Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biotecnología en el Sector Agropecuario y Agroindustrial
Print version ISSN 1692-3561
Rev.Bio.Agro vol.12 no.1 Popayán Jan./June 2014
EFFECT OF EXTRINSIC FACTORS ON THE ANTIMICROBIAL ACTIVITY OF W10B METABOLITE PRODUCED BY Weissella confusa
EFECTO DE FACTORES EXTRINSECOS SOBRE LA ACTIVIDAD ANTIMICROBIANA DEL METABOLITO W10b PRODUCIDO POR Weissella confusa
EFEITO DUS FATORES EXTRINSECOS NA ATIVIDADE ANTIMICROBIANA DO METABOLITO W10B PRODUZIDO PELA Weissella confusa
LILIANA SERNA-COCK1*, LEIDY JOHANA VALENCIA H.2, ANGELA VIVIANA RUALES S.2
1 Universidad Nacional de Colombia Sede Palmira, Facultad de Ingeniería y Administración. Ph.D. Ingeniería de Alimentos. Palmira, Valle, Colombia.
2Universidad Nacional de Colombia, Sede Palmira, Facultad de Ingeniería y Administración. Ingeniera Agroindustrial. Palmira, Valle, Colombia.
Correspondencia: lserna@unal.edu.co
Recibido para evaluación: 13-01-2014. Aprobado para publicación: 29-04-2014.
ABSTRACT
Antimicrobial substances produced by lactic acid bacteria (LAB) may lose activity due to extrinsic factors. This study determined the effects of temperature, pH, and exposure to enzymes, on the activity of antimicrobial compounds (W10b) isolated from LAB Weissella confusa. W10b were active against Staphylococcus aureus and Streptococcus agalactiae. W. confusa was obtained by fermentation at 32°C for 4 h and W10b by centrifugation, microfiltration, and lyophilization from the resulting cell-free supernatant. W10b was exposed to acidic, neutral and basic pH, for two residence times (10 and 30 minutes) and four temperatures (27, 40, 60, and 100°C). The effect of the enzymes pepsin and amyloglucosidase on W10b was also determined. Significant differences (P<0,05) on the antimicrobial activity of W10b due to pH and temperature were found. The highest antimicrobial activity was obtained against S. aureus at basic pH and 27°C, and against S. agalactiae at neutral pH and 27°C. W10b presented sensitivity to the pepsin and amyloglucosidase enzymes, indicating the proteinaceous nature and chemical complexity of the compounds. Although further research is needed, antimicrobial compounds from W. confusa have a strong potential to be used in industrial setups.
KEYWORDS: Bacteriocin, Lactic acid bacteria, Staphylococcus aureus, Streptococcus agalactiae.
RESUMEN
Las bacterias ácido lácticas producen sustancias antimicrobianas que pueden perder su actividad debido a factores extrínsecos. En este estudio se determinaron los efectos de temperatura, pH, tiempo de exposición y enzimas sobre la actividad antimicrobiana del metabolito W10b producido por la bacteria ácido láctica Weissella confusa. W10b es activo contra Staphylococcus aureus y Streptococcus agalactiae. W. confusa se produjo mediante fermentación a 32°C por 4 horas y el metabolito W10b se obtuvo a partir del sobrenadante libre de células mediante centrifugación, filtración y liofilización. W10b se sometió a pH ácido, neutro y básico, para dos tiempos de exposición (10 y 30 minutos) y diferentes temperaturas (27, 40, 60 y 100°C). Además se midió el efecto de las enzimas pepsina y amiloglucosidasa sobre W10b. Se encontró diferencias significativas (p<0,05) de la actividad antimicrobiana de W10b por efectos de pH y temperaturas, donde la mayor actividad antimicrobiana se obtuvo, contra S. aureus en medio básico a 27°C y contra S. agalactiae en medio neutro a 27°C. W10b presentó sensibilidad frente a las enzimas pepsina y amiloglucosidasa, indicando la naturaleza proteica y complejidad química del metabolito. Estos resultados orientan el uso industrial o comercial del compuesto antimicrobiano y señala el camino de futuras investigaciones.
