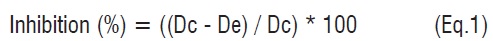Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biotecnología en el Sector Agropecuario y Agroindustrial
Print version ISSN 1692-3561
Rev.Bio.Agro vol.12 no.1 Popayán Jan./June 2014
BIOCATALYTIC PRODUCTION OF PERILLYL ALCOHOL USING WHOLE CELLS OF Aspergillusniger DSM 821
PRODUCCIÓN BIOCATALÍTICA DE ALCOHOL PERÍLICO UTILIZANDO CÉLULAS COMPLETAS DE Aspergillus niger DSM 821
PRODUÇÃO DE ÁLCOOL PERÍLICO BIOCATALÍTICA USAR CÉLULAS COMPLETOS Aspergillus niger DSM 821
GLORIA ASTRID PRIETO S.1, YANNETH AIDE PEREA V.2, CLAUDIA CRISTINA ORTIZ L.3*
1 Universidad Pedagógica y Tecnológica de Colombia, Escuela de Ciencias Químicas.Química PhD. Tunja, Colombia.
2 Universidad Industrial de Santander, Escuela de Química.Química PhD. Bucaramanga, Colombia.
3 Universidad Industrial de Santander, Escuela de Bacteriología y Laboratorio Clínico. Microbióloga PhD. Bucaramanga, Colombia.
Correspondencia: ortizc@uis.edu.co
Recibido para evaluación: 25-09-2012. Aprobado para publicación: 28-04-2014.
ABSTRACT
In this research, biotransformation of (R)-(+)-limonene for production of perillyl alcohol (POH) at lab scale was studied using whole cells of Aspergillus niger DSM 821. Effects of fungal growth phase, inductive effect of the substrate, pH, type of biotransformation medium and (R)-(+)-limonene concentration on both biotransformation selectivity and yield were evaluated. Using Malt Extract Agar (MEA) as culture medium at 28°C, it was possible to achieve higher specific growth rate of the fungi. Moreover, by using of Malt Yeast Extract Broth (MYB) and 12 mM of (R)-(+)-limonene as substrate in a liquid reaction medium at pH 5,0, it was obtained 246 mg/L of POH. This POH production was 1,9 and 3,1 times higher than yields obtained in liquid medium constituted by Yeast and Malt Extract, Peptone and Glucose (YMPG) and Broth Yeast Extract and Glucose (YG), respectively. Higher concentrations of POH (405 mg/L) were obtained by adding 50 mM of limonene in the exponential phase (at 72 h) of A. niger DSM 821 grown in MYB at pH 5,0, 28°C, 300 rpm and 6 days of biotransformation. Other by-products, such as: limonene-1,2-diol, linalool, carvone, phenyl ethanol and ethyl esters of palmitic, oleic and linoleic acids, were also obtained.
KEYWORDS: Biotransformation, Bioconversion, (R)-(+)-limonene, Aspergillus niger.
RESUMEN
En esta investigación se estudió la producción de alcohol perílico (AP) mediante biotransformación del (R)-(+)-limoneno utilizando células completas de Aspergillus nigerDSM 821 a escala de laboratorio. Para tal fin se determinóla influencia de diferentes variables sobre la selectividad del microorganismo hacia un producto determinado y el rendimiento de biotransformación. Se evaluó el efecto de la fase del crecimiento del hongo y el efecto inductor del sustrato. De igual forma se incluyeron las siguientes variables: pH, medio de biotransformación y concentración del sustrato. La mayor velocidad específica de crecimientose alcanzó utilizando Agar Extracto Malta(MEA) como medio de cultivo a 28°C. Además se obtuvieron 246 mg/L de AP, utilizando 12 mM de (R)-(+)- limoneno encaldo Malta y Extracto de Levadura (MYB). Esta producción fue 1,9 y 3,1 veces mayor que la obtenida en caldo de Levadura, Extracto de Malta, Peptonay Glucosa (YMPG) y Caldo de Extracto de Levadura y Glucosa (YG), respectivamente. Las mayores concentraciones de AP (405 mg/L) fueron obtenidas cuando A. nigerDMS 821 fue cultivado en medio suplementado con 50 mL de limoneno durante la fase exponencial (a las 72 h), en MYB a pH 5,0, 28°C, 300 rpm y 6 días de biotransformación. Adicionalmente se obtuvieron otros subproductos: limoneno-1,2-diol, linalol, carvona, fenil etanol y etil ésteres de ácidos palmitico, oleico y linoleico.
PALABRAS CLAVE: Biotransformación, Bioconversión, (R)-(+)-limoneno, Aspergillus niger.
