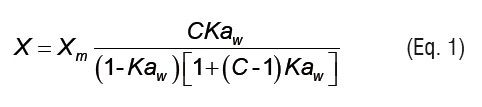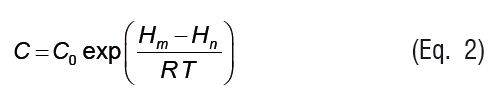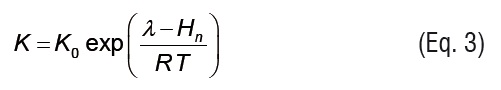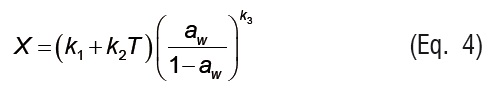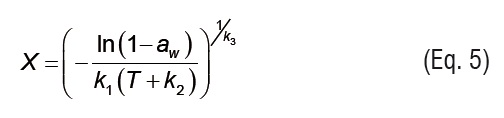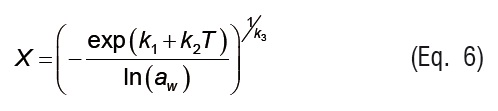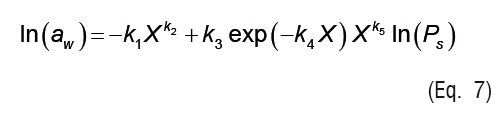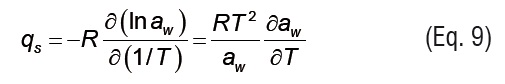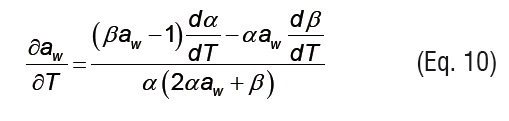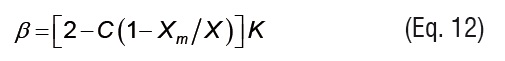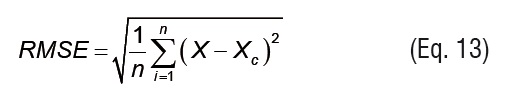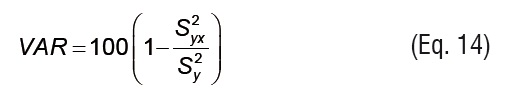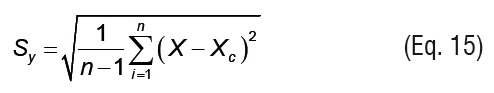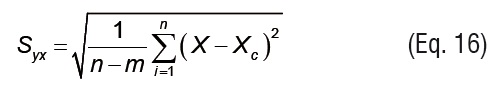Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biotecnología en el Sector Agropecuario y Agroindustrial
Print version ISSN 1692-3561
Rev.Bio.Agro vol.10 no.2 Popayán July/Dec. 2012
MODELING SORPTION ISOTHERMS AND ISOSTERIC HEAT OF SORPTION OF MANGO PULP cv. TOMMY ATKINS
MODELADO DE LAS ISOTERMAS DE SORCIÓN Y DEL CALOR ISOSTÉRICO DE SORCIÓN EN PULPA DE MANGO cv. TOMMY ATKINS
MODELAGEM DE ISOTERMAS DE SORÇÃO E CALOR ISOSTÉRICO DE SORÇÃO DE POLPA DE MANGA cv. TOMMY ATKINS
1 Grupo de Análisis y Simulación de Procesos Agroalimentarios ASPA, Departamento de Tecnología de Alimentos, Universidad Politécnica de Valencia, Cno de Vera s/n, 46071, Valencia, Spain.
2 Facultad de Ingeniería Agronómica, Universidad del Tolima, B. Santa Helena A.A. 546, Ibagué, Colombia
Correspondencia: havaquiro@ut.edu.co
Recibido para evaluación: 30/08/2011. Aprobado para publicación: 18/04/2012
ABSTRACT
Mango is an agricultural product of great economic importance for various developing countries, which has increased demand for its nutritional properties and exotic characteristics. The relationship between moisture content and water activity provides useful information for mango processing, especially for drying and storage operations. Water activity and moisture content of mango pulp (Mangiferaindica L. cv. Tommy Atkins) were analyzed to determine the sorption isotherms. The water activity was measured using an electric hygrometer at five different temperatures (10, 20, 30, 40 and 50 °C) and wide ranges of moisture content (2.829–0.031 kg kg-1 d.b.) and water activity (0.977–0.215). One theoretical (GAB) and four empirical equations (Oswin, Henderson, Halsey and Ratti) were used for modeling the sorption isotherms. After following several criteria to evaluate the models, the GAB model appeared as the best option to represent the hygroscopic equilibrium of mango pulp (RMSE = 0.057, VAR = 0.996). The sorption isotherms behaved as expected in agro-foodstuffs and similarly with the mango pulp of other cultivars. The isosteric heat of sorptionwas computed using the Clausius-Clapeyron equation by means of the GAB model.A decrease in the isosteric heat of sorption was observed when the equilibrium moisture content increase, with a strong moisture effect at values lower than 0.8 kg kg-1 (d.b.).