PALABRAS CLAVE: Bacteriocinas, Bacteria ácido Láctica, Staphylococcus aureus, Streptococcus agalactiae.RESUMO
As substâncias antimicrobianas produzidas por bactérias do ácido láctico (LAB) podem perder a atividade devido a fatores extrínsecos. Este estudo determinou os efeitos da temperatura, pH, exposição a enzimas, sobre a actividade dos compostos antimicrobianos (W10b) isoladas da LAB Weissella confusa. W10b tinham atividade contra Staphylococcus aureus e Streptococcus agalactiae. A W. confusa foi obtida por fermentação a 32°C durante 4 h e W10b por centrifugação, a microfiltração e liofilização a partir do sobrenadante resultante de células livres. W10b foi exposta a pH ácido, neutro e básico, emdois tempos de residência (10 e 30 minutos) e quatro temperaturas (27, 40, 60 e 100°C). O efeito das enzimas pepsina e amyloglucosidase sobre W10b foi também determinada. Foram encontradas diferenças significativas (P <0,05) da atividade antimicrobiana de W10b devido ao efeito de pH e temperatura. A maior actividade antimicrobiana foi obtida contra S. aureus, no pH básico e 27°C, e contra S. agalactiae no pH neutro e 27°C. W10b apresentou sensibilidade a pepsina e enzimas amloglucosidase, indicando a natureza proteica e complexidade química dos compostos. Embora seja necessárias mais pesquisas, os compostos antimicrobianos de W. confusa têm um forte potencial para ser usados nas industriais.
PALAVRAS-CHAVE: Bacteriocina, Bactérias lácticas, Staphylococcus aureus, Streptococcus agalactiaeINTRODUCTION
Bacteriocins are proteinaceous compounds ribosomally synthesized by bacteria, which are important due to their bacteriostatic and/or bactericidal effect [1]. These compounds are effective against bacterial strains closely related to the strain producing the antimicrobial or to other less related bacterial populations [1]. These characteristics make of bacteriocins, particularly those produced by lactic acid bacteria (LAB), of special interest for the food and pharmaceutical industries.
The antimicrobial activity of bacteriocins is highly variable and affected by enzymatic activity, temperature, and pH among others; being the last two factors the most important in affecting bacteriocin stability. This is because environmental factors promote differential structural changes [2], even in bacteriocins that are structurally similar [3].
The total or partial loss of antimicrobial activity in the presence of proteolytic enzymes is consistent with the protein nature of bacteriocins. However, loss of activity may also occur in the presence of glycolytic and lipolytic enzymes [4] due to their structural complexity (e.g., presence of carbohydrates or lipids in their structure). Similarly, pH affects the stability of bacteriocins. Some bacteriocins are effective in certain pH ranges (e.g., acidic, neutral, or basic) whereas others (e.g., Pediocin AcM) are effective in pH ranging from 1,0 to 12,0 [1]. Most bacteriocins lose partial or total antimicrobial activity when subjected to high temperature, but the sensitivity varies depending on the specific bacteriocin. Although the combination of environmental factors may result in a synergistic effect over the activity of a given bacteriocin, changes in antimicrobial activity may be strain specific and cannot be extrapolated to all the susceptible bacterial populations [5].
Weissellastrains have been isolated from various sources, such as wheat, garlic, carrot juice, banana leaf, fermented vegetables like kimchi, kimraw milk and liquid rumen from cattle [6, 7, 8]. Its metabolites were tested with promising results against the bacteria Staphylococcus aureus, Streptococcus agalactiae and Escherichia coli [9, 8] and the fungi Penicillium roqueforti and Aspergillus niger, been reported on the effect of extrinsic factors on the antimicrobial activity of metabolic compounds produced by W. confusa.The objective of this study was to determine the effect of the extrinsic factors temperature, pH, and exposure to enzymes on the antimicrobial activity of W10b, a group of metabolic compounds isolated from W. confusa, to assess their potential use by the food and pharmaceutical industries.
