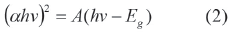Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Iteckne
Print version ISSN 1692-1798
Iteckne vol.10 no.1 Bucaramanga Jan./June 2013
Synthesis and characterization of TiO2 thin films doped with copper to be used in photocatalysis
Síntesis y caracterización de películas delgadas de TiO2 dopadas con cobre para ser usadas en fotocatálisis
Carlos Enrique Díaz-Uribe1, William Andrés Vallejo Lozada2, Fernando Martínez Ortega3
1 Dr. Química Universidad Industrial de Santander. Docente Tiempo Completo, Investigador Grupo de Investigación en Fisicoquímica Aplicada y Estudios Ambientales, Laboratorio de Fotoquímica y Fotobiología, Universidad del Atlántico. Barranquilla, Colombia. carlosdiaz@mail.uniatlantico.edu.co.
2 Dr. Ciencias Químicas Universidad Nacional. Docente Tiempo Completo, Investigador Grupo de Investigación en Fisicoquímica Aplicada y Estudios Ambientales, Laboratorio de Fotoquímica y Fotobiología, Universidad del Atlántico. Barranquilla, Colombia. williamvallejo@mail.uniatlantico.edu.co.
3 Dr. Chimie Université de Poitiers, Docente Tiempo Completo, Investigador Centro de Investigaciones en Catálisis. Universidad Industrial de Santander. Bucaramanga, Colombia. fmartine@uis.edu.co.
ABSTRACT
In this work we studied the influence of incorporation of copper into TiO2 thin films on structural, optical and surface properties of TiO2 thin films. The as-grown TiO2 was synthesized by sol gel method using titanium isopropoxide, and the TiO2 thin films were deposited by spin coating method. TiO2 copper-doped (Cu:TiO2) was synthesized by impregnation method using Cu(NO3).H2O as source of Cu(II), the Cu:TiO2 thin films were deposited by spin coating method. The properties of the compounds obtained were evaluated by measurements of X-ray diffraction (XRD) and diffuse reflectance.
The XRD results showed that Cu doping change the crystalline phase radio of the films, XRD pattern of TiO2 indicated that films grow with anatase structute, while Cu:TiO2 thin films presented a polycrystalline mixture of anatase/rutile. Reflectance analysis indicated that TiO2 presents an energy band gap of 3.25 eV and the Cu:TiO2 presents a shift-red of the band gap to 2,9 eV. The results suggest that doping with copper improved the harvesting of the TiO2 to visible radiation.
KEYWORDS: TiO2, Photocatalysis, metal doping, X-Ray, Diffuse Reflectance.
1. INTRODUCTION
Nowadays the uncontrolled growth of the population increases both the water consumption and the amount of pollutants in the water resources; water treatment is an important research topic around the world. The development of mechanisms of water treatment is a necessity because water is not a renewable resource [1]. In the last decades, advanced oxidation processes (AOPs) have presented different kinds of methodologies for remediation of water and heterogeneous photocatalysis have become a promising method for purification. In this field Titanium oxide (TiO2) is one of the most important photocatalytic materials. There currently exists a better understanding and improvement of catalytic reactions, which is a main driving force for surface investigations on TiO2. However, two drawbacks limit the practical application of TiO2 technology: (a) it is effective only under ultraviolet irradiation (λ < 380 nm) and (b) the low-quantum efficiency of this process [2]. To solve these, different methodologies have been used: sensitization with organic dyes, natural and synthetic [3], metal ion implantation [4] nobel metal loading [5], metal ion doping [6], anion doping [7], composite semiconductors [8]. All of these present both advantages and drawbacks, however, metal ion doping is one of the most promising because it shifts the TiO2 responses towards longer wavelengths and an enhanced photoactivity is obtained from incorporation of metallic dopants. These advantages can be explained because a dopant ion acts as an electron trap or hole trap; this could prolong the lifetime of the generated charge carriers, resulting in an enhancement of photocatalytic activity [9, 10]. The ionic metallic doping could be done through different ways such as hydrotermal precipitation [11], sol-gel [12], chemical vapor deposition [13], impregnation method [14] and sputtering [15]. The impregantion method has proven to be convenient for the modification of TiO2 due to its low cost of implementation, the low synthesis temperatures, and because it easily allows the coating large areas.
In this work, we studied the influence of incorporation of Cu into TiO2 on its structural, optical properties.