KEYWORDS: Hygroscopic equilibrium, Water activity, Mathematical modeling, Thermodynamic properties.
RESUMEN
El mango es un producto agrícola de gran importancia para diferentes países en vías de desarrollo, el cual ha incrementado su demanda por sus propiedades nutricionales y características exóticas. La relación entre el contenido de humedad y la actividad de agua suministra información útil para el procesamiento del mango, especialmente en las operaciones de secado y almacenamiento. La actividad de agua y el contenido de humedad de la pulpa de mango (Mangifera indica L. cv. Tommy Atkins) fue analizada para determinar las isotermas de sorción. La actividad de agua fue medida utilizando un higrómetro eléctrico a cinco diferentes temperaturas (10, 20, 30, 40 y 50 °C) y amplios rangos de contenido de humedad (2.829–0.031 kg kg-1 b.s.) y actividad de agua (0.977–0.215). Un modelo teórico (GAB) y cuatro ecuaciones empíricas (Oswin, Henderson, Halsey y Ratti) fueron utilizados para representar las isotermas de sorción. Después de considerar diferentes criterios, el modelo de GAB fue evaluado como la mejor opción para representar el equilibrio higroscópico de la pulpa de mango (RMSE = 0.057, VAR = 0.996). Las isotermas mostraron un comportamiento acorde a los materiales agroalimentarios y similar al reportado para pulpa de mango de otras variedades. El calor isostérico de sorción fue calculado utilizando la ecuación de Clausius-Clapeyron a partir del modelo de GAB. Se observó una disminución en el calor isostérico de sorción al aumentar el contenido de humedad de equilibrio, con gran efecto de la humedad a valores por debajo de 0.8 kg kg-1 (b.s.).
PALABRAS CLAVE: Equilibrio higroscópico, Actividad de agua, Modelización matemática, Propiedades termodinámicas.
RESUMO
A manga é um produto agrícola em países de desenvolvimento econômico, com grande aceitação devido a suas propriedades nutricionais e características exóticas. A relação entre o conteúdo de umidade e atividade de água proporciona informação útil para o processamento do mango, especialmente nas operações de secagem e armazenamento. A atividade de água e o conteúdo de umidade da polpa de manga (Mangifera indica L. cv. Tommy Atkins) foi analisada para determinar as isotermas de sorção. A atividade de água foi medida empregando um higrômetro elétrico a cinco diferentes temperaturas (10, 20, 30, 40 e 50 °C) e amplas faixas de conteúdo de umidade (2.829 – 0.031 kg kg-1 b.s.) e atividade de água (0.977 – 0.215). O modelo de teórico de GAB e os modelos empíricos de (Oswin, Henderson, Halsey e Ratti) foram empregados para modelar as isotermas de sorção. Depois de considerar diferentes critérios, o modelo de GAB apresentou a melhor opção para representar o equilíbrio higroscópico da polpa de manga (RMSE = 0.057, VAR = 0.996).As isotermas mostraram um comportamento acorde aos materiais agroalimentícios e similar ao reportado para polpa de manga de outras variedades. O calor isostérico de sorção foi calculado utilizando a equação de Clausius-Clapeyron a partir do modelo de GAB. Observou-se uma diminuição no calor isostérico de sorção quando aumentou o conteúdo de umidade de equilíbrio, com um grande efeito da umidade em valores abaixo de 0.8 kg kg-1 (b.s.).PALAVRAS-CHAVE: Equilíbrio higroscópico, Atividade de água, Modelagem matemática, Propriedades termodinâmicas.
INTRODUCTION
Worldwide, the mango (Mangiferaindica L.) occupies the third place within the tropical fruits as to production and commercial levels. It plays an important economic role for several countries, mainly inthose in developing ones [1, 2]. Additionally to its nutritional contributions of dietary fiber and A-vitamin, its exotic characteristics have led an increase in the demand for products obtained from its processing [3, 4].