RESUMO
A produção de álcoolperílico (POH) por biotransformação de (R)-(+)- limoneno usando células íntegras de Aspergillus niger DSM 821 foiestudada. Foramavaliados os efeitos da fase de crescimento do fungo, o efeitoindutivo do substrato, o pH do meio de biotransformação e a concentração do substrato sobre a seletividade e a produtividade da biotransformação. Usando agar extrato de malte (MEA), como meio de cultura a 28°C, foipossívelobter a maiortaxa específica de crescimento. Alemdissoobtiveram-se 246 g/mL de AP, usando 12 mM de (R)-(+)- limoneno no caldo malta y extrato de levedura (MYB). Esta produçãofoi 1,9 e 3,1 vezesmaior do que a obtidaem caldo de levedura, extrato de malte, peptona e glicose (YMPG) e caldo de extrato de levedura e glicose (YG), respetivamente. As maioresconcentrações de AP (405 mg/L) foramobtidasquando A. niger DMS 821 foi cultivado emmeio suplementado com 50 mL de limoneno durante a fase exponencial (às 72 h), em MYB pH 5,0, 28°C, 300 rpm e 6 dias de biotransformação. Adicionalmente, obtiveram-se outrosprodutos: limoneno-1,2-diol, linalol, carvona, fenil etanol e etil ésteres de ácidos palmítico, oléico e linoléico.
PALAVRAS-CHAVE: Biotransformação,Bioconversão, (R)-(+)-Limoneno,Aspergillus niger.
INTRODUCTION
Nowadays,industrial application of biocatalysis(e.g. cells or enzymes) has increased its use in organic chemistry, because of their properties of high catalytic activity, specificity and regio/selectivity in mild reaction conditions [1]. For these reasons, it has begun to use more biocatalytic process to synthesize and/or biotransform chemical and pharmaceutical compounds [2]. For example, (R)-(+)-limonene, the most abundant monoterpene in the essential oil extracted from citrus peels (96%), can be biotransformed for obtaining compounds with high economic added value [3].
In general, monoterpenes possess preserving, antioxidant and anti-carcinogenetic activities [4,5,6,7]. Limonene and some of its oxygenated derivatives (OD), such as: perillyl alcohol (POH), menthol, carveol and carvone, can inhibit the progression of metastatic melanome cells and also destroy existing malignant tumors [8]. Specifically, C-7 limonene, POH and perillic acid (PCOOH) are well recognized as non-toxic agents. They have demonstrated antitumor and therapeutic activitiesfor the treatment of lung, liver, colon, skin, prostate pancreas, and also breast andovariancancers [9, 10, 11, 12, 13, 14].
In recent years, the number with cancer risks has been increased in the world. For this reason, several alternatives solutions have been raised. For example, minimal exposures to environmental carcinogens and use of exogenous factors (dietary constituents, supplements or drugs and immunization) have positively impacted in reducing tumor growth in early stages of the carcinogenic process. Therefore, the demand for anti-cancer compounds has greatly increased [15].
The POH with anti-carcinogenetic potentialcan be extracted from natural sources, such as essential oils of citrus plants, lavender, mint, caraway, cherry and blackberry. However, this process is not economically viable, due to the low content of essential oils (less than 0,1%w/w)[16,17]. Therefore, the production of POH and its derivatives (aldehyde and acid)at industrial scaleis carried outby using chemical catalysis ofβ-pinene and limonenemonoterpenes. In the case of β-pinene, conversion yields are very low. Additionally, chemical synthesis and reaction conditions are not environmentally friendly[18,19a,20,21].In the case of limonene, terpenoid mixtures may occur. This is because the allylic methylene and methyl carbons in the molecule of limonene can introduce hydroxyl groups and carbonyls nonspecifically. Because of these limitations, use of biocatalysisin monoterpenes oxy-functionalization is a promising methodological strategy, mainly for their additional benefits such as the lower toxicity of the process and inherent chemo and stereoselectivity [1].
Limonene biotransformation has been traditionally carried out by using of whole cells from different microorganisms, such as: Mesophilic fungi A. niger[22] and A. cellulosae[23];psycrotrophic fungi M. minutissina, C. pannorum, M. alpina, P. chrysosgenum, P. cyclopiumand P. islandicum[24]; bacteria P. putida MTCC 1072 [25] and DSM 12264 [26],Mycobacterium HXN-1500 [27] and yeasts C. parapsilosis and Y. lipolytica[28]. These microorganisms can produce POH and PCOOH by regioselectivehydroxylation C-7-limonene methyl group.Applications of bacteria as biocatalysts for production of POH have been also published in some Patents [19b,29,30].
On the other hand, genetic engineering techniques have improved the enzymatic activity of biocatalysts for oxy-functionalization of limonene at C-7. By using of Mycobacteriumalkanehydroxylasegenes,co-expressed in P. putida to producethe enzyme with a specific activity of 3U/g of dry cells, was possible to hydroxylateC-7-limonene [27]. PCOOH productionfrom limonene has been carried out on a continuous packed bed bioreactor by using of immobilized cells of P. putida DSM 12264. Product recovery wascarried out in situ (ISPR). With this configuration was obtained 31 g of PCOOH/L[26].
In this work, biotransformation of (R)-(+)-limonene to POH by whole cells of A. niger DSM 821 was evaluated. We studied addition of limonene in various stages of growth of the organism to induce limonene biotransformation. Finally, it wasevaluated best conditions for POH production, such of pH, culture medium, reaction time and substrate concentration.
METHOD
Chemical and reagents
(R)-(+)-limonene (98%) was obtained from Merck (Germany) and (-)-perillyl alcohol from Fluka (Switzerland).All reagents and solvents were of analytical grade and obtained from Merck (Germany).
