Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biotecnología en el Sector Agropecuario y Agroindustrial
Print version ISSN 1692-3561
Rev.Bio.Agro vol.15 no.1 Popayán Jan./June 2017
https://doi.org/10.18684/BSAA(15)76-84
DOI: http://dx.doi.org/10.18684/BSAA(15)76-84
ANALYTICAL APPROACH OF THE VOLATILE FRACTION OF Solanum quitoense BY HSSPME/GC-MS
APROXIMACIÓN ANALÍTICA DE LA FRACCIÓN VOLÁTIL DE Solanum quitoense POR HSSPME/GC-MSZ
ABORDAGEM ANALÍTICA DA FRAÇÃO VOLÁTIL DE Solanum quitoense PELA HS-SPME/GC-MSz
EDUARDO CORPAS-IGUARÁN1, GONZALO TABORDA-OCAMPO2, OMAR TAPASCO-ALZATE3
1 Universidad Católica de Manizales, Instituto Investigación en Microbiología y Biotecnología Industrial IMBA, Grupo de Investigación y Desarrollo Tecnológico para el Sector Agroindustrial y Agroalimentario INDETSA. Ph.D en Ciencias Agrarias. Manizales, Colombia.
2 Universidad de Caldas, Facultad de Ciencias exactas y Naturales, Grupo de Investigación en Cromatografía y Técnicas Afines GICTA. Ph.D. en Ciencias Químicas. Manizales, Colombia.
3 Universidad de Caldas, Facultad de Ciencias exactas y Naturales, Grupo de investigación en Estadística y Matemáticas. Mgr. en Enseñanza de la Matemática línea de Estadística. Manizales, Colombia.
Correspondencia: ecorpas@ucm.edu.co
Recibido para evaluación: 28 de Marzo de 2016. Aprobado para publicación: 10 de Enero de 2017.
ABSTRACT
The species of lulo fruit (Solanum quitoense), predominant in Colombia, is a promising fruit for both national and international market due to its flavor and nutritional characteristics, which generated the interest to know the volatile composition of its pulp. After adjusting, the chromatographic conditions necessary to analyze volatile fraction of this fruit, the effect of the temperature and time of adsorption was measured through the headspace - solid phase microextraction (HS-SPME) and gas chromatography - mass spectrometry (GC-MS), on the area of volatile compounds of S. quitoense, by applying the experimental design of a factor. The descriptive analysis suggested that the adsorption at 60°C and 30 minutes promoted optimal recovery of volatiles as well as internal standard (1-Octanol, with recovery of 99,66% at 60°C), while the non-parametric test Kruskal-Wallis showed statistical differences in the effect of time (P= 0,018), but not of the temperature adsorption (P= 0,058) upon the volatiles compounds area. A predominance of esters (48,98%), aldehydes (18,37%), and alcohols (14,29%) was observed and also were found compounds of greatest area such as 3-hexen-1-ol acetate, acetic acid methyl ester, and acetic acid hexyl ester. These metabolites determine the characteristic aroma from lulo pulp and influence the consumer preference.
KEYWORDS: Chemical analysis, Chromatographic conditions, Lulo, Volatile organic compounds, Solid phase microextraction.
RESUMEN
La especie de lulo (Solanum quitoense), predominante en Colombia, es una fruta promisoria para el mercado nacional e internacional debido a su aroma y características nutricionales, lo cual generó el interés por conocer la composición volátil de su pulpa. Después de ajustar las condiciones cromatográficas necesarias para analizar los compuestos volátiles de esta fruta, se midió el efecto de la temperatura y tiempo de adsorción a través de la microextracción en fase sólida por espacio de cabeza (HS-SPME), sobre el área de los volátiles, aplicando un diseño experimental de un factor. El análisis descriptivo sugirió que la adsorción a 60°C y 30 minutos promovía una recuperación óptima de volátiles así como del estándar interno (1-Octanol, con recuperación del 99,66%), mientras la prueba no paramétrica de Kruskal-Wallis mostró diferencias estadísticas en el efecto del tiempo (P= 0,018), pero no de la temperatura de adsorción (P= 0,058), sobre el área de los compuestos volátiles. Además, se observó un predominio de ésteres (48,98%), aldehídos (18,37%) y alcoholes (14,29%) y se encontraron compuestos con áreas superiores como acetato de 3-hexen-1-ol, acetato de metilo y acetato de hexilo. Estos metabolitos determinan el aroma característico del lulo e influencian la predilección por el consumidor.