The water activity and sorption properties of foods provide information which is useful for operations such as drying, mixing, packaging and storage. Foodstuffs present an inherent relationship between equilibrium moisture content and water activity, known as a sorption isotherm, which is dependent on the structure and composition of the food material, as well as pressure and temperature [5]. A sound knowledge of sorption isotherms helps not only to explain the water sorption mechanism and interaction between food components and water, but also to estimate the energy requirements of drying processes and also to provide information about the state of water within the dried product [4, 6-14]. Due to the complexity of foodstuffs, there is no one model that fits all the cases well. As a consequence, there have been many proposed models, and it is interesting to check how well some of them fit the experimental data. Among the most commonly used is the GAB model, with a sound theoretical basis, and others which are more empirical: the Oswin, the Henderson, the Halsey and the Ratti models.
Literature provides reports on sorption isotherms for different mango varieties: [4], for Nam Dok Mai, [15], for Manila [9], for Haden cultivars.[4], determined moisture sorption isotherms using a dynamic method at temperatures from 30 to 50 °C and water activity from 0,11 to 0,97. [9], used a static gravimetric method at temperatures from 30 to 70°C and water activities from 0,02 to 0,97. In these studies, the GAB model with temperature dependent parameters was used to represent sorption isotherms and presented accurate fitting results. [15], reported a modification of the Henderson model without taking into account the temperature effect.
The isosteric heat of sorption is a measurement of the energy required to break the intermolecular attractive forces between the molecules of moisture vapor and the material surface [6]. Knowing the isosteric heat of sorption is important for the drying process. It can be used to determine the energy requirements and provide information on the state of water within the dried product. The level of moisture content of a material at which the isosteric heat of sorption reaches the evaporation enthalpy of water is often considered as an indicator of 'bound-water' existing in the food [4].
The aims of this work were to provide experimental sorption isotherm data of mango cv. Tommy Atkins at various temperatures, to test empirical and theoretical sorption models, and to assess the isosteric heat of sorption.
METHOD
Experimental set up. Fresh, ripe mangoes (Mangiferaindica L. cv. Tommy Atkins) of Brazilian origin were obtained from a local market in Valencia (Spain). The initial moisture content, according to AOAC method 934,06 [16], was 85,9 ± 1,7 % (wet basis). In order to select fruits at a similar stage of ripeness, soluble solids (AOAC method 932,14C) and titratable acidity (AOAC method 934.06) were considered (°Brix = 11.3 ± 0,8, acidity = 0,72 ± 0,06 % malic acid) [16].
Dried mango samples were prepared and analyzed according to the methodology used by [13], to determine sorption isotherms. Thus, pulp was separated from the peel and kernel of the mango fruits and mixed in a food grinder (Type D56, Moulinex, Seb Group, France). Thin layers of the ground samples were dried at 70 °C using an air forced tray oven (Model FD115 Oven Forced Air, Binder, Tuttlingen, Germany). The dried pulp was taken out from the oven at different times in order to reach a wide range of moisture contents. The dried material was again ground, wrapped in a plastic film and put in glass jars for 48 hours at refrigeration temperature (∼4°C) in order to ensure sample homogenization before taking measurements of water activity and moisture content.
The water activity of each sample was measured using a standardized conductivity hygrometer (model AW Sprint TH 500, Novasina, Pfäffikon, Switzerland) at five different temperatures (10, 20, 30, 40 and 50 °C). The equipment was previously calibrated, according to the calibration procedure of the equipment manufacturer, using the following salts: LiCl, MgCl2, Mg(NO3)2, NaCl, BaCl2 and K2Cr2O7.
When each sample was placed in the hygrometer, the water activity in equilibrium with the sample moisture content was measured in triplicate from the lowest to the highest temperature. This procedure provides a rapid and reliable means of measuring water activities and avoids degenerative changes in the sample, such as when it takes a long time to attain equilibrium [10].
Once water activity was measured, sample moisture content was determined in triplicate.
The arithmetic average of the replicates for both water activity and moisture content were used for data analysis.
Using this methodology, eleven experimental points (water activity/moisture content) were obtained for each temperature. Data ranged from 0,031 to 2,829 kg kg-1 d.b. for moisture contents, and 0,215 to 0,977 for water activities.
Modelingsorption isotherms. In order to model the water sorptionbehavior of mango, one theoretical (GAB) and four empirical equations (the Oswin, the Henderson, the Halsey and the Ratti) were used [13, 17, 18]. These models have been frequently used to describe isotherms of foodstuffs and biological materials [6-8, 10, 11].