Microorganism and culture media. Aspergillusniger DSM 821was obtained from German Collection of Microorganisms and Cell Cultures DSMZ (Braunschweig, Germany). The culture mediums PDA(potato dextrose agar), MEA(malt extract agar) and raw materials required to prepare the YGA complex liquid medium (yeast extract 3,0 g/L, malt extract 20 g/L, glucose 20 g/L, bacteriological peptone 1,0 g/L and agar 20 g/L), the YMPG (yeast extract 5,0 g/L, malt extract 10 g/L, glucose 10 g/L and bacteriological peptone 10 g/L), the MYB (yeast extract 3,0 g/L, malt extract 20 g/L, glucose 20 g/L and bacteriological peptone 10 g/L) and the YG (yeast extract 3,0 g/L, malt extract 20 g/L, glucose 20 g/L and bacteriological peptone 1,0 g/L, were purchased from OXOID (England).
Microbial growth kinetics ofA. niger DSM 821 in solid medium. A. niger DSM 821 was grown in PDA, MEA and YGA media at 28°C for 10 days, adding 10 µL of 1*107 spores/mL suspension. Growth kinetics of A. nigerwere determined by monitoring the colony diameter. Radial Growth Rate (RGR, mm/h) was estimated according to the method described by Pirt[31]. The best growth medium for microbial cultures was selected according RGR values and it was used for the maintenance of the strain. All experiments were performed in triplicate.
Evaluation of antifungal effect of substrate. Different concentrations of (R)-(+)-limonene (between 0 and 50,0 mM) were added to the MEA medium before gelation. Subsequently, the media were inoculated with 20 µL of spore suspension. Microbial growth was determined by measuring the change in diameter of the colonies after 8 days [31]. Medium without (R)-(+)-limonene was used as a negative control. The inhibition growth was calculated using the expression Eq1, where Dc is the average diameter of fungal colony in presence of inhibitor and De is the average diameter of the control. The limonene concentration required to inhibit the growth of A. nigerup to a maximum of 25% was defined as the Minimum Inhibitory Concentration (MIC) and the concentration that inhibited up to 100% of growth, as the lethal concentration (LC) [32].
Preparation of spore suspension. Spores from A. niger DSM 821were harvested from MEA medium a 28°C after 8 days of culture. Subsequently, they were re-suspended in 10 ml of saline 0,85% NaCl and 0,1% Tween 80. The initial concentration of spores in the biotransformation medium was 1*107spores/mL.
General process for biotransformation of limonene at lab scale. The bioassays were carried out in 22 mL vials fitted with Teflon stoppers, containing 5 mL of sterile liquid medium (YMPG, MYB and YGA) and inoculated with 50 µL of a 107 spores/mL suspension. Culture media were pre-incubated at 28°C for 72 h and 300 rpm of stirring using an orbital shaker (Heidolph Vibramax 100). After pre-incubation, (R)-(+)-limonene was added at different concentrations according to each experiment. Samples were taken periodically during 6 days. At the same time, a control biomass (spores suspended in the reaction medium without substrate) and control substrate (reaction medium and substrate without suspension of spores) were made to determine acid catalysis reactions. The reaction products and remaining substrate were extracted using ethyl acetateand identifiedby GC-MS. All experiments were performed in duplicate using independent samples. The reported value is the average of the two measurements.
Evaluation of the inducible effect from (R)-(+)-limonene on biotransformation. To determine the possible inducible effect of (R)-(+)-limonene on expression of oxidative enzymes, biotransformation experiments were carried out inoculating the medium YMPG with 50 µL of 1,0*107 spores/mL suspension, obtained from a fungal growth in 20 mL of MEA medium cultivated at 28°C in presence of inducible (0,74 mM of (R)-(+)-limonene). Biotransformations reactions were performed during 72 h at 28°C and 300 rpm stirring speed, using 12 mM (R)-(+)-limonene (dissolved in 20% ethanol) in the culture medium. Periodically, samples were taken from the reaction medium for monitoring the biotransformation during 6 days and analyzed by GC-MS. Spore suspension grown without inducible was used in a control experiment.
Evaluation of the effect of pH on biotransformation. For these experiments, initial pH of the liquid medium YMPG was adjusted to values of 3,5, 5,0 y 6,5 using 0,1 M citrate-phosphate buffer. Culture media were inoculated with as described above. Subsequently, biotransformation reactions were performed according to the previously described methodology. The experiments were carried out by duplicated.
Growth kinetics of A. nigerin liquids medium. The growth kinetics of A. niger DSM 821 was estimated by quantification of cell concentration by using dry weight cell method [35] during 15 days. Liquid media YMPG (broth malt extract 1%, yeast extract 0,5%, peptone 0,5% and glucose 1%), MYB (broth malt extract 2%, yeast extract 0,3%, peptone 1% and glucose 1%) and YM (broth malt extract 2%, yeast extract 0,3%, peptone 0,1% and glucose 2%) were adjusted at pH 5,0 using 0,1 M citrate-phosphate buffer and inoculated as previously described. The specific growth rate (m) of fungi in each culture medium was determined [31].The experiments were carried out by duplicated.
Evaluation of the effect of culture medium composition. The effect of the culture media YMPG, MYB and YM of pH 5,0 on the biotransformation of (R)-(+)-limonene was studied. The experiments were performed in the liquid media under the conditions described before. The biotransformation experiments were initiated by adding 12 mM (R)-(+)-limonene. Microbial kinetics biotransformation was monitored each 48hfor six days. The culture medium with highest bioconversion values was selected to evaluate other biotransformation variables.