PALABRAS CLAVE: Análisis químico, Compuestos orgánicos volátiles, Condiciones cromatográficas, Lulo, Microextracción en fase sólida.
RESUMO
A espécie de lulo (Solanum quitoense), predominante em Colômbia, é uma fruta pertinente para o mercado nacional e internacional devido ao seu aroma e nutricionais características, o que gerou o interesse em conhecer a composição volátil da sua polpa. Depois de ajustar as condições cromatográficas necessárias para analisar os voláteis desta fruta, o efeito da temperatura e do tempo de adsorção foi medido pela microextração em fase sólida, por espaço de cabeça (HS-SPME), na área voláteis, aplicando um desenho de um fator. A análise descritiva sugere que a adsorção para 60°C e 30 minutos promoveu uma ótima recuperação de voláteis assim como do padrão interno (1-Octanol, com recuperação de 99,66%), enquanto que o teste não paramétrico de Kruskal-Wallis mostrou diferenças estatísticas no efeito do tempo (P= 0,018), mas não da temperatura de adsorção (P= 0,058), sobre a área dos voláteis. Além disso, observou-se uma predominância de ésteres (48,98%), aldeídos (18,37%) e álcoois (14,29%) e foram encontrados compostos com áreas superiores como acetato de 3-hexen-1-ol, acetato de metilo e acetato de hexilo. Estes metabolitos determinam o aroma característico do lulo e influenciam a preferência pelo consumidor.
PALAVRAS-CHAVE: Análise Química, Compostos Orgânicos Voláteis, Condições cromatográficas, Lulo, Microextração em fase sólida.
INTRODUCTION
During its maturation, fruits undergo biochemical, physiological and structural alterations, which include the production of volatile compounds [1], which are responsible of its aroma [2,3], and consequently on its quality and the preference by consumers [4,5,6].
Among the species of lulo, S. quitoense Lam., native from South America [7], is the species cultivated and consumed in Colombia due to its pleasant aroma and nutritional characteristics [8,9,10], conditions that have aroused the interest in taking advantage of its productive potential in industrial processing [11,12].
For the analysis of volatile compounds in fruit pulp by solid phase microextraction (SPME) different conditions of time and temperature absorption have been used. In fruits where have been used temperatures of 25°C or lower, such as Mobola plum (Parinari curatellifolia) [13] and monkey orange (Strychnos cocculoides) [14], times were predominantly greater than 30 minutes; whereas in fruits like pear (Pyrus ussuriensis) [15], the strawberry tree (Arbutus unedo L.) [16], melon (Cucumis melo L.) [17], avocado (Persea americana Mill., cv. Hass) [18], among others, where the temperatures used were equal to or higher than 40°C, times were predominantly equal or lower than 30 minutes.
In order to obtain higher performance in the extraction of SPME fiber, in some studies it was considered to optimize the time and the adsorption temperature. A referent for the purple passion fruit (Passiflora edulis Sims), yellow passion fruit (P. edulis Sims f. flavicarpa) and banana passion fruit (P. mollissima), in which a temperature of 50°C was used, with adsorption time of 10, 20, 40 and 60 minutes, obtained optimum extraction time of 40 minutes [19], whereas in common tomato (Lycopersicon esculentum var. commune) an extraction of 60 minutes at room temperature was chosen for its optimal efficiency. Similarly, in citrus juice extraction optimal conditions were obtained, corresponding to 60°C for 40 minutes [20].