METHOD
Strains and culture conditions
A cryo-preserved W. confusa strain previously isolated by Serna-Cock et al., (2011) [8] was used. Ten percent (v/v) of the W. confusa culture was inoculated in 5 mL of MRS broth (Scharlau Microbiology, Barcelona, Spain) supplemented with 40 g/L of glucose (Merck, Darmstadt, Germany) and then incubated at 32°C for 24 h.
To assess the antimicrobial activity of the W. confusa metabolic compounds W10b, two indicator strains, S. aureus ATCC 2592 and S. agalactiae ATCC 13813, previously shown to be susceptible were used [8]. The indicator strains were grown in 5 mL nutrient broth (Scharlau Microbiology, Barcelona, Spain), which was inoculated with 10% (v/v) of each microorganism and subsequently incubated at 32°C for 24 h.
Isolation of W10b metabolic compounds
The W10b compounds were isolated as described by Serna-Cock et al. [8]. W. confusa, culture grown for 24 h was inoculated (10% v/v) in 500 mL MRS broth supplemented with 40 g/L glucose. Fermentation was then conducted under continuous agitation in an ellipsoidal shaker (VWR, Radnor, PA) set at 32°C and 100 rpm for 4 h, regularly adjusting the pH to 6,0 using NaOH 1M (Mol Labs, Bogotá, Colombia). The fermented substrate was centrifuged (Eppendorf, Hamburg, Germany) at 2700xG and 4°C for 2 h. After centrifugation, the precipitate (biomass) was removed and the cell-free supernatant was filtered using a 0,45 µm crossflow filter (Titan, Pittsburgh, PA) and concentrated by lyophilization (Labconco, Fort Scott, KS) (sample frozen at -70°C, -50°C condenser temperature, 0,120 mbar vacuum pressure). The cell free lyophilized isolate was labeled “W10b” and stored at -4°C.Effect of pH, temperature and residence time on the antimicrobial activity of W10b
The antimicrobial activity of W10b against indicator microorganisms was evaluated at various pH (4,5, 5,0, 7,0, 8,0, and 9,0), temperature (27, 40, 60 and 100°C), and two residence times (10 and 30 min). For statistical analysis, the results were grouped into three pH levels: basic (8 and 9), neutral (7) and acidic (4,5 and 5). For controls, microorganisms were exposed to 27°C and 0 min residence time at each pH.The lyophilized W10b was reconstituted in phosphate buffer (1:9 W10b in buffer v/v) and adjusted to the different pH values. The temperature and residence time were evaluated by pouring 200 µL of the reconstituted W10b at each pH, in 2 mL vials. Then 9 hermetically sealed vials were placed in a container with water (Julabo, Germany) and inside an oven set to 40, 60, or 100°C (Binder, Germany) for 10 min or 30 min. Once the residence time of each treatment was reached, antimicrobial activity tests were performed.
