Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biosalud
Print version ISSN 1657-9550
Biosalud vol.11 no.2 Manizales July/Dec. 2012
EVALUACIÓN DE CINCO FUNCIONES DE ESCANEADO PARA EL ANÁLISIS DE CARNITINA EN PLASMA HUMANO POR ESPECTROMETRIA DE MASAS EN TANDEM
José Henry Osorio1
1 Laboratorio de Bioquímica Clínica y Patología Molecular, Departamento de Ciencias Básicas de la Salud, Universidad de Caldas. Correo electrónico: jose.osorio_o@ucaldas.edu.co
Abstract: the determination of carnitine levels through electrospray tandem mass spectrometry using human plasma for the detection of alteration in mitochondrial oxidation of fatty acids and organic acidemias, has been described; however improving precision in the method used for such end is desirable. Materials y methods: carnitine in plasma was measured by tandem mass spectrometry using five different methods: derivatized (4 methods) and underivatized (1 method). Results: no significant difference was observed between the five functions evaluated. Conclusion: any of the functions evaluated can be used to determine carnitine in plasma.
Palabras clave: carnitine, tandem mass spectrometry, analysis.
Introducción: la determinación de los niveles de carnitina mediante el uso de espectrometría de masas en tándem, utilizando plasma humano, para la detección de alteraciones en la oxidación mitocondrial de ácidos grasos y acidurias orgánicas, ha sido descrita, sin embargo es deseable mejorar la precisión de los métodos usados para tal fin. Materiales y métodos: la carnitina en plasma fue analizada mediante espectrometría de masas en tándem mediante 5 diferentes métodos: derivatizando (4 métodos) y sin derivatizar (1 método). Resultados: no se observó diferencia significativa entre las cinco funciones evaluadas. Conclusión: cualquiera de las funciones aquí evaluadas, puede ser utilizada para determinar carnitina en plasma.
Key words: carnitina, espectrometría de masas en tándem, análisis.
The altered b-oxidation is followed by the accumulation of representative acylcarnitines depending on the type of enzymatic deficiency in the pathway (1). However, the production of these acylcarnitines is dependent on the availability of free carnitine in the mitochondria, thus leaving the possibility of having undetectable alterations in acylcarnitine accumulation if the cell becomes carnitine depleted (2). It is necessary to know the occurrence and distribution of free carnitine and acylcarnitines in blood as an indication of unusual metabolic conditions. Therefore the diagnosis and management of these metabolic diseases requires knowledge of both free carnitine and acylcarnitine concentrations.
Carnitine and acylcarnitines contain a quaternary ammonium functional group, making them preformed positive ions (cations) that are polar and non-volatile (3). Ions produced in the source are selected by MS1 for transmission to the collision cell. The fragments produced after CID are transmitted to MS2 where they are again selected for transmission to the detector. Ions transmitted by MS1 to the collision cell are called precursor ions (commonly referred as "parent" ions), and the fragments produced from CID are product ions (known as "daughter" ions) (4). During the derivatization process butyl esters of acylcarnitines are formed. These butyl esters are well suited for analysis by MS/MS since they already carry a positive charge and accordingly no additives are needed in the mobile phase. Both butyl esters derivatives and underivatised carnitine and acylcarnitines share a common product ion upon CID, which is singly charged with a mass of 85 Da, corresponding to +CH2-CH=CH-COOH. This fragment results from the loss of elements of both (CH3)3N and C4H8 and the side chain acyl group as RCOOH. Selective analysis of only those molecules that produce and 85 Da product ion is accomplished using Pre ion scan mode. For the analysis of L-carnitine by MS/MS it is possible to obtain a characteristic fragment of 103 Da derived from the loss of both the trimethylamino group and the elements of C4H8, and both the unlabelled and labelled forms of carnitine as butyl esters exhibit prominent molecular cations at m/z 218 and 221 respectively (5). Some authors use Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry (HILIC), for quantification of total and free carnitine in human Plasma (6), while others used flow injection tandem mass spectrometry (7). In the present work electrospray tandem mass spectrometry is used for plasma carnitine measurement as it was performed before by our group (8) and others (9). At present there is a need for reliable, rapid, and automated procedures for quantitation of free carnitine and individual acylcarnitines from the same sample (10). There are several technical factors which can influence the concentrations obtained in tandem mass spectrometry analysis, as the obtained chart shows the carnitine peak, related to an internal standard. During initial experiments it appeared that the choice of scan function during analysis had some effect on the intensities of carnitine measured (11). Then increasing precision in any measurement method is desired. That is the goal of the present study.