II. EXPERIMENTAL
A. Materials synthesis
The as-grown TiO2 Thin films were synthesized by the sol-gel method for the as-grown TiO2 synthesis, the titanium isopropoxide [Ti(OC2H5)4] was used as reagent, ethanol (CH3CH2OH) and water were used as solvent and nitric acid (HNO3) was used as buffer. The molar ratio was [CH3CH2OH:H2O:HNO3:Ti(OC2H5)4] 55:1,5:0,3:1 respectively. The Cu:TiO2 powder was obtained by adding copper (II) nitrate and titanium tetrachloride as source of copper and titanium respectively; furthermore, HCl was used as a buffer solution, a solution of cetyltrimethyl ammonium bromide (CATBr) was used as surfactant and water-ethanol mix was used as solvent in the following molar ratio [Ti:CATBr:HCl:H2O:EtO:Cu] 1:0,16:1,7:1,7:20:0,025 respectively. The asgrown TiO2 and the Cu:TiO2 thin films were deposited on the substrates from a coating solution by spin-coating technique in a nitrogen atmosphere. The substrates coated with films were annealed at 450ºC for 1 hour in air.
B. Characterization of materials synthesized
The X-ray powder diffraction (XRD) patterns were recorded in X-ray powder diffractometer (MSAL-XDII) using Kα radiation of the Cu (λ =0,15406nm) for operating at a 30 kV voltage with a current of 20 mA. The optical properties of the as-grown TiO2 and the Cu:TiO2 thin films were studied through diffuse reflectance measurements. The diffuse reflectance absorption spectrum of the as-grown TiO2 and the Cu:TiO2 thin films were measured using a Lambda 4 Perkin Elmer spectrophotometer equipped with an integrating sphere. Kubelka-Munk model and analysis based on differential reflection spectra were used to independently determine the energies of the fundamental optical transitions. FT-IR spectra (KBr) of the compounds were recorded on a Bruker Tensor 27 spectrometer in the spectrum region of 3500-500 cm-1.
III. RESULTS AND DISCUSSION
A. Diffuse transmittance measurements
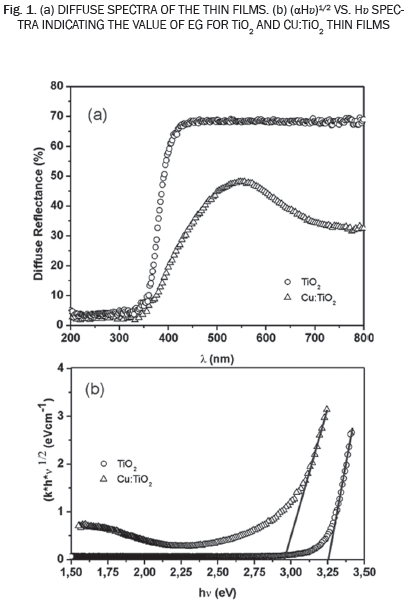
The optical properties of the as-grown TiO2 thin film and Cu:TiO2 thin films were determined from diffuse reflectance measurements in the range of 200-800 nm. Fig. 1(a) shows the diffuse reflectance spectra of the as-grown TiO2 thin films, The results indicate that about 70% of the visible radiation is reflected in the visible range (after 350nm). Furthermore, a sharp absorption edge is observed near to the 340 nm, indicating the good crystallinity and a low defect density near to the band edge. This behavior is typical of thin films of TiO2 [16]. The results of diffuse reflectance spectra were analyzed with Kubelka-Munk remission function, given by the equation below [17]:
Where Rα is the reflectance and F(Rα) is proportional to the constant absorption of the material, an indicative of the absorbance of the sample in a particular wavelength. The optical band gap of the films was determined by extrapolating the linear portion of the (αhv)2 versus hv plot on the x-axis [18].
Where Eg is the band gap energy and A is a constant depending on the transition probability.
Fig. 1(b) shows the (αhv)2 versus hv for the as grown TiO2 thin films and Cu:TiO2 thin films.
It is observed that the band gap of as-grown TiO2 thin film was 3,25 eV, which corresponds to the typical value of energy of the anatase TiO2; this result is according to XRD measurements presented afterwards. The results also show a shift of absorption band edge towards visible region upon doping TiO2 with copper, Cu:TiO2 thin films present a band gap energy of 2,9 eV. These results suggest that copper could be incorporated into the crystalline TiO2 network modifying its band structure and therefore its electrical properties. According to the optical results, it can be assumed that Cu-doping onto TiO2 may enhance the visible-light absorption and it could improve the photocatalytic activity of TiO2.