Evaluation of the effect of addition of limonene at different growth phases of A. niger. To determine the inducing effect of the addition of (R)-(+)-limonene at different growth phases of A. nigerDSM 821, on its biotransformation, the fungi were grown inMYB at pH 5,0 during different times period: 6 h and 1, 3, 5 or 7 days. These timescorresponding with all sections of microbial growth curve (the lag, early, medium and final exponential and stationary phases) which were determined previously.When A. niger had reached the desired growth phase, the reaction was initiated by the addition of 12 mM (R)-(+)-limonene into the culture medium. The biotransformation kinetics was monitored every 72 h fornine days to each growth phase. The growth phase with highest concentration of POH was selected for evaluation of further variables. The tests were carried out by duplicated.
Evaluation of the effect of substrate concentration. The effect of (R)-(+)-limonene concentration on biotransformation by A. nigerDSM 821 was evaluated using different concentrations (between 0 and 100 mM) in the MYB media at pH 5,0 during 9 days. The optimum concentration of substrate was determined according to the selectivity (to a single compound produced) and the concentration of POH produced.
Extraction, identification and quantification of products. DO and remaining substrates formed by biotransformation were extracted two times with ethyl acetate (2x2,5 mL) followed by centrifugation at 4000 rpm for 5 min. The organic phase was collected and dried with anhydrous Na2SO4 and concentrated by N2 stream. Subsequently, 3μL of n-tetradecane (istd) were added and diluted to 1mL. Substrate and DO were identified and quantified by GC-MS using Agilent model 6890N (Palo Alto, CA, USA) chromatograph coupled to a mass selective detector Agilent Technologies 5975C with a detector system of electron impact ionization (70eV) and a quadrupole mass analyzer, operated in full scan mode from 40-400 Dalton (m/z). An automatic injector HP 7683 Serieswith a split ratio of 1:13 and a DB-WAX capillary column (60mx0,25mmx0,25 μm) were used. Temperature was programmed from 45°C maintained for 10 minutes, with a heating rate of 3°C/min until 220°C, maintaining this temperature for 30 min. The identification of compounds was performed comparing mass spectra of the samples with spectra from compound library data from the databases ADAMS, NSB 75K, 138K NIST 05 and WILEY available in the data systems G1701BA HP Enhanced Chemstation.
RESULTS
Growth kinetics of A.niger DSM 821 in solid culture media
Firstly, we evaluated fungal growth of A. niger DSM 821 in different culture media (PDA, MEA and YGA).The growth kinetics of A. niger was affected by the composition of the media. Best results were obtained in the MEA medium followed by the PDA and YGA media.This may be because the medium MEA has a higher carbon content, different sources of nitrogen and slightly more acidic pH compared with PDA and YGA media.
Highest RGR of A. niger DSM 821 was obtained in the MEA media (0,46±0,05)and was 1,1 and 2 times higher than in PDA (0,41 ±0,05) and YGA media (0,23±0,07), respectively. Therefore, MEA medium was selected for periodic maintenance of A. nigerand obtaining spore solution used in the experiments of biotransformation.
Evaluation of antifungal activity of substrate
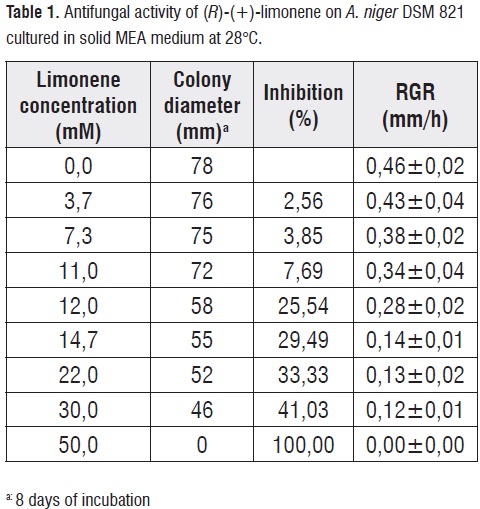
To determine the effect of (R)-(+)-limonene on the growth of A. niger DSM 821, substrate concentration in MEA culture media was varied (between 0 and 50 mM). It was observed that the presence of limonene as an additional source of carbon and energy affects negatively fungal growth decreasing the RGR (table 1). There was a minimum inhibitory concentration (MIC) of 12 mM, while 50 mM limonene or more in the culture medium were lethal for fungal growth (LC). The results are indicating toxicity of (R)-(+)-limonene, probably due to the polarity of thiscompound,which has a partition coefficient in n-octanol-water (Log Pow) of 4,8 [33,34,35]. In previous studies, it has been described that terpenes with Log Pow between 1-5 can cause a loss of specific permeability and integrity of the cell membrane [35,36,37].