Effect of enzymes on the antimicrobial activity of W10b
The effect of the enzymes pepsin (Merck, Darmstadt Germany) and amyloglucosidase (Sigma Aldrich, Steinheim, CITY, Germany) on the antimicrobial activity of the W10b was evaluated. S. aureus was considered the indicator microorganism since preliminary experiments showed that this bacterium had greater susceptibility to W10b. W10b was reconstituted in phosphate buffer at pH 6,0, since it was demonstrated from preliminary trials that the compounds have the best stability at this pH. 200 µL of rehydrated W10b and 2 µL of each selected enzyme (100 mg/mL concentration) were combined. The dilution of the pepsin and amyloglucosidase enzymes was done in phosphate buffer at pH 4,5 (optimal pH of activity). Subsequently, the vials were placed in water bath (Julabo, Germany) at 37°C for 1 h, simulating the gastrointestinal temperature. The control treatment consisted on rehydrating W10b at pH 6,0 without the addition of any enzyme, and incubating at 37°C for 1 h. After the incubation time, the antimicrobial activity was determined.Determination of the antimicrobial activity
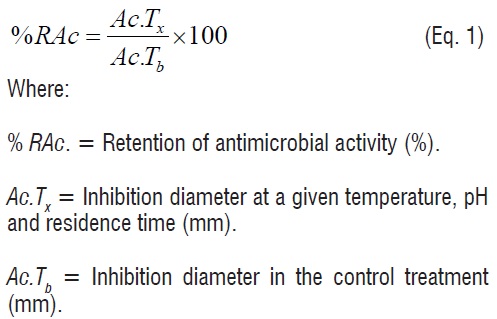
The antimicrobial activity was determined using the diffusion method [10]. Nine holes with a sterile 7 mm diameter punch were done in Petri dishes containing 20 mL of solidified agar. Beard Parker Agar (Scharlau Microbiology, Barcelona, Spain) was used for S. aureus and M-17 Agar (Merck, Darmstadt, Germany) for S. agalactiae. Using a sterile swab, 100 µL of a 24 h culture of the indicator strain (106 CFU/mL) were spread in each Petri dish. For each treatment, 60 μL of the antimicrobial media were poured in each hole. The inoculated Petri dishes were incubated at 32°C for 24 h. The quantification of the antimicrobial activity was done by measuring the inhibition diameter (mm). Inhibition was expressed as the diameter of inhibition less the diameter of the hole. In addition, the retention of antimicrobial activity (percent basis) was calculated using equation 1.
Experimental design
To evaluate the effect of pH, temperature and residence time on the antimicrobial activity of the W10b, an unbalanced completely randomized design was used in a factorial array (32x4), with three factors: pH (three levels: basic, neutral, and acidic), temperature (four levels: 27, 40, 60, and 100°C), and residence time (three levels: 0, 10, and 30 min). To evaluate the effect of the two enzymes on the antimicrobial activity of W10b, an unifactorial design was used with two levels: pepsin and amyloglucosidase. In both cases, each treatment was conducted in triplicate, with antimicrobial activity as the response variable. Analysis of variance was done using SAS software (version 9.1.3, SAS, Cary, NC). The comparison of means was done with the Duncan multiple range test (α= 5%).
RESULTS
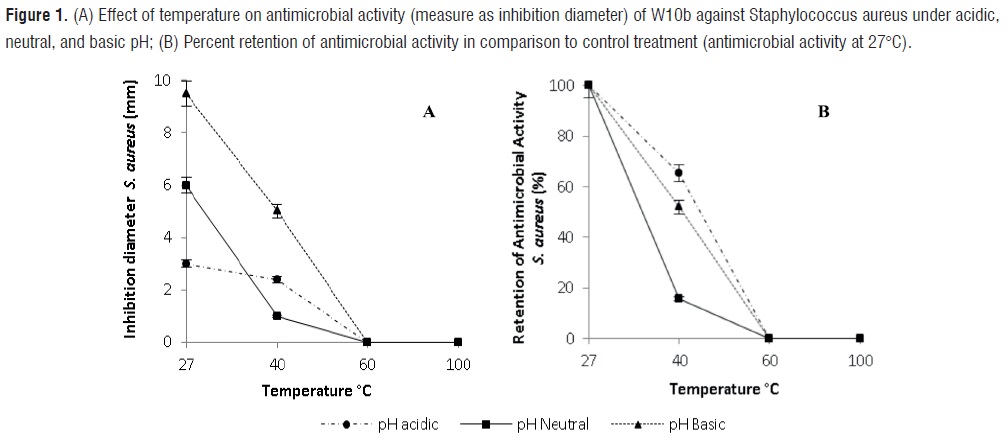
Effect of pH, temperature, and residence time on the antimicrobial activity of W10b against Staphylococcus aureus The analysis of variance showed a significant effect of the interaction of pH and temperature on the antimicrobial ability of W10b (P<0,05). However, no significant effect due to residence time (0, 10 and 30 min) was found (P=0,2383; table 1).