The Guggenheim-Anderson-de Boer(GAB) theoretical model (Eq. 1) is an adequate representation of the experimental data to be found in the range of water activity which is of most practical interest in foods. The GAB equation has been recommended by the European Project Group COST90 (European Cooperation in Scientific and Technical Research) as the fundamental equation for the characterization of water sorption of food materials [13, 17-19]:
where X is the equilibrium moisture content (kg kg-1 d.b.), Xm is the monolayer equilibrium moisture content (kg kg-1 d.b.), and aw is the water activity. K and C are GAB model parameters that can be written as temperature dependent functions using Arrhenius type relationships:
Where, C0 and K0 are GAB model parameters, Hm is the monolayer sorption heat (kJ mol-1), Hn is the multilayer sorption heat (kJ mol-1), is the evaporation enthalpy of water(kJ mol-1), T is the absolute temperature (K), and R is the universal gas constant (kJ mol-1 K-1).
In Equations 4 to 7, ki are the parameters of empirical isotherm models, andPs is the saturation vapor pressure.
Temperature effect is introduced in Eq. 7 through the vapor pressure term (Ps), which was calculated from ASAE Standards [20].
Isosteric heat of sorption. The isosteric heat of sorption (Qs, kJ mol-1) represents the addition of the net isosteric heatof sorption (qs) to the evaporation enthalpy of water (λ) (Eq. 8) [21]. To assess the isosteric heat, the net isosteric heat of sorption was computed using the Clausius-Clapeyron equation (Eq. 9), and the ASAE Standards [20] were used to estimate the evaporation enthalpy of waterin relation with the temperature. To compute the Clausius-Clapeyron equation, the GAB model (Eqs. 1-3) fitted with the experimental data was used to determine the analytical first derivative of the awwith respect to T (Eqs. 10-12) [22].The errors associated with experimental work, also linked to natural variability, constitute the main disadvantage of this technique as compared to those based on calorimetric methods [23], which are a good alternative if proper instrumentation is available [24, 25].
where α and β are written as dependent functions of the GAB parameters:
In addition to isosteric heat of sorption, important thermodynamic properties, as the Gibbs free energy and the differential entropy [26, 27], can be estimated directly from the analytical solution of Clausius-Clapeyron equation. This direct procedure avoids the uncertainty associated with linearization used by the conventional procedures [12, 24, 28].
Parameter estimation and statistical analysis. Model parameters were estimated by fitting the equations to experimental data using 'nlinfit' function of the Statistic Toolbox of Matlab® 7.1 (The MathWorks Inc., Natick, MA, USA). This function uses the Gauss-Newton algorithm with Levenberg-Marquardt modifications that iteratively reweights response data and recomputes a least-square fit of a non-linear model.
Root mean squared error and explained variance statistics were used to evaluate the accuracy of fit. Root mean squared error (RMSE) (Eq. 13) is a measure of the standard error of the random component in the estimation. Explained variance (VAR) (Eq. 14) indicates the proportion of variance that is accounted for by the model.
The total standard deviation (Sy) and the standard deviation of the estimation (Syx) are defined by Eqs.15 and 16, respectively.
where m is the number of model parameters, n is the number of data, and Xc is the estimated equilibrium moisture content (kg kg-1 d.b.).
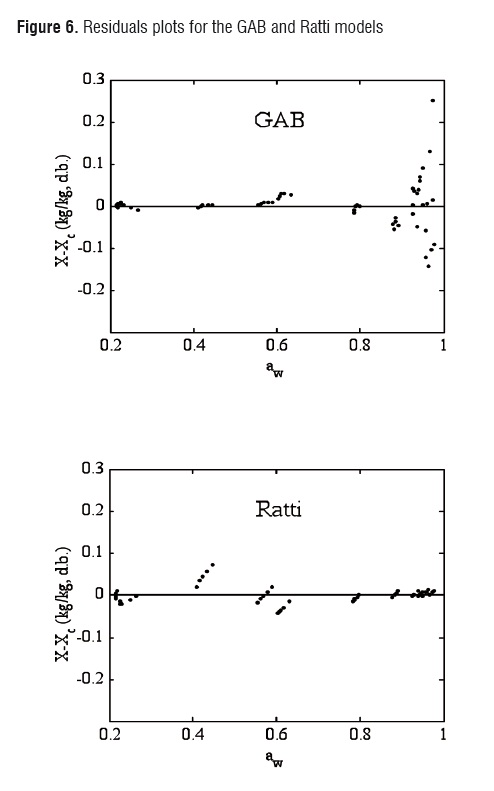
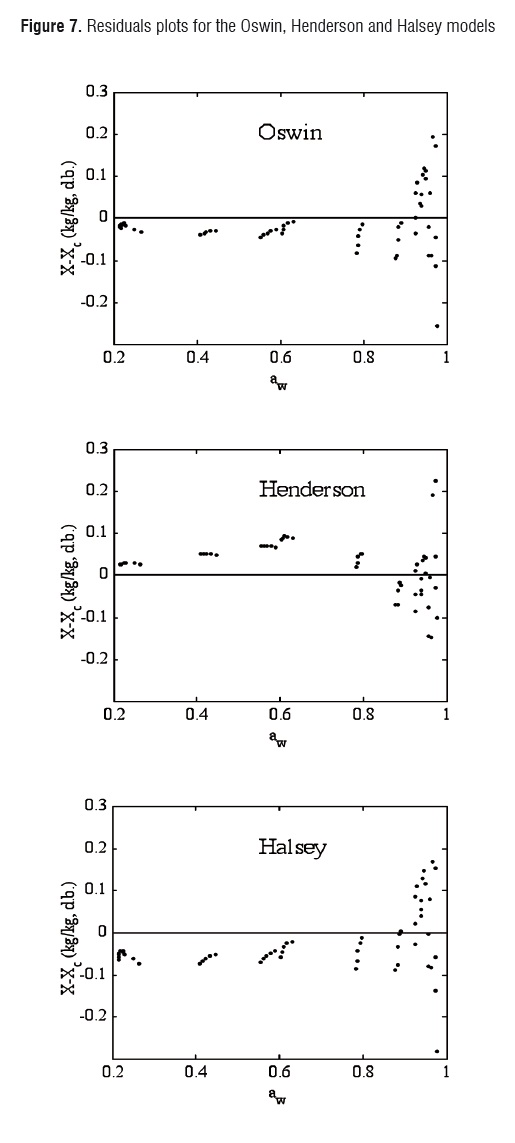
Plotting residuals (X - Xc) against the independent variable (aw) may also contribute to evaluate the closeness goodness of the fit [29]. If the model fits well, then the residuals should only be random independent errors with a zero mean [30].
RESULTS
Modeling sorption isotherms. Five isotherm models (Eqs. 1-7), which consider the influence of temperature, were tested to represent the sorption isotherm data. Estimated model parameters and statistical results are shown in Table 1.
The comparisons between the experimental and estimated data of the isotherms at 10 and 50 °C for the GAB, Oswin, Henderson, Halsey and Ratti models are shown in Figures 1, 2, 3, 4 and 5, respectively.
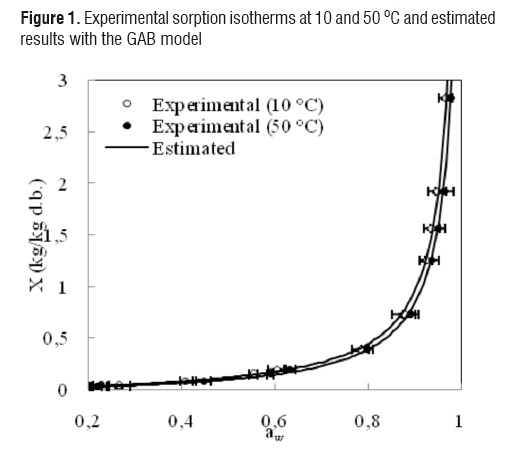
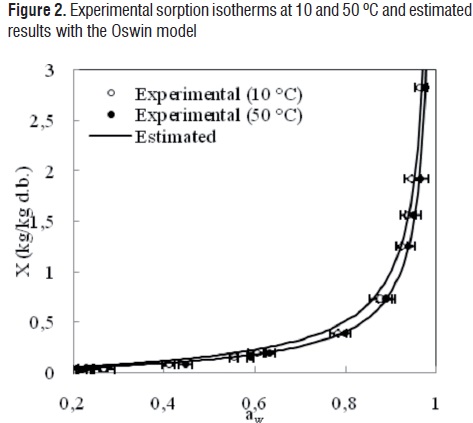
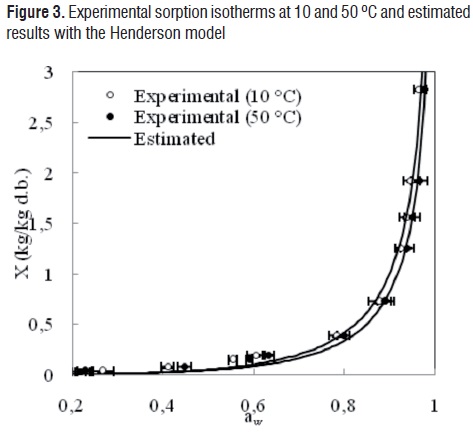