Effect of the inductor (R)-(+)-limonene on biotransformation
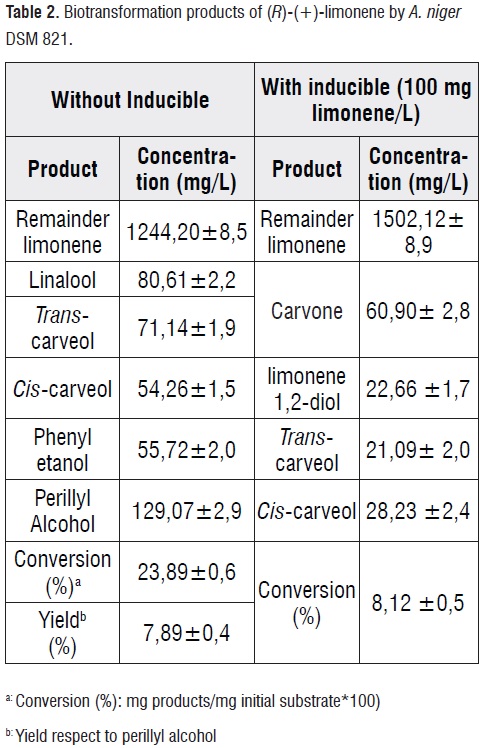
We evaluated the inductive effect of (R)-(+)-limonene in the biotrasnformation of A. niger DSM 821 according to Tan and Day[33], using a substrate concentration of 0,74 mM. The experiments in YMPG medium at 12mM limonene, 28°C and 300 rpm, showed that biotransformation was different when A.niger was pre-exposed to limonene. In the presence of inducer, the oxidation and hydroxylation reactions led to the production of OD such as carvone, cis/trans-carveol and limonene1,2-diol (table 2). By contrast, in the absence of inducer,only hydroxylation reactions were performed with POH as main product. Other derivatives were cis/trans-carveol, linalool and phenyl ethanol. Under these conditions was produced 129 mg POH/L and it was demonstrated that production of POH by A. niger by biotransformation of limonene is not inducible.
Effect of pH onthe biotransformation
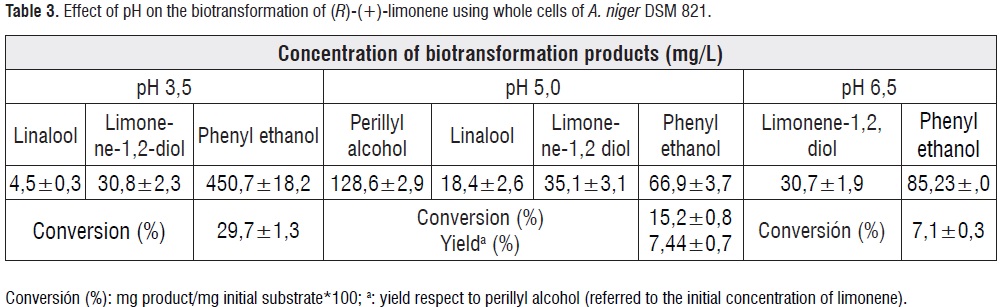
Biotransformation of (R)-(+)-limonene by A. niger DSM 821 was performed at pH 3,5, 5,0 and 6,5, in the YMPG medium. Selectivity of the biotransformation was affected by the different conditions of pH and hydroxylated derivatives were produced. Only at pH 5 was produced POH.At pH 3,5, mainly phenyl ethanol (450 mg/L) was obtained whit a concentration 15 to 100 times higher than linalool and limonene-1,2-diol, respectively (see table 3). It is important to note thatPOH was the main product with a concentration of 129 mg/L at pH 5,0. Other products were alcohols such as linalool, limonene-1.2-diol and phenyl ethanol. At pH 6,5 the reaction was more selective because only phenyl ethanol was formed and bioconversion of limonene-1,2-diol, was lower.
Effects of the culture medium on the growth of A. niger and the biotransformation of limonene
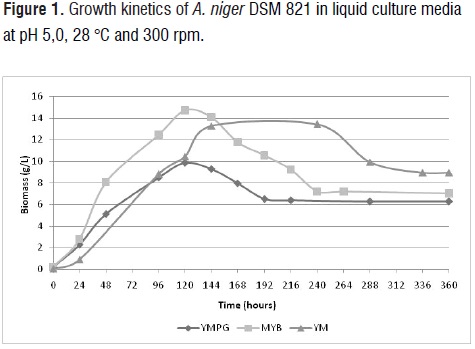
Growth kinetics of A. niger DSM 821 in liquid media YMPG, MYB and YG at pH 5,0 (figure 1) showed that the composition of the culture medium influences microbial growth of the fungus.Better results of microbial growth were achieved in the medium MYB. Fungal growth was higher in the medium MYB compared to YMPG and YG. However,
specific growth rates (μ) inthe three media were relatively similar (0,03 h-1 for MYB and YG, and 0,02 h-1 for YMPG).