The best antimicrobial activity of W10b against S. aureus was found at basic pH and 27ºC (9,5 mm inhibition diameter; figure 1). This temperature is close to the growth temperature of W. confusa. However, for the same temperature (27°C), no significant differences were found on the antimicrobial activity when the W10b was exposed to acidic and neutral pH, suggesting similar antimicrobial activity against S. aureus at these conditions. Under basic, acidic and neutral pH levels and 40°C, there was a significant reduction (P<0,05) in the antimicrobial activity against S. aureus, with inhibition diameters averaging 5,0, 2,4, and 1,0 mm, respectively. At 60°C and 100°C the loss of antimicrobial activity was total (figure 1). Inhibition diameters of 3,0 mm and 2,4 mm were observed in acidic pH at 27°C and 40°C, respectively. When compared to the control sample (27°C, 100% of antimicrobial activity), a 65,3% retention of antimicrobial activity was observed at 40oC. Similarly, in the neutral pH at 27°C and 40°C, inhibition diameters of 6,0 mm and 1,0 mm were observed, respectively, indicating 15,6% retention of antimicrobial activity at 40°C.
Effect of pH, temperature, and residence time on the antimicrobial activity of W10b against Streptococcus agalactiae
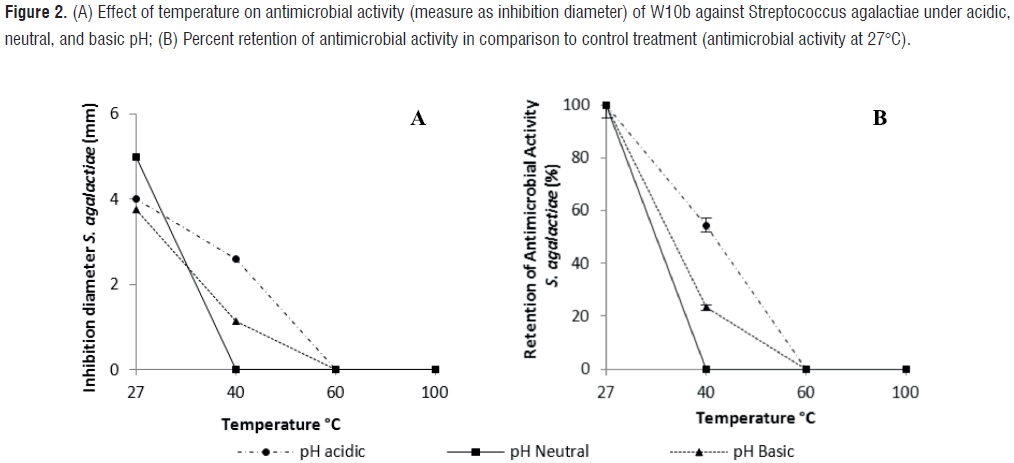
Significant interaction effects (P<0,05) due to pH and temperature were found, suggesting a synergistic action of the two factors on the antimicrobial activity of W10b against S. agalactiae. However, when only pH varied, no effect on the antimicrobial activity was observed (P=0,5052). On the other hand, no significant differences (P=0,3809) were found on the antimicrobial activity of W10b against S. agalactiae at different exposure times (0, 10 and 30 min; table 1).