Because the SPME is supported in the distribution equilibrium of the analytes between the food matrix and the fiber coating [21], and since it the equilibrium conditions are different in each compound, the total recovery of the concentrations of the metabolites in the sample becomes impossible, nevertheless, optimizing the extraction allows an appropriate efficiency to obtain a compounds profile that reflects the volatile composition of its matrix, including the analytes present in limited quantities (minoitary compounds). This study aimed at the implementation of the HS-SPME coupled to analysis by Gas Chromatography / Mass Spectrometry for identification and quantification of the volatile compounds in fresh pulp of S. quitoense.
METHOD
Place of the study
Samples were selected from the farm 'Villa Malicia', located at 1 km from Manizales. The analysis of the samples was carried out in the laboratory of chromatography of the University of Caldas in Manizales (Colombia).
Chemicals and distilled water
The distilled water was prepared using a purification system Direct-Q3 model ZRQS0P030 of Merck Millipore (Darmstadt, Germany). Sodium chloride (99%) was supplied from Carlo Erba Reagents (Barcelone, Spain). The internal standard 1-octanol was obtained from Sigma-Aldrich® (Saint Louis, USA). SPME holder used for manual sampling as well as the fibres employed for adsorption of volatile metabolites from pulp of S. quitoense were purchased in Supelco TM® (Bellenfonte, PA, USA).
Experimental
Initially, assays were done to adjust the optimum conditions of analysis by GC/MS, like: temperature ramp, injection mode, split radius to be used (considering that the sample contained majority and minority compounds), voltage of the detector and others. For each variable, temperature and adsorption time, the experimental design of a factor was applied, taking as the response variable the total area of volatiles. Were evaluated in duplicate the temperatures of 20, 40, and 60°C and the times 5, 15, and 30 minutes in both, the split injection mode 50 and 200, considering that some of the majority compounds saturated the detector of the mass spectrometer, which made it impossible to determine its area in split 50. The analysis of data took into account the behavior of the total area of the volatiles, chemical classes, internal standard, and compounds of greater area.
Lulo fruit characteristics
The selected samples were in the in stage five of ripening, diameter of 5 ± 1 cm, degrees Brix of 10,3 ± 1, and absence of spoilage due to the microorganisms or insects. In order to keep the integrity of samples, plastic separators placed between lulo fruits were used.
The fruits were stored at room temperature and the analyzes were performed in the following 24 hours. Conditions of sample preparation and volatiles extraction 10 g of the pulp of S. quitoense and 1 g of sodium chloride were weighed in an extraction vial. 5 µL of a solution of 1-Octanol with concentration of 0,0018 mol/L were added to the extraction vial. The container was sealed and placed in water bath with exposition of the SPME fiber in the headspace of the sample, under temperature and the time allotted for every test. The fiber was withdrawn and let to desorb during 1 minute at 230°C in the injection port of the GC/MS equipment, time after which this was retired. For extracting, a fiber Supelco TM® of 75 µm Carboxen (CAR)/Polydimethylsiloxane (PDMS) was used. The fiber employed was previously conditioned by its subjection to 235°C over 60 minutes to activate the functional groups present in the stationary phase.
Analysis and quantification conditions
The chromatographic analysis of the volatile compounds from the pulp of S. quitoense was carried out with a gas chromatograph Shimadzu GCMS/QP 2010 Plus, provided with a mass spectrometry detector (MSD) and it was used a column Shimadzu® SHRVGC (30 m x 0,25 mm ID x 1,4 µm DF). with temperature range of 40-240/260 °C under the following conditions:
Chromatographic conditions.
Injection temperature 30°C; Flow control mode linear velocity (36 cm s-1); Pressure of 55,2 kPa; Split injection mode (50 and 200); Column flow of 0,98 min-1; Purge flow of 3 min-1; equilibration time of 1 minute; Full timeout of 3,1 minutes. The temperature ramp was as follows: 50 °C during 1 min, increase at a rate of 2,5 min to reach 150°C with sustained of 7 min, increase at a rate of 15 min up to 220 °C with sustained of 3 min and and increase at a rate of 15 min to achieve 230 °C maintained in this for 2 min.