In all experiments, the POH was the main product (table 4). However, in the MYB medium, the concentration of POH was 246 mg/L, which is 1,7 and 3,4 higher than obtained in YMPG and YM media
The highest yield of POH in MYB is possibly due to higher nitrogen content represented in
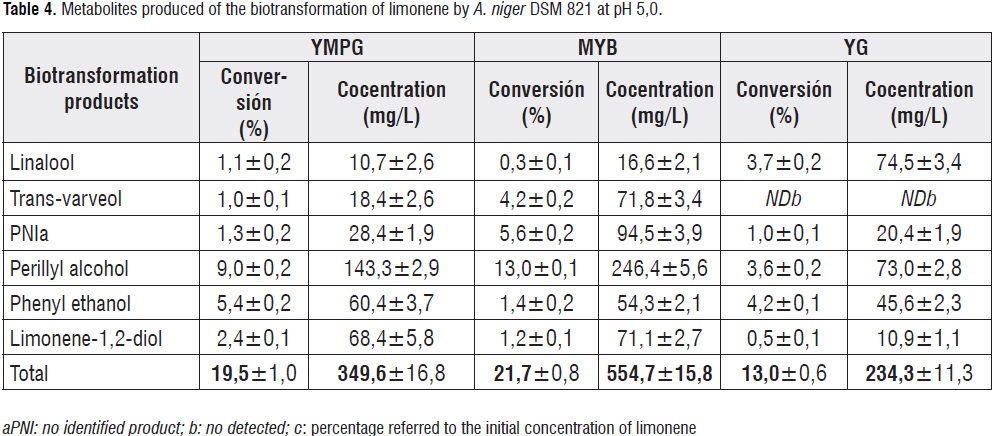
the peptone. In this way, would influence the biomass concentration and induction of oxidative enzymes. Obtained results are very interesting when compared with those reported in the literature. For example, 72,6 mg/L of POH (14,7%) were obtained from biotransformation of 4,93 mM limonene using A. niger grown in YMPG [38,39]. The studies withM. minutissinaproduced 125 mg POH/L in the YMPG media using 58,71 mM of substrate[25], while another strain of A. nigerBFQU 68 transformedlimonene (37 mM) in TSB (tryptone-soya broth) medium to produce POH with a 28,5% of bioconversion[22].In addition, cells of A.nigerDSM 821 were able to biotransform(R)-(+)-limonene when they were in the exponential phase media (72 h), reaching a maximum concentration of POH of 246 mg/L at 6 days of bioreaction (figure 2). In the final exponential phase (120 h), biotransformation of limonene was directed towards production of trans-carveol(100 mg/L) 6 days after adding of substrate. In other phases, e.g. lag, early exponential and stationary, no biotransformation of (R)-(+)-limonene into POH as observed.
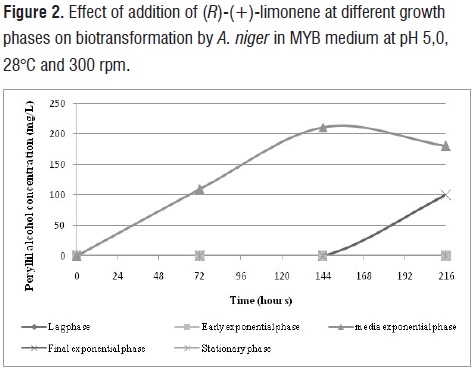
Effect of the substrate concentration on the biotransformation of (R)-(+)-limonene. Substrate concentration affected the selectivity of the reaction and level of bioconversion. Selectivity decreased with increasing concentrations of limonene in the medium (figure 3). However, at 50 mM (R)-(+)-limonene, POH production was
increased, reaching a concentration of 405 mg/L. Compared with previously results, we could obtain POH production 1,7 times higher than obtained in effects of pH, medium of biotransformation and growth phase of A. niger.
The formation of the oxygenated compounds of limonene by means of hydroxylation reactions in different carbons of limonene, oxidation reaction and break cycles it is shown in figure4.
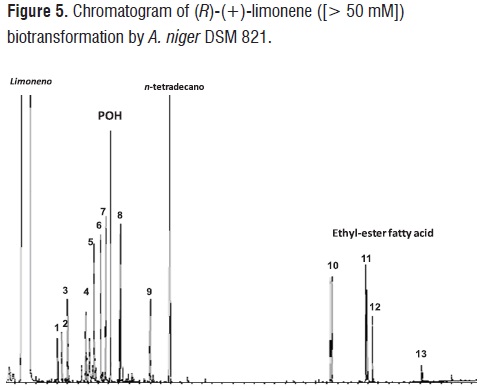
Higher concentrations of limonene (> 50 mM) produced hydroxylated derivatives of limonene such as linalool,trans/cis-menth-2,8-dien-1-ol, cis/trans-carveol, carvone, perillyl aldehyde, limonene-1,2-diol, limonene 1,2 epoxide, phenyl ethanol, and ethyl esters of palmitic, oleic, linoleic and stearic (peak 1,2,3,4,5,6,7,8,9,10,11,12,13,respectively), as it is shown in figure 5. These ethyl esters may be formed by the action of oxygenases synthesized by A. niger and subsequent reaction using as co-solvent. Menéndez et al., have reported for the rmation biotransformation of limonene with A. niger BFQU 68, the production of short chain fatty acids (propanoic, isobutyric and isopentane) in absence of co-solvents [22]. Production of ethyl esters of higher fatty acids during terpene biotransformation processes has not been reported to date. In a recent work, esterification of POH with fatty acids catalyzed by lipases was carried out. The presence of ethyl esters of higher fatty acids in the natural extract obtained from the biotransformation of (R)-(+)- limonene by A. niger DSM 821 probably can broad its spectrum of application [34].
Finally, it is noteworthy that this work has allowed to reach a concentration of POH of 405 mg/L, equivalent to the maximum amount of this oxygenated limonene derivatives obtained by biotransformation processes using fungi.