B. XRD measurements
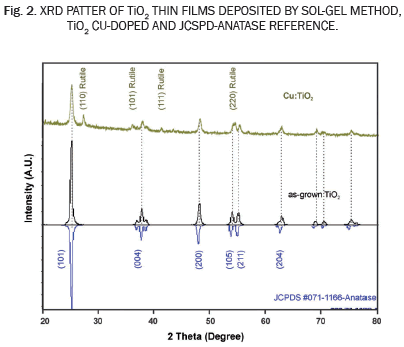
Fig. 2 shows experimental XRD pattern corresponding to as-grown TiO2 thin films and the Cu:TiO2 thin films deposited on soda lime glass substrates by spin coating. The XRD measurements show that as-grown TiO2 thin films were polycrystalline and present only one crystalline phase corresponding to the anatase phase (Fig. 1 includes a JCPDS-#071-1166 pattern of reference), the pattern of as-grown TiO2 thin films presents different planes of growth and all diffraction signals correspond to the anatase-pattern indicating that only one crystalline phase is present. Furthermore, XRD results showed that the as-grown TiO2 thin films grow in a preferential orientation in the crystalline plane (110), typical of the anatase phase. These results could occur due to the method used to obtain the compound, according to other reports [19]. Fig. 2 also shows the XRD pattern of the Cu:TiO2. The diffraction pattern shows three additional diffraction signals at 2θ=27,9, 2θ=37,9, 2θ=42,4, 2θ=56,9; these reflections can be associated with the planes (110), (101), (111) and (220) respectively. These crystalline planes can be associated with the rutile phase (JCPDS #021-1276); this happens because the rutile phase is thermodynamically the most stable crystalline phase and it possesses the highest density with a compact atomic structure. The presence of Cu is a disadvantage for the formation of the metastable anatase phase and so the rutile growth can occur [20]. Furthermore, not a signal associated with CuO or compund of Cu is observed, indicating that the compound could be amorfous or that it could be incorporated in the crystalline network of the doped TiO2. However, the change in the way of the crystalline growth indicates that the Cu participates in the growth of the TiO2 and it could be incorporated in the crystalline network of the final compoud according to other reports. This assertion is comfirmed for optical results [21].
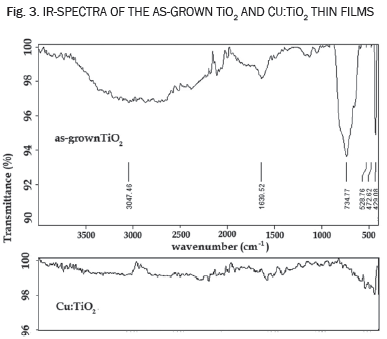
C. IR measurements
Fig. 3 shows the IR-spectra of the as-grown TiO2 films annealed at 500°C. The chemical bonding of the powders was scrutinized by correlating the developed peaks in the spectrum to the vibration or stretching of various functional groups. Results show two strong absorption signals in the frequency region of 429,1 cm-1 and 734,7 cm-1 corresponds to Ti-O-Ti bonding and indicates the formation of a titanium oxide network [22], furthermore, a broad band at 3400 cm-1 is observed, which is characteristic of associated hydroxyl groups (absorbed molecular water), weakly chemisorbed and disappearing at temperatures of 200°C; a corresponding weak bending vibration band at near 1630 cm-1 is also observed [23]. Finally, fig 3 shows the IR spectra of the Cu:TiO2 thin films. In these spectra the intensity of the signals of TiO2 decrease significantly, indicating that water has been desorbed and not a signal associated to the stretching mode of Cu-O is detected, which demonstrates that copper could have been incorporated into the TiO2 network as proved by the optical results.
III. CONCLUSIONS
Thin films of TiO2 were doped with copper and the optical and structural properties were investigated. The optical results indicated that TiO2 doped with copper presents a red-shift of the transmittance spectra increasing the absorption of the photocatalyst in the visible region. The band gap increased by about 12% from 3.25 eV TiO2 to 2,9 eV TiO2 doped with copper. The XRD analysis showed that TiO2 grows in the anatase phase while thin films of TiO2 doped with copper present a polycrystalline mixture of anatase, rutile, and brookite. Results indicated that TiO2 doped with copper can be used as an active photocatalyst in a visible range of the electromagnetic spectra.
ACKNOWLEDGEMENTS
Authors are grateful to the Laboratory of XRD in the Parque Tecnológico Guatiguará of the Universidad Industrial de Santander, Bucaramanga, Colombia.