At neutral pH and 27oC, W10b showed strong antimicrobial activity with a 5 mm inhibition diameter against S. agalactiae (Figure 2). However, at 40°C no antimicrobial activity was observed. Under acidic pH at 27°C and 40°C, the antimicrobial activity was 4,0 and 2,6 mm, respectively (with 54,5% retention of antimicrobial activity at 40°C). Whereas the best antimicrobial activity against S. aureus was found at basic pH and 27ºC (9,5 mm; figure 1), the same conditions resulted in the lowest antimicrobial activity against S. agalactiae (inhibition diameter = 3,8 mm). When W10b was exposed to basic pH and 40°C, the inhibition diameter was 1,1 mm (23,3% retention of antimicrobial activity).
Results for both strains were analogous for the effect of temperature on retention of antimicrobial activity. The W10b antimicrobial compounds showed the best retention activity at 40ºC and acidic pH with 65,3% for S. aureus and 54,5% for S. agalactiae. The lowest retention activity was obtained at 40°C and neutral pH with rates of 15,6% for S. aureus and 0% for S. agalactiae. However, the best antimicrobial activity resulted from the exposure of W10b to basic pH and 27°C for S. aureus and neutral pH and 27°C for S. agalactiae (figures 1 and 2).Effect of enzymes on the antimicrobial activity of W10b against Staphylococcus aureus
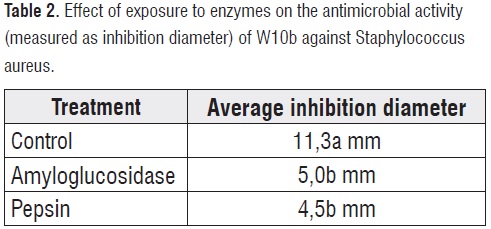
Significant differences for the antimicrobial effect of W10b against S. aureus were found between treated (exposed to enzymes) and control samples for both enzymes amyloglucosidase and pepsin (P<0,05; table 2). However, no significant differences between treated samples (exposure to amyloglucosidase vs. exposure to pepsin) were found.W10b treated with amyloglucosidase showed average inhibition diameters of 5 mm while the control treatment showed diameters of 11,3 mm (44% retention of antimicrobial activity). When W10b was treated with pepsin the inhibition diameter was 4,5 mm, a 39,8% retention of antimicrobial activity vs. the control treatment.
Values followed by different letters are significantly different using the multiple range Duncan test (α=0,05)Discussion
The objective of this research was to determine the potential commercial use of the antimicrobial W10b metabolic compounds from LAB W. confusa by studying changes in antimicrobial activity when exposed to extrinsic factors (temperature, pH, and enzymes). W10b was able to inhibit S. aureus under a wide range of pH (from 4,5 to 9,0) and temperature (27°C and 40°C). W. confuse metabolic products can potentially be used as biological preservatives in foods susceptible to contamination with S. aureus (e.g., yogurt, juices, vegetables, fermented foods, eggs, fresh meat, cheese and pasta) as well as in the therapeutic sector for products developed under the pH and temperature range studied in this work.
Since the exposure to temperature equal or higher than 60°C destroyed the antimicrobial activity of W10b against S. aureus, further research (e.g., protective encapsulation) is needed for agro industrial processes were higher processing temperatures are needed.
The antimicrobial activity of W10b against S. aureus in a wide pH range was comparable to previous studies using bioactive peptides. Todorov et al. [11] found that the ST16Pa bacteriocin produced by Lactobacillus plantarum maintained their activity after 2 h of incubation in pH ranging from 2 to 12 [12] reported that the stability of Bacteriocin F1 was not affected by a pH range from 3 to 9. Although, it has been reported that some bacteriocins are generally stable at acidic, neutral, and basic pH [13], the antimicrobial activity of W10b against S. agalactiae was dependent of the interaction between pH and temperature, similarly to results by Borges et al [14].When W10b was exposed to neutral, acidic and basic conditions and exposed to temperatures of 60°C and 100°C, no antimicrobial activity against S. agalactiae and S. aureus was found. These results contrast with those obtained by Todorov et al. [15], who reported that the activity of the bacteriocin produced by Lactobacillus sakei R1333 isolated from smoked salmon, was not affected when exposed to 100°C and pH 5,5. Similarly, Papagianni et al. [16] found that Wessellin A, a bacteriocin produced by Weissella paramesenteroides DX, was resistant to commercial sterilization conditions (121°C for 1 h) and pH ranging from 2 to 10.
