnitine in human plasma using tandem mass spectrometry. Clin Chem 2000;46(5):727-9. [ Links ]
8. Kodo N, Millington DS, Norwood DL, Roe CR. Quantitative assay of free and total carnitine using tandem mass spectrometry. Clin Chim Acta 1990;186(3):383-90. [ Links ]
9. Osorio JH, Pourfarzam M. Diagnostic mistake of neurometabolic mental retardation confirmed by mass sequential spectrometry. Rev Neurol 2000; 30(8): 728-730. [ Links ]
10. An M, Dai J, Wang Q, Tong Y, Ji J. Efficient and clean charge derivatization of peptides for analysis by mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(13):1869-74. [ Links ]
11. Jensen OM, Podtelejnikov A, Mann M. Delayed extraction improves specificity in database searches by matrix-assisted laser desorption/ionization peptide maps. Rap. Commun. Mass Spectr 1996;101:371-78. [ Links ]
12. Holub M, Tuschl K, Ratschmann R, Strnadová KA, Mühl A, Heinze G, et al. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin Chim Acta 2006;373:27-31. [ Links ]
13. Sewell AC. Methods of carnitine analysis. In: Seim H, Löster H, editors. Carnitine-Pathobiochemical Basics and Clinical Applications. Bochum: Ponte Press; 1996. p. 73-84. [ Links ]
14. Osorio JH, Pourfarzam M. Hydrolysis of acylcarnitines during measurement in blood and plasma by tandem mass spectrometry. Acta Bioquim Clin Latinoam 2010;44(2):15-20. [ Links ]