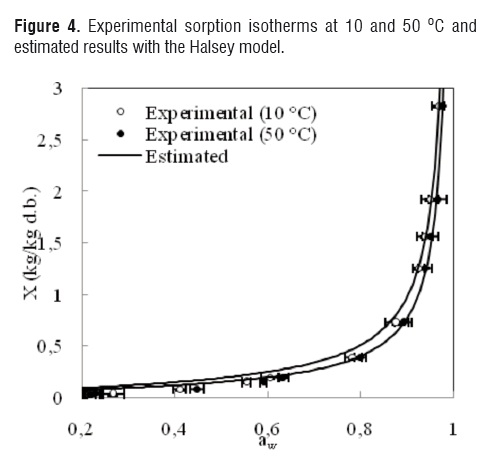
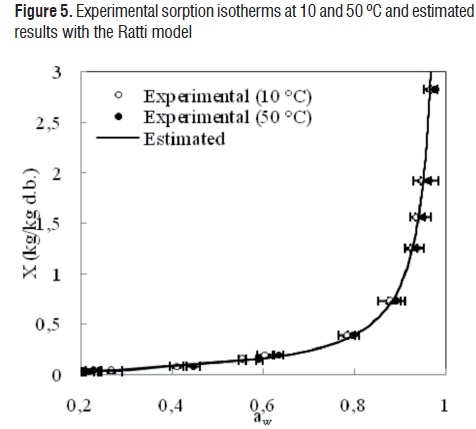
Although the agreement between experimental and estimated data was satisfactory (RMSE< 0.09, VAR> 0.99), the GAB and Ratti models presented the highest explained variance, the lowest average square root, and random residual distribution (Figure 6). The Oswin, Henderson, and Halsey methods showed patterned residuals (Figure 7). The usefulness of the models may also be assessed by considering the number of parameters involved. In order to model the sorption isotherms of mango cv. Tommy Atkins, it seems that the GAB model was the best option. It not only provided a high explained variance and a random residual distribution, but also useful information may be extracted from its parameters.Although the Ratti model showed good statistical results, the trend provided by this equationwas not able to represent the temperature effect on the experimental data.
The observed behavior is typical of foodstuffs, since water activity increases as temperature rises at constant moisture content [4, 8-11, 25]. This indicates that mango pulp becomes less hygroscopic when temperature is increased due to the increase in kinetic energy associated with the water molecules of the material [4]. The sorption isotherms of mango behaved in a way characteristic of foods rich in soluble components [8, 10, 11].
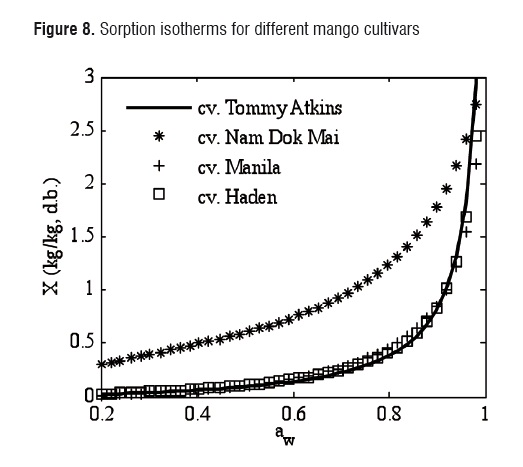
The estimated sorption isotherms for different mango cultivars are compared in Figure 8. A GAB isotherm was used at 50°C for Tommy Atkins, Nam Dok Mai [4], and Haden [9] cultivars. A modified Henderson model was used for Manila [15] cultivar, but without taking the temperature effect into account. The sorption isotherm values of Tommy Atkins, Haden and Manila cultivars were very similar. The Nam Dok Mai cultivar was very different from the other ones, which could be due to variety characteristics or the physiological state of the fruits.
Isosteric heat of sorption. Isosteric heats of sorption at temperatures of 10 and 50°C are shown in Figure 9. Isosteric heat values figures decreased as the moisture content increased, and were close to the evaporation enthalpy at moisture contents of over 0.8 kg kg-1 d.b.