Spectrometric conditions.
Temperature of the ion source of 235°C; Interface temperature of 240°C; Solvent cutting time of 3 minutes; threshold of 1000 pA; Mass ratio initial and final of 33 - 350 (m/z).
Identification of the volatile compounds.
It was conducted from the comparison of mass spectra of the sample with the NIST® library, with a concordance at or above the 93%.
Data Analysis
For data analysis, the SPSS® software version 22 was used. The coefficients of variation in all groups were lower than 12%.
RESULTS
Compounds of S. quitoense obtained by HS-SPME to different times and adsorption temperatures.
The extraction process in the different times and adsorption temperatures used favored a recovery of 48 different volatile compounds (Table 1), with a predominance mainly of esters, represented by 24 compounds (48,98%), followed by 9 aldehydes (18,37%) and 7 alcoholic compounds (14,29%).
Effect of temperature and the adsorption time on the total area of volatile compounds
An increase in total volatile area according as the temperature was increased (Figure 1, left) can be observed in split 50, whereas in split 200 was a greater recovery denoted at 40°C (Figure 1, right). In both cases, the adsorption process was lower at 20°C, temperature nearby to environment of Manizales, where the studies were carried out, due to the increased temperature rose the concentration of analytes in the headspace, also favoring, a greater recovery of compounds with lower vapor pressure [21].
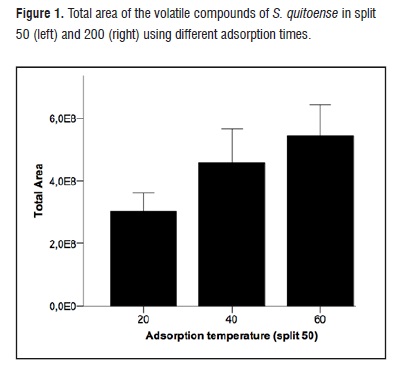 Figure 1
Figure 1Some studies show that the use of temperatures of 40 or 60°C is suitable for the recovery of volatile compounds in fruits such as pear (P. ussuriensis) [15], the strawberry tree (A. unedo L.) [16], melon (C. melo L.) [17], and avocado (P. americana Mill., cv. Hass) [18].
When establishing statistical differences between the areas by Kruskal-Wallis test (the nonparametric test was chosen because of the violation the assumption of homogeneity of variance by the data), differences were not found in the effect of adsorption temperature over the area of volatile compounds obtained (P= 0,058). In contrast, differences in the effect of adsorption time over the area of volatile compounds were found (P= 0,018).
Effect of temperature and adsorption time on the areas of chemical classes.
Concerning the effect of temperature, in 50 split was noted that except for the alcohols, which showed an upper area at higher adsorption temperature, a trend of increase or decrease of the area in the chemical classes is not perceived as the temperature was raised, because temperature causes an individual effect on each compound, related to its molecular weight, polarity, vapor pressure and boiling point [19], which in turn can affect the general behavior of the areas in each chemical class. This way, while compounds 2-Hexenal and 3-Pentanol increased as the temperature was raised, others such as methyl propionate and toluene had greater area in the test at 40°C. Similar behavior was appreciated in 200 split, where also was presented an increase in the area of alcohols as the temperature was raised. As to the adsorption time, in split 50 a growing trend was presented in the area of the chemical classes when this increased, which was appreciable mainly to the total area of esters, alcohols, hydrocarbons and aldehydes. These same groups of compounds also appeared increasingly when increased the adsorption time in split 200.
Behavior of the compounds with greatest relative abundance in the volatile fraction of S. quitoense at different adsorption temperatures.