Random blood samples from a normal adult volunteer were collected by venopuncture and centrifuged (2300 g x 5 min) to separate the plasma.
Preparation of a plasma sample free of endogenous carnitine: dialysis membrane bags containing 1 ml of plasma were placed in isotonic Phosphate Buffered Saline (PBS) (pH 7.4) (250 ml PBS / 1 ml sample) for 24 hours at 4ºC and gently stirred (PBS composition: 136.9 mM NaCl, 2.68 mM KCl, 1.47 mM KH2PO4, 8.1 mM Na2HPO4). The PBS solution was changed every 6 hours until 24 hours of dialysis.
Card preparation for free and total carnitine measurement by tandem mass spectrometry: an internal standard volume of 20 l (300 mM [d3]Cn in 50% (v/v) methanol:water) was added to 180 ml of plasma, and the solution was mixed before being left to stand for 15 min. Aliquots of 20 ml from this plasma were spotted on filter paper card, and the cards were put in the fume-cupboard for 60 min to dry. These cards were used for the measurement of free carnitine concentrations.
Plasma sample analysis in microtitre plates: spots were punched from the card (6.35 mm diameter, corresponding to 12 ml of plasma) into microtitre plates and 500 ml of pure methanol was added to each sample. The plates were placed on an orbital shaker (setting 750 rpm) for 30 min, and sonicated for 15 min. The plates were returned to the shaker for a further 2 hours and sonicated again for another 30 min. The discs were removed and the resulting eluate was evaporated under air at 450C until dry. To calculate free and total carnitine concentration with derivatization, the procedure was as follows: 50 ml of 1 M Butanolic HCl was added to each sample and incubated at 60ºC for 15 min. Samples were immediately returned to the fume cupboard and evaporated under air at 45ºC until dry and redissolved in 100 ml of 70% (v/v) acetonitrile in water prior to analysis by ESI -MS/MS. In the infusion mode, the sample is simply introduced into a continuous liquid stream (typically a mixture of organic and aqueous liquid such as the mixture used in the present work: 70:30 acetonitrile: H2O) via an injection. Flow rates are usually between 0.5 and several microliters per minute. For the analysis, a scan range of 210-230 (m/z), and for samples without derivatization, a scan range of 150-170 (m/z). At those scan ranges can be found the parents of the ions of m/z 85 and m/z 103 with derivatization and parents of m/z 85 without derivatization respectively. The ratio value for carnitine/internal standard intensities was obtained and multiplied by 30 mM after smoothing the peaks twice (peak width 0.80 Da- Savitzky Golay method), centering (min peak width at half height 2 channels, centroid top 90%) without background subtraction.
Tandem mass spectrometry instrumentation and analysis: all the analyses were performed using a Quattro II, triple quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with an ion source (ESI) and a micro mass MassLynx data system. The samples were injected into the mass spectrometer using a Jasco AS980 auto sampler and a Jasco PU980 HPLC pump (Jasco International Co., Tokyo, Japan). To evaluate the linearity of the ion intensity ratio relative to carnitine concentration, samples of 24 hours dialysed plasma specimens were enriched with L-carnitine at concentrations of 5, 10, 20, 40, 60, 80, and 100 mM, and cards for free carnitine analysis by MS/MS were prepared. Each sample was extracted and derivatized in triplicate as described above. In addition one set of standards was extracted but not derivatized.
Derivatized samples were analysed using four different scan functions as follows:
1: Parents of 85 Da, scan range 210-230 (m/z), collision energy 25 eV, cone 30 V, scan time 2.0 sec, interscan time 0.1 sec.
2: Parents of 103 Da, scan range 210-230 (m/z), collision energy 15 eV, cone 30 V, scan time 2.0 sec, interscan time 0.1 sec.
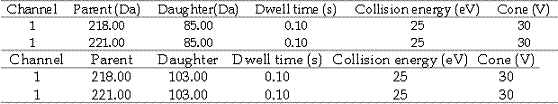
3: Multiple Reaction Monitoring of two mass pairs:
Samples without derivatization were analysed using the following scan function:
4: Multiple Reaction Monitoring of two mass pairs:
5: Parents of 85, scan range 150-200 (m/z), collision energy 25 eV, cone 30 V, Scan time 2.0 sec, interscan time 0.1 sec.