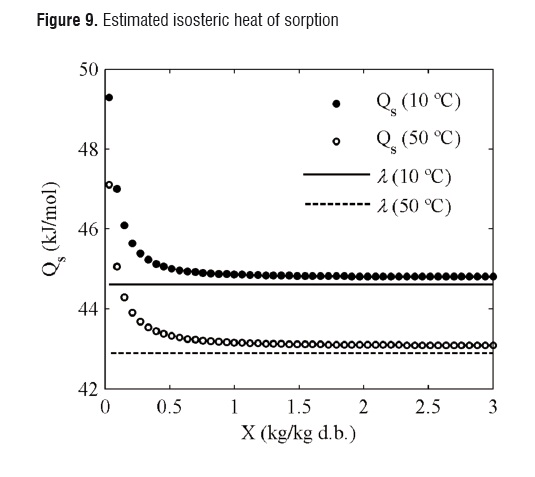
This trend is similar to those reported by Janjai et al., 2007 [4], and Telis-Romero et al., 2005 [9], for Nam Dok Mai and Haden mango cultivars.
The high isosteric heat figures at low equilibrium moisture contents indicate strong interactions of water-food components [4, 17]. The increase in the sorption heat at low moisture contents is explained by the existence of highly active polar sites on the material surface. Such sites are filled when the moisture content increases, resulting in lower figures of isosteric heat [4, 13].
CONCLUSIONS
The equilibrium moisture content decreases when temperature increases at constant water activity. Among the theoretical (GAB) and the empirical equations (Oswin, Henderson, Halsey and Ratti) used for modelingsorption isotherms, the GAB model was considered the best equation for describing sorption isotherms of mango cv. Tommy Atkins, due to the high explained variance attained, the physical meaning of its parameters and the fact that it presented a random residual distribution. The isotherms behaved in a way typical of foods rich in soluble components.
The isosteric heat of sorption, assessed by using the Clausius-Clapeyron equation and the GAB model, was found to decrease as the moisture content rise, being close to the evaporation enthalpy of waterat moisture contents of over 0.8 kg kg-1 d.b. The trends of the sorption isotherms and isosteric heat were similar to those reported for Manila and Haden mango cultivars.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support of MCyT project (ref: AGL2006-14146-C02-01).
REFERENCES
[1] FAO. Current situation and medium-term outlook for tropical fruits [Online]. Available: http://www.fao.org/es/esc/en/15/217/highlight_218.html. [cited 2011 Feb 25] [ Links ].
[2] FAO. FAO statistical databases and data-sets FAOSTAT [Online]. Available: http://faostat.fao.org/site/406/default.aspx. [cited 2011 Feb 25] [ Links ].
[3] MANEEPUN, S. and YUNCHALAD, M. Developing processed mango products for international markets. Acta Hort (ISHS), 645, 2004, p. 93-105. [ Links ]
[4] JANJAI, S., BALA, B.K., TOHSING, K., MAHAYOTHEE, B., HAEWSUNGCHARERN, M. and MÜLLER, J. Moisture sorption isotherms and heat of sorption of mango (Mangifera Indica L. cv. Nam Dok Mai). Int Agr Eng J, 16 (3-4), 2007, p. 159-168. [ Links ]
[5] MULET, A., GARCÍA-PASCUAL, P., SANJUÁN, N. and GARCÍA-REVERTER, J. Equilibrium isotherms and isoteric heats of morel (Morchela esculenta). J Food Eng, 53 (1), 2002, p. 75-81. [ Links ]
[6] AL-MUHTASEB, A.H., MCMINN, W.A.M. and MAGEE, T.R.A. Moisture sorption isotherm characteristics of food products: a review. Food Bioprod Process, 80 (2), 2002, p. 118-128. [ Links ]
[7] KAYMAK-ERTEKIN, F. and GEDIK, A. Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. LWT-Food Sci Technol, 37 (4), 2004, p. 429-438. [ Links ]
[8] RIZVI, S.S.H. In: Engineering Properties of Foods. Thermodinamic properties of food in dehydration. 3 edn. New York, USA: CRC Press, 2005, p. 133-214. [ Links ]
[9] TELIS-ROMERO, J., KOHAYAKAWA, M.N., SILVEIRA JR, V., PEDRO, M.A.M. and GABAS, A.L. Enthalpy-entropy compensation based on isotherms of mango. Ciencia Tecnol Alime, 25 (2), 2005, p. 297-303. [ Links ]
[10] SAHIN, S. and SUMNU, S.G. Physical properties of foods. USA: Springer, 2006, 257 p. [ Links ]
[11] BASU, S., SHIVHARE, U.S. and MUJUMDAR, A.S. Models for sorption isotherms for foods: a review. Dry Technol, 24 (8), 2006, p. 917-930. [ Links ]