REFERENCES
[1] K. Nakata, A. Fujishima. TiO2 photocatalysis: Design and Applications. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 13 (2012) 169-189. [ Links ]
[2] U. Diebold. The Surface Science of titanium dioxide. Surface science reports 48 (2003) 53229. [ Links ]
[3] C.C. Chen, C.S. Lu, Y.C. Chung, J.L. Jan. UV light induced photodegradation of malachite green on TiO2 nanoparticles. Journal of Hazardous Materials, Volume 141, Issue 3, 22 March 2007, Pages 520-528. [ Links ]
[4] H. Yamashita, M. Harada, J. Misaka, H. Nakao, M. Takeuchi, M. Anpo. Application of ion beams for preparation of TiO2 thin film photocatalysts operatable under visible light irradiation: Ionassisted deposition and metal ion-implantation. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 206 (2003) 889-892. [ Links ]
[5] X. Li, Z. Zhuang, W. Li, H. Pan. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Applied Catalysis A: General 429-430 (2012) 31-38. [ Links ]
[6] Y. Gurkan, E. Kasapbasi, Ze. Cinar. Enhanced solar photocatalytic activity of TiO2 by selenium(IV) ion-doping: Characterization and DFT modeling of the surface. Chemical Engineering Journal, 214 (2013) 34-44. [ Links ]
[7] Y. Chen, X. Cao, B. Lin, B. Gao. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies. Applied Surface Science, 264 (2013) 845-852. [ Links ]
[8] S. Chin, E. Park, M. Kim, G. Bae, J. Jurng. Synthesis and visible light photocatalytic activity of transition metal oxide (V2O5) loading on TiO2 via a chemical vapor condensation method. Materials Letters 75 (2012) 57-60. [ Links ]
[9] A. Zajac, M. Radecka, M. Jasinski, K.A. Michalow, M. Rekas, E. Kusior, K. Zakrzewsk, A. Heel, T. Graule, K. Kowalski. Influence of Cr on structural and optical properties of TiO2: Cr nanopowders prepared by flame spray synthesis. Journal of Power Sources 194 (2009) 104-111. [ Links ]
[10] S. Ghasemi, S. Rahimnejad, S. R. Setayesh, S. Rohani, M.R. Gholami. Transition metal ions effect on the properties and photocatalytic activity of nanocrystalline TiO2 prepared in an ionic liquid. Journal of Hazardous Materials 172 (2009) 1573-1578. [ Links ]
[11] H. Dang, X. Dong, Y. Dong, Y. Zhang, S. Hampshire. TiO2 nanotubes coupled with nano-Cu(OH)2 for highly efficient photocatalytic hydrogen production. International Journal of Hydrogen Energy 38 (2013) 2126-2135. [ Links ]
[12] K. Wilke, H.D. Breuer. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. A: Chem. 121 (1999) 49-53. [ Links ]
[13] H.A. Foster, D.W. Sheel, P. Sheel, P. Evans, S. Varghese, N. Rutschke, H.M. Yates. Antimicrobial activity of titania/silver and titania/copper films prepared by CVD. Journal of Photochemistry and Photobiology A: Chemistry 216 (2010) 283-289. [ Links ]
[14] U.G. Akpan, B.H. Hameed. The advancements in sol-gel method of doped-TiO2 photocatalysts. Applied Catalysis A: General 375 (2010) 1-11. [ Links ]
[15] W. Zhang, Y. Li, S. Zhu, F. Wang. Copper doping in titanium oxide catalyst film prepared by dc reactive magnetron sputtering. Catalysis Today, 93-95 (2004) 589-594. [ Links ]
[16] C. Tsai, H. Hsi, T. Kuo, Y. Chang, J. Liou. Preparation of Cu-Doped TiO2 Photocatalyst with Thermal Plasma Torch for Low-Concentration Mercury Removal. Aerosol and Air Quality Research DOI: 10.4209/aaqr.2012.07.0196. In press. [ Links ]
[17] P. Pongwan, B. Inceesungvorn, K. Wetchakun, S. Phanichphant, N. Wetchakun. Highly Efficient Visible-Light-Induced Photocatalytic Activity of Fe-doped TiO2 Nanoparticles. Engineering Journal. DOI: 10.4186/ej.2012.16.3.143. in press. [ Links ]
[18] J. Tauc (F. Abeles ed.), Optical Properties of Solids, North-Holland (1972). 19-92. [ Links ]
[19] A. Cesnovar, P. Paunovic, A. Grozdanov, P. Makreski, E. Fidancevska. Preparation of nanocrystalline TiO2 by sol-gel method using titanium tetraisopropoxide (TTIP). Advanced Natural Science: Theory and Applications 01/2012; 1(2):133-142. [ Links ]
[20] A. K. Ray, S. M. Tracey, B. McQuillin, S. N. B. Hodgson, IEEE Proc. Sc., Meas. Technol. 147(6), 301 (2000). [ Links ]
[21] B. Xu, L. Dong, Y. Chen, J. Chem. Soc., Faraday Trans. 94 (13) (1998) 1905. [ Links ]
[22] M. Burgos, M. Langlet. The sol-gel transformation of TIPT coatings: a FTIR study. Thin Solid Films Vol. 349 (1999) 19. [ Links ]
[23] P. M. Kumar, S. Badrinarayanan, M. Sastry. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: correlation to presence of surface states. Thin Solid Films 358 (2000) 122-130. [ Links ]