To expose any possible differences between the scan functions, the results of measuring 20 samples using each scan function were compared by student t-test.
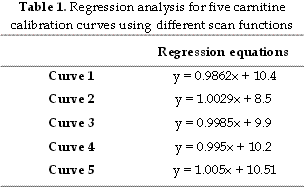
Standard curves for free carnitine (from 0 to 100 mM) are shown in Figure 1. The correlation coefficient values for the 5 scan functions were all close to 1 and the best correlation was observed using parent ions of m/z 85 for the derivatized samples. The correlation coefficient values for the scan functions with and without derivatization were analysed by regression analysis although in the presence of a very marginal difference. No significant difference was found at any significance level. Regression analysis for the five carnitine calibration curves using different scan functions is shown in Table 1.
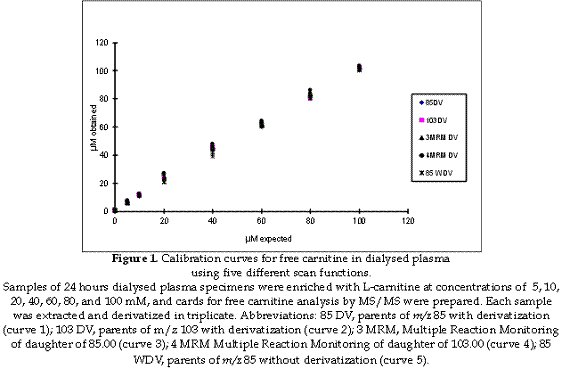
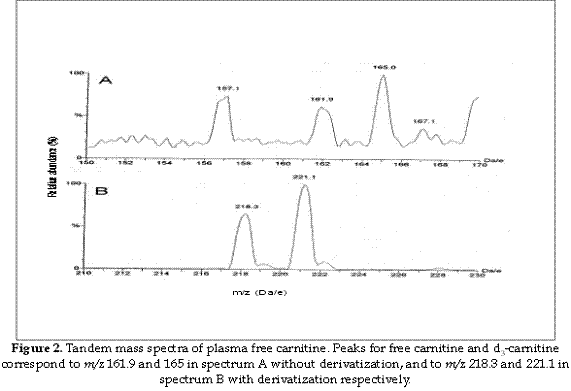
The determination of carnitine levels by electrospray tandem mass spectrometry using dried blood spot in filter paper, for the detection of defects in fatty acid oxidation and organic acidaemias, has been described (12-14), the butylester derivatives and underivatized carnitine and acylcarnitines share a common product ion, which is singly charged with a mass of 85 Da, and thus allows the use of MS/MS for the analysis of these compounds (15), however the methods used for measuring carnitine and acylcarnitines can be influenced by several factors when samples are obtained or even during the laboratory procedure when samples are analysed (16). It has been shown that the temperature used during the procedure can affect the carnitine/acylcarnitine ratio when measured by tandem mass spectrometry (17), or that increasing concentrations of acetonitrile during measurement increased significantly the peaks observed (18). Others works analysed the influence of the anticoagulant used during sampling (19) or the effect of adding the internal standard to samples, prior to the preparation of cards for analysis (20), but there is no any report in the scientific literature related to the comparison of different scan functions for carnitine measurement when using tandem mass spectrometry. Typically, MS/MS analysis of carnitine has utilized an acid-catalyzed reaction with butanol to form butyl esters. This process is not only time consuming, but the acidic reaction conditions promote the hydrolysis of acylcarnitines, and can result in an overestimation of the carnitine concentration. Then according to the results of the present work, the underivatized process can be recommended, as it does not require pre- or post-analytical derivitization. Furthermore, the assay is sensitive, robust, and amenable to high throughput formats as total sample preparation time is less than 5 min. However it is important to remark that the main advantage of derivatizing samples is that peaks in the spectrum are most clear as the chemical noise is minor, and the spectrum is cleaner (Figure 2). Using Multiple Reaction Monitoring (MRM) can have advantages as it is a mode by which data is recorded in MS/MS. Instead of scanning a mass range both analysers (MS1 and MS2) are programmed to record the current of one or several ions with selected m/z values, hence increasing the sensitivity. Dwell time is the length of time that the mass spectrometer monitors a selected ion.