CONCLUSIONS
Biotransformation of (R)-(+)-limonene by A. niger DSM 821 was directed mainly towards the production of perillyl alcohol, reaching a maximum concentration of 405 mg/L, using the following conditions: the fungus in the exponential phase (72 h), no prior exposure to limonene, MYB adjusted to pH 5,0 (using citrate-phosphate buffer 0,1 M), substrate concentration of 50 mM, 28°C and 300 rpm.In these experimental conditions, had the highest concentration of POH reported to date.A. nigerDSM 821 also showed the ability to hydroxylate limonene at a carbon center than C-7 leading to terpenoids, cis/trans-carveol, limonene1,2-diol, linalool and phenyl ethanol. Additionally the biotransformation in vivo gives rise to ethyl esters of higher fatty acids, palmitic, oleic and linoleic.
ACKNOWLEDGEMENTS
The authors thank to COLCIENCIAS for financial support of this research through contract RC4322004 with the National Center for Research on Agro-industrialization of Aromatic and Medicinal Plant Tropical, CENIVAM. We also thank to Universidad Industrial de Santander and Universidad Pedagógica y Tecnológica de Colombia – UPTC - Tunja, for a PhD fellowship for Gloria Astrid Prieto. Suggestions and technical comment from Drs. Martha Daza, Markus Doerr and Rodrigo Torres are acknowledged.
REFERENCES
[1] DE CARVALHO, C. Enzymatic and whole cell catalysis: finding new strategies for old processes. BiotechnologyAdvances, 29, 2011, p. 75-83. [ Links ]
[2] HELD, M., SCHMID, A., VAN BEILEN, J. and WITHOLT, B. Biocatalysis. Biological Systems for the Production of Chemicals.Pure and AppliedChemistry,72(7), 2000, p. 1337-1343. [ Links ]
[3] MARÓSTICA, M., ROCHA, T., FRANCHI, G., NOWILL, A., PASTORE, G. and HYSLOP, S. Antioxidant potential of aroma compounds obtained by limonene biotransformation of orange essential oil. Food Chemistry,116, 2009, p. 8-12. [ Links ]
[4] CROWELL, P. Prevention and therapy of cancer by dietary monoterpenes. Journal of Nutrition, 129, 1999, p. 775S-778S. [ Links ]
[5] FISHER, K. and PHILLIPS, C. Potential antimicrobial uses of essential oils in food: is citrus the answer. Trends in Food Science and Technology, 19, 2008, p. 156-164. [ Links ]
[6] SANDRI, I., ZACARIA, J. and FRACARO, F. Antimicrobial activity of the Essential oils of Brazilian species of the genus Cunila against foodborne pathogens and spoiling bacteria. Food Chemistry, 103, 2007, p. 821-828. [ Links ]
[7] SCHELTZ, Z., MOLNAR, J. and HOHMANN, J. Antimicrobial and Antiplasmid Activities of Essential Oils. Fitoterapia, 77, 2006, p. 279-285. [ Links ]
[8] DE CARVALHO, C., and DA FONSECA, M. Carvone. Why and how should one bother to produce this terpene. Food Chemistry, 95, 2006, p. 413-422. [ Links ]
[9] YURI, T., DANBARA, N., TSUJITA-KYUTOKU, M., KIYOSUKA, Y. and SENSAKI, H. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Research and Treatment, 84, 2004, p. 251-260. [ Links ]
[10] PEFFLEY, D., SHARMA, C., HENTOSH, P. and BUECHLER, R. Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Archives of BiochemistryBiophysics, 465, 2007, p. 266-273. [ Links ]
[11] CHUNG, B., LEE, H., SEOK, J. and YOUNG, C. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Letters,236, 2006, p. 222-228. [ Links ]
[12] BELANGER, J. Perillyl Alcohol: Applications in Oncology. Alternative Medicine Review, 3, 1998, p. 448-457. [ Links ]
[13] GUPTA, A. and MYRDAL, P. Development of a perillyl alcohol topical cream formulation. International Journals of Pharmaceutics, 269, 2004, p. 373-383. [ Links ]
[14] XU, M., FLOYD, H., GRETH, S., CHANG, W., LOHMAN, K. and STOYANOVA, R. Perillyl alcohol-mediated inhibition of lung cancer cell line proliferation: potential mechanisms for its chemotherapeutic effects. Toxicology andApplied Pharmacology, 195, 2004, p.232-246. [ Links ]
[15] HODEK, P., KRIZKOVA, J., BURDOVA, K., SULC, M., KIZEK, R., HUDECEK, J. and STIBOROVA, M. Chemopreventive compounds- View from the other side. Chemico-Biological Interactions, 180, 2009, p 1-9. [ Links ]
[16] NAKAYAMA, M., YAMAGUCHI, Y., MACHIDA, H., IWASAKI, S., KOMATSU, A. and SHINODA, A. Preparation of L-menthol racemate. Patent JP53044691.Tokio (Japan):1978. [ Links ]
[17] WANG, X., LIU, Y., NAIR, U., ARMSTRONG, D. and ELLIS, B. Enantiomeric composition of monoterpenes in conifer resins. TetrahedronAsymmetry, 8, 1997, p. 3977-3984. [ Links ]