Among the compounds with greatest area in the volatile profiling of S. quitoense, four of them correspond to esters (3-hexen-1-ol acetate, acetic acid methyl ester, acetic acid hexyl ester and ethyl acetate). In general, as the temperature was raised these five volatile compounds represented a lower percentage of area regarding the total area of volatile of the fruit, since at 20°C accounted for 85,5% of the total volatile fraction and at 60°C corresponded to 62,4% of the respective average profile; This behavior is related to low polarity compounds which required an increase of their kinetic energy through thermal supply to move from the gas phase and be adsorbed and analyzed. When analyzing the areas of these compounds individually, a trend of growing or decline with increasing of temperature is not clearly appreciated, except for acetic acid methyl ester where it was appreciated a decrease of the compound area with increasing temperature. As was indicated previously, temperature causes an individual effect on each of these compounds, related to its polarity and boiling point, and therefore some compounds like 3-hexen-1-ol acetate shown a greater area when the adsorption was developed at 40°C.
The relative standard deviation (RSD) was less than 12% in the groups corresponding to the compounds obtained.
Behavior of the compounds with highest relative abundance in the volatile fraction of S. quitoense at different adsorption times.
Just like was presented with the effect of temperature, a decrease in the percentage of area obtained for these five compounds in relation to the time of adsorption was denoted, so that in an adsorption of five minutes these compounds represented 85,2%, whilst at desorption of 30 minute, corresponded to a percentage of 76,5% of the total of compounds identified. This indicates that volatile compounds found in lower concentrations in the food matrix require greater time to be adsorbed so that they can represent a greater percentage with respect to the total area. Furthermore, when areas of these five compounds were analyzed individually, it was appreciated that except for acetic acid methyl ester, the other four compounds had a higher average area from the maximum adsorption time used (30 minutes). Figure 2 shows the chromatographic separation of volatile compounds from lulo pulp.
Effect of temperature and adsorption time on the recovery of the internal standard
Figure 3 shows the effect of temperature and adsorption time on the adsorption of the 1-Octanol used as internal standard, which had a recovery of 99,66% at 60°C for 30 min. This compound was recovered to a greater extent as the temperature was increased, with a superior level using 60°C, whereas at 20 °C, recovery levels were significantly limited (Figure 3, left). Similarly, it was appreciated that the recovery of 1-Octanol was greater as it was increased exposure time of the fiber towards the pulp of S. quitoense, therefore the time required for a suitable adsorption of standard was 30 minutes (Figure 3, right). There is precedent for the use of 1-Octanol as the internal standard, carried out in dry milled leaves of rosemary, at 60°C for 30 minutes, in which the recovery of this standard was 15% [19].
The volatile composition of the lulo pulp has a relationship with the sensory quality of this fruit, but also with the preference by the consumer, therefore it is important to know which compounds exist in the profile of this fruit. Moreover, processing companies of lulo pulp require to collect this fruit with specific characteristics in terms of the aroma compounds, since this influences the quality of the processed product.
Regarding the technological advances that represents the methodology employed, HS-SPME has been used for extraction of volatile compounds in different fruit pulps [15,16,17,18, 22], in which, as well as in the current study, could be obtained volatiles with low molecular weight and high volatility. These features of the volatile profiles are relevant to the sensorial characterization, since some volatiles metabolites had high impact on sensory perception and allow to characterize the aroma of a particular fruit. These conditions result in the relevance of maintaining the quality of the volatile profile from fruit pulps in industries such as food and fragrances.
CONCLUSIONS
After establishing the analytical conditions by CG/MS adequate to the study of the volatile fraction of S. quitoense, trends of descriptive analysis suggested that HS-SPME extraction process using a temperature of 60°C and time of 30 minutes promoted a proper recovery of volatile compound, although only statistical differences were evident in the effect of adsorption time over volatile compounds area. Given that the greater recovery of the internal standard was obtained at 60 °C, and its area is used for determining the concentration of the volatile compounds in the sample, is considered that this temperature is suitable between the evaluated ones, for the quantification of the volatile compounds of S. quitoense. Through the test, a predominance of esters was appreciated, followed by aldehydes and alcohols, and the compounds of greater area were 3-hexen-1-ol acetate, acetic acid methyl ester, acetic acid hexyl ester, and ethyl acetate. HSSPME is an alternative extraction method for obtaining the volatile compounds from lulo pulp without using solvents and avoiding the forming artifacts. Moreover, he nature of the extraction method used promoted the obtaining of a profile of volatile compounds, according to the analytical requirements of the food, flavors, and fragrances industries.