1. Osorio JH. Patología molecular de los errores hereditarios de la B-oxidación mitocondrial de los ácidos grasos: alcances en el diagnóstico y tratamiento. Biosalud 2006; 5:71-83. [ Links ]
2. Osorio JH. Carnitine a very important osmolite for mitocondrial fatty acid oxidation. Biosalud 2006; 5:85-92. [ Links ]
3. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem Mass Spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inher Metab Dis 1990; 13:321-324. [ Links ]
4. Millington DS, Chace DH. Carnitine and acylcarnitines in metabolic disease diagnosis and management. In: Desiderio DM, ed. Mass spectrometry: clinical and biomedical applications. Vol. I. New York: Plenum Press, New York; 1992. p. 299-316. [ Links ]
5. Kodo N, Millington DS, Norwood DL, Roe CR. Quantative assay of free and total carnitine using tandem mass spectrometry. Clin Chim Acta 1989; 186:383-390. [ Links ]
6. Sowell J, Fuqua M, Wood T. Quantification of Total and Free Carnitine in Human Plasma by Hydrophilic Interaction Liquid Chromatography Tandem Mass Spectrometry. J Chromatogr Sci 2011; 49(6):463-8. [ Links ]
7. Johnson DW. An acid hydrolysis method for quantification of plasma free and total carnitine by flow injection tandem mass spectrometry. Clin Biochem 2010; 43(16-17):1362-7. [ Links ]
8. Osorio JH, Pourfarzam M. Plasma free and total carnitine measured in children by tandem mass spectrometry. Braz J Med Biol Res 2002; 35:1265-1271. [ Links ]
9. Hardy DT, Preece MA, Green A. Determination of plasma free carnitine by electrospray tandem mass spectrometry. Ann. Clin. Biochem 2001; 38: 665-670. [ Links ]
10. Osorio JH, Loango N, Landazuri P. El tamizaje metabólico en los errores innatos del metabolismo. Biosalud 2008; 7:131-40. [ Links ]
11. Osorio JH, Pourfarzam M. Implementación y estandarización de un método para la determinación de carnitina en plasma como herramienta diagnóstica de enfermedades hereditarias. Arch Med (Manizales) 2008; 8(2):126-133. [ Links ]
12. Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid B-oxidation. J Inherit Metab Dis 2010; 33(5):469-77. [ Links ]
13. Osorio JH, Pourfarzam M. Early diagnosis of neurometabolic diseases by tandem mass spectrometry. Acylcarnitine profile from cord blood. Rev Neurol. 2004; 38(1):11-6. [ Links ]
14. Thompson JW, Zhang H, Smith P, Hillman S, Moseley MA, Millington DS. Extraction and analysis of carnitine and acylcarnitines by electrospray ionization tandem mass spectrometry directly from dried blood and plasma spots using a novel autosampler. Rapid Commun Mass Spectrom. 2012; 26(21):2548-54. [ Links ]
15. Lepage N, Aucoin S. Measurement of plasma/serum acylcarnitines using tandem mass spectrometry. Methods Mol Biol 2010; 603:9-25. [ Links ]
16. Osorio JH. Uses of tandem mass spectrometry as a novel tool for research and diagnosis of intermediary metabolism diseases. Biosalud 2007; 6(1):161-74. [ Links ]
17. Osorio JH, Pourfarzam M. Hydrolysis of acylcarnitines during measurement in blood and plasma by tandem mass spectrometry. Acta Bioquim Clin Latinoam 2010; 44(2):15-20. [ Links ]
18. Osorio JH. Effect of acetonitrile concentration on acylcarnitines measurement by tandem mass spectrometry. Biosalud 2010; 9(1):9-16. [ Links ]
19. Osorio JH. Effect of anticoagulant type during the blood sample collection process on the determination of the acylcarnitine profile in blood and plasma. Biosalud 2010; 9(2):9-13. [ Links ]
20. Osorio JH, Pourfarzam M. Effect of adding the internal standard to blood samples, prior to the preparation of blood spots for acylcarnitine analysis. Biosalud 2010; 9(2):14-20. [ Links ]