Since differences in the antimicrobial activity of W10b against S. aureus and S. agalactiae were found (W10b best performed when exposed to basic pH against S. aureus and neutral pH against S. agalactiae). It was hypothesized that different receptor structures are needed in each microorganism [2] and different mechanisms of action are triggered when W10b is subjected to specific environmental and restrictive conditions.
A reduction on the antimicrobial activity against S. aureus was observed when W10b was exposed to the enzymes pepsin and amyloglucosidase. As previously reported, most bacteriocins are polypeptides, but others are sensitive to amylases [13] that evaluated Cell-free supernatants of Lactobacillus plantarum and loss of its activity when these were treated with proteinase K, trypsin, pronase E, suggest it is proteinaceous.The partial loss of antimicrobial activity when using pepsin also suggests that the W10b is associated to other non-protein compounds (possibly carbohydrates) which surround the molecule and restrict the hydrolytic action of the enzyme. For this reason, the loss of antimicrobial activity found in the treatment with pepsin was not total. On the other hand, the treatment with amyloglucosidase alters the compound structure affecting its activity.
CONCLUSIONS
The antimicrobial activity of the W10b metabolic compounds produced by W. confusa was affected by the extrinsic factors pH, temperature, and enzyme activity. The greatest antimicrobial activity against S. aureus and S. agalactiae was obtained at 27°C under basic and neutral pH, respectively. The greatest retention of antimicrobial activity for the two pathogens was obtained with acidic pH and the lowest activity retention was found with neutral pH. Therefore, future research should focus on assessing the antimicrobial activity of W10b at temperatures below 27°C, pH below 4,5, and exposure times longer than 30 min. These investigations will allow understanding changes in W10b stability, therefore expanding the application spectrum for this antimicrobial. Similarly, a thorough analysis of W. confusa genome and its mechanism of adaptation to temperature would allow understanding whether this bacterium and its metabolites can be used for the control foodborne pathogens.
These results focus on providing a potential industrial or commercial use of W10b to a wide range of products, which require moderate, or no heat treatments.ACKNOWLEDGEMENTS
The authors acknowledge the support from the “Young Researchers and Innovators - Virginia Gutiérrez de Pineda - Colciencias program - Generation of the bicentennial (2009), and the "Lactic acid bacteria for biotechnological - industrial applications" research group.
REFERENCES
[1] BATDORJ, B., DALGALARRONDO, M., CHOISET, Y., PEDROCHE, J., MÉTRO, F., PRÉVOST, H., CHOBERT, J.M. and HAERTLÉ, T. Purification and characterization of two bacteriocins produced by lactic acid bacteria isolated from Mongolian airag. Journal of Applied Microbiology, 101(4), 2006, p. 837-848. [ Links ]
[2] PAL, V., PAL, A., PATIL, M., RAMANA, K.V. and JEEVARATNAM, K. Isolation, biochemical properties and application of bacteriocins from Pediococcus pentosaceous isolates. Journal of Food Processing and preservation, 34(6), 2010, p. 1064-1079. [ Links ]
[3] DRIDER, D., FIMLAND, G., HÉCHARD, Y., Mc MULLEN, L.M. and PRÉVOST, H. The Continuing story of class IIa bacteriocins. Microbiology and Molecular Biology Reviews, 70(2), 2006, p. 564-582. [ Links ]