[18] CAGNOLI, M., CASUSCELLI, S., ALVAREZ, A., BENGOA, J., GALLEGOS, N. and CRIVELLO, N. Ti-MCM-41 silylation: Development of a simple methodology for its estimation silylation effect on the activity and selectivity in the limonene oxidation with H2O2. CatalysisToday, 107, 2005, p. 397-403. [ Links ]
[19a] DUETZ, W., BOUWMEESTER, H. and VAN BEILEN, J. Using proteins in their natural environment: potential and limitations of microbial whole cell hydoxylations in applied biocatalysis. CurrentOpinion in Biotechnology, 12, 2003,p. 419-425. [ Links ]
[19b] DUETZ, W., WITHOLT, B. and JOURDAT, C. Process for the preparation of perillyl alcohol.PatentUS20040077063 A1. Zurich (Switz): 2003. [ Links ]
[20] GALLEZOT, P. Catalytic routes from renewable to fine chemicals. Catalysis Today, 121, 2007, p. 76-91. [ Links ]
[21] OLIVEIRA, P., ROJAS, M., RAMOS, A. and FONSECA, I. Limonene oxidation over V2O5/TiO2 catalysts. Catalysis Today, 118, 2006, p. 307-314. [ Links ]
[22] MENÉNDEZ, P., GARCÍA, C., RODRÍGUEZ, P., MOYNA, P. and HEINZEN, H. Enzymatic Systems Involved in D-limonene Biooxidation. Brazilian Archivesof Biology and Technology, 45, 2002, p. 11-114. [ Links ]
[23] NOMA, Y., YAWASAKI, S. and ASAKAWA, Y. Biotransformation of limonene and related compounds by Aspergilluscellulosae. Phytochemistry, 31, 1992, p. 2725-2727. [ Links ]
[24] TRYTEK, M. and FIEDUREK, J. A novel psychrotrophic fungus, Mortierellaminutissima, for D-limonene biotransformation. Biotechnology Letters, 27,2005, p. 149-153. [ Links ]
[25] CHATTERJEE, T. and BHATTACHARYYA, D. Biotransformation of limonene by Pseudomonas putida. AppliedMicrobiology andBiotechnology, 55, 2001, p. 541-546. [ Links ]
[26] MIRATA, M., HEERD, D. and SCHRADER, J. Integrated bioprocess for the oxidation of limonene to perillic acid with Pseudomonasputida DSM 12264. Process Biochemistry, 44, 2009, p. 764-771. [ Links ]
[27] VAN BEILEN, J., HOLTACKERS, R., LÜSCHER, D., BAUER, U., WITHOLT, B. and DUETZ, W. Biocatalytic production of perillyl alcohol from limonene by using a novel Mycobacterium sp. Cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Applied andEnvironmental Microbiology, 71, 2005, p. 1737-1744. [ Links ]
[28] VAN RENSBURG, E., MOLELEKI, N., VAN DER WALT, J., BOTES, P. and VAN DYK, M. Biotransformation of (+)-limonene and (-)-piperitone by yeast and yeast-like fungi. BiotechnologyLetters, 19, 1997, p. 779-782. [ Links ]
[29] CHASTAIN, D., MODY, N. and MAJETICH, G. Method of preparing perillyl alcohol and perillyl acetate.Patent US5994598A. Florida (USA):1999. [ Links ]
[30] JOURDAT, C., DUETZ, W. and WITHOLT, B. Process for the preparation of perillyl alcohol. PatentAU2002232294. Zurich (Switz): 2000. [ Links ]
[31] PIRT, S. Principles of microbes and cell cultivation. Oxford (England): Blackwell Scientific Publications,1975, p. 268. [ Links ] [ Links ]
[33] TAN, Q. and DAY, D. Bioconversion of limonene to α-terpineol by P. digitatum (NRRL 12002). Process Biochemistry, 49, 1998, p. 96-101.
[34] MARTINS, R., KAWAI, S. and CHACÓN, D. Estudio de la biotransformación del D-limoneno por fermentación en fase líquida. XXII Interamerican Congress of Chemical Engineering. Buenos Aires (Argentina): 2006. [ Links ]
[35] ONKEN, J. and BERGER, R. Effects of R-(+)-limonene on submerged cultures of the terpene transforming basidiomycetePleurotussapidus. Journal of Biotechnology, 69, 1999, p. 163-168. [ Links ]
[36] BICAS, J., BARROS, F., WAGNER, R., GODOY, H. and PASTORE, G. Optimization of (R)-(+)-α-terpineol production by the biotransformation of (R)-(+)-limonene.Journal of IndustrialMicrobiology and Biotechnology,35, 2008, p. 1061-1070.
[37] KRAIDMAN, G., MUKHERJEE, B. and HILL, I. Conversion of D-limonene into an optically active isomer of a-terpienol by a Cladosporium species. Bacteriological Proceedings, 69, 1969, p. 63. [ Links ]
[38] CASTELLANOS, F., PEREA, J.y ORTIZ, C. Obtención de alcohol perílico por biotransformación del limoneno. ScientiaEtTechnica,XIII(033), 2007, p. 137-140. [ Links ]
[39] DEMYTTENAERE, J., VAN BELLEGHEM, K. and DE KIMPEN. Biotransformation of (R)-(+) and (S)-(-)-limonene by fungi and the use of solid phase microextraction for screening. Phytochemistry 57, 2001, p. 199-208. [ Links ]