[12] CHEN, C. Obtaining the isosteric sorption heat directly by sorption isotherm equations. J Food Eng, 74 (2), 2006, p. 178-185. [ Links ]
[13] GARCÍA-PÉREZ, J.V., CÁRCEL, J.A., CLEMENTE, G. and MULET, A. Water sorption isotherms for lemon peel at different temperatures and isosteric heats. LWT-Food Sci Technol, 41 (1), 2008, p. 18-25. [ Links ]
[14] VEGA-GÁLVEZ, A., LÓPEZ, J., MIRANDA, M., DI SCALA, K., YAGNAM, F. and URIBE, E. Mathematical modelling of moisture sorption isotherms and determination of isosteric heat of blueberry variety O'Neil. Int J Food Sci Tech, 44 (10), 2009, p. 2033-2041. [ Links ]
[15] HERNÁNDEZ, J.A., GARCÍA, M.A., TRYSTRAM, G. and HEYD, B. Neural networks for the heat and mass transfer prediction during drying of cassava and mango. Innov Food Sci Emerg, 5 (1), 2004, p. 57-64. [ Links ]
[16] AOAC. Official methods of analysis of AOAC International. 16 edn. Gaithersburg, USA: Association of Official Analytical Chemist International AOAC, 1997. [ Links ]
[17] EIM, V.S., ROSSELLÓ, C., FEMENIA, A. and SIMAL, S. Moisture sorption isotherms and thermodynamic properties of carrot. Int J Food Eng, 7 (3), 2011, p. Article 13. [ Links ]
[18] BELLAGHA, S., SAHLI, A., GLENZA, A. and KECHAOU, N. Isohalic sorption isotherm of sardine (Sardinella aurita): Experimental determination and modeling. J Food Eng, 68 (1), 2005, p. 105-111. [ Links ]
[19] TIMMERMANN, E.O., CHIRIFE, J. and IGLESIAS, H.A. Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? J Food Eng, 48 (1), 2001, p. 19-31. [ Links ]
[20] ASAE. 1999. Psychrometric data, D271.2 DEC99. American Society of Agricultural Engineers ASAE, USA. [ Links ]
[21] PERDOMO, J., COVA, A., SANDOVAL, A.J., GARCIA, L., LAREDO, E. and MULLER, A.J. Glass transition temperatures and water sorption isotherms of cassava starch. Carbohyd Polym, 76 (2), 2009, p. 305-313. [ Links ]
[22] VILLA-VÉLEZ, H.A., VÁQUIRO, H.A., BON, J. and TELIS-ROMERO, J. Modelling thermodynamic properties of banana waste by analytical derivation of desorption isotherms. Int J Food Eng, 8 (1), 2012, p. [ Links ]
[23] CORREA, P.C., GONELI, A.L.D., JUNIOR, P.C.A., DE OLIVEIRA, G.H.H. and VALENTE, D.S.M. Moisture sorption isotherms and isosteric heat of sorption of coffee in different processing levels. Int J Food Sci Tech, 45 (10), 2010, p. 2016-2022. [ Links ]
[24] MULET, A., GARCÍA-REVERTER, J., SANJUÁN, R. and BON, J. Sorption isosteric heat determination by thermal analysis and sorption isotherms. J Food Sci, 64, 1999, p. 64-68. [ Links ]
[25] CLEMENTE, G., BON, J., BENEDITO, J. and MULET, A. Desorption isotherms and isosteric heat of desorption of previously frozen raw pork meat. Meat Sci, 82 (4), 2009, p. 413-418. [ Links ]
[26] SIMAL, S., FEMENIA, A., CASTELL-PALOU, Á. and ROSSELLÓ, C. Water desorption thermodynamic properties of pineapple. J Food Eng, 80, 2007, p. 1293-1301. [ Links ]
[27] TELIS, V.R.N., GABAS, A.L., MANEGALLI, F.C. and TELIS-ROMERO, J. Water sorption thermodynamic properties applied to persimmon skin and pulp. Thermochim Acta, 343, 2000, p. 49-56. [ Links ]
[28] SÁNCHEZ, E., SANJUÁN, N., SIMAL, S. and ROSSELLÓ, C. Calorimetric techniques applied to the determination of isosteric heat desorption for potato. J Sci Food Agric, 74 (1), 1999, p. 57-63. [ Links ]
[29] KALEEMULLAH, S. and KAILAPPAN, R. Moisture sorption isotherms of red chillies. Biosyst Eng, 88 (1), 2004, p. 95-104. [ Links ]
[30] PAGANO, A.M. and MASCHERONI, R.H. Sorption isotherms for amaranth grains. J Food Eng, 67 (4), 2005, p. 441-450. [ Links ]