[4] NARBUTAITE, V., FERNANDEZ, A., HORN, N., JUODEIKIENE, G. and NARBAD A. Influence of baking enzymes on antimicrobial activity of five bacteriocin-like inhibitory substances produced by lactic acid bacteria isolated from Lithuanian sourdoughs. Letters in Applied Microbiology, 47(7), 2008, p. 555-560. [ Links ]
[5] THOMAS, L.V. and WIMPENNY, J.W.T. Investigation of the effect of combined variations in temperature, pH, and NaCl concentration on nisin inhibition of Listeria monocytogenes and Staphylococcus aureus. Applies Environment Microbiology, 62(6), p. 2006-2012. [ Links ]
[6] FUSCO, V, QUERO, G.M., STEA, G., MOREA, M. and VISCONTI, A. Novel PCR-based identification of Weissella confusa using an AFLP-derived marker. International Journal of Food Microbiology, 145(2-3), 2011, p. 437-443. [ Links ]
[7] CHOI, H., KIM, Y.W., HWANG, I., KIM, J. and YOON, S. Evaluation of Leuconostoc citreum HO12 and Weissella koreensis HO20 isolated from kimchi as a starter culture for whole wheat sourdough. Food Chemistry, 134(4), 2012, p. 2208-2216, [ Links ]
[8] SERNA-COCK, L., VALENCIA-HERNÁNDEZ, L.J. and CAMPOS-GAONA, R. Lactic acid bacteria with antimicrobial activity against pathogenic agent causing of bovine mastitis. Revista Biotecnología en el Sector Agropecuario y Agroindustrial, 9(1), 2011, p. 97-104. [ Links ]
[9] RUBIANO, L.F. y SERNA-COCK, L. Actividad antimicrobiana de Weissella confusa contra Escherichia coli. Vitae, 19(1), 2012, p.102-104. [ Links ]
[10] PARENTE, E., BRIENZA, C., MOLES, M. and RICCIARDI, A. A comparison of methods for the measurement of bacteriocin activity. Journal of Microbiological Methods, 22(1), p. 95-108. [ Links ]
[11] TODOROV, S.D., PRÉVOST, H., LEBOIS, M., DOUSSET, X., LEBLANCC, J.G. and FRANCO, B.D.G.M. Bacteriocinogenic Lactobacillus plantarum ST16Pa isolated from papaya (Carica papaya) from isolation to application: characterization of a bacteriocina. Food Research International, 44(5), 2011a, p. 1351-1363. [ Links ]
[12] MIAO, J., GUO, H., OU, Y., LIU, G., FANG, X., LIAO, Z., KE, C., CHEN, Y., ZHAO, L. and CAO, Y. Purification and characterization of bacteriocin F1, a novel bacteriocin produced by Lactobacillus paracasei subsp. tolerans FX-6 from Tibetan kefir, a traditional fermented milk from Tibet, China. Food Control, 42, 2014, p. 48-53. [ Links ]
[13] HONGXING, Z., LI, L., YANLING, H., SIQIONG, Z., HUI, L., TAO, H. and YUANHONG, X. Isolation and partial characterization of a bacteriocin produced by Lactobacillus plantarum BM-1 isolated from a traditionally fermented Chinese meat product. Microbiology and Immunology, 57(11), 2013, p. 746-755. [ Links ]
[14] BORGES, S., BARBOSA, J., SILVA, J. and TEIXEIRA, P. Characterization of a Bacteriocin of Pediococcus pentosaceus SB83 and Its Potential for Vaginal Application. Anti-Infective Agents, 12(1), 2014, p. 68-74. [ Links ]
[15] TODOROV, S.D., RACHMAN, C., FOURRIER, A., DICKS, L.M.T., VAN REENEN, C.A., PRÉVOST, H. and DOUSSET, X. Characterization of a bacteriocin produced by Lactobacillus sakei R1333 isolated from smoked salmon. Anaerobe, 17(1), 2011b, p. 23-31. [ Links ]
[16] PAPAGIANNI, M. and PAPAMICHAEL E.M. Purification, amino acid sequence and characterization of the class IIa bacteriocina Weissellin A, produced by Weissella paramesenteroides DX. Bioresource Technology, 102(12), 2011, p. 6730-6734. [ Links ]