Finally, it is recommended the use of wider ranges of temperature and time as used in this study, as well as exploring diverse internal standards, to compare recovery rates obtained with 1-octanol.
ACKNOWLEDGEMENTS
The Ph.D. in Agrarian Sciences, Eduardo Corpas Iguarán, thanks to the Colciencias, for their financial contribution to the study process.
REFERENCES
[1] EL-SHARKAWY, I. et al. Stimulated auxin levels enhance plum fruit ripening, but limit shelf-life characteristics. Postharvest Biology and Technology, 112(1), 2016, p. 215-223. [ Links ]
[2] DU, X. and ROUSEFF, R. Aroma Active Volatiles in Four Southern Highbush Blueberry Cultivars Determined by Gas Chromatography-Olfactometry (GC-O) and Gas Chromatography-Mass Spectrometry (GC-MS). Journal of Agricultural and Food Chemistry, 62(20), 2014, p. 4537-4543. [ Links ]
[3] CONDE-MARTINEZ, N., SINUCO, D., and OSORIO, C. Chemical studies on curuba (Passiflora mollissima (Kunth) L. H. Bailey) fruit flavour. Food chemistry, 357(1), 2014, p. 356-363. [ Links ]
[4] GARCIA, C. et al. Changes in the bound aroma profiles of 'Hayward' and 'Hort16A' kiwifruit (Actinidia spp.) during ripening and GC-olfactometry analysis. Food Chemistry, 137(1-4), 2013, p. 45-54. [ Links ]
[5] DU, X., SONG, M., BALDWIN, E. and ROUSEFF, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chemistry, 171(1), 2015, p. 306- 314. [ Links ]
[6] BUTTARA, M., INTATAPICHET, K. and CADWALLADER, K. Characterization of potent odorants in Thai chempedak fruit (Artocarpus integer Merr.), an exotic fruit of Southeast Asia. Food Research International, 66(1), 2014, p. 388-395. [ Links ]
[7] FORERO, D., ORREGO, C., GRANT, D. and OSORIO, C. Chemical and sensory comparison of fresh and dried lulo (Solanum quitoense Lam.) fruit aroma. Food Chemistry, 169(1), 2015, p. 85-91. [ Links ]
[8] CORPAS, E., TABORDA, G., ORTÍZ A. y TAPASCO, A. Compuestos volátiles de la fracción volátil en pulpa de lulo (S. quitoense L.) bajo diferentes condiciones de almacenamiento. Vitae, 23(1), 2016, p. 831-836. [ Links ]
[9] MESSINGER, J. and LAUERER, M. Solanum quitoense, a new greenhouse crop for Central Europe: Flowering and fruiting respond to photoperiod. Scientia horticulturae, 183(1), 2015, p. 23-30. [ Links ]
[10] FLÓREZ-VELASCO, N., BALAGUERA-LÓPEZ, H. and RESTREPO-DÍAZ, H. Effects of foliar urea application on lulo (Solanum quitoense cv. septentrionale) plants grown under different waterlogging and nitrogen conditions. Scientia horticulturae, 186(1), 2015, p. 154-162. [ Links ]
[11] IGUAL, M., RAMIRES, S., MOSQUERA, L. and MARTÍNEZ-NAVARRETE, N. Optimization of spray drying conditions for lulo (Solanum quitoense L.) pulp. Powder Technology, 256(1), 2014, p. 233- 238. [ Links ]
[12] NGO, M. et al. Assessing crossmodal correspondences in exotic fruit juices: The case of shape and sound symbolism. Food Quality and Preference, 28(1), 2013, p. 361-369. [ Links ]
[13] SHOKO, T., SAKA, J. and APOSTOLIDES, Z. Headspace volatiles of the edible fruit pulp of Parinari curatellifolia growing in Malawi using solid phase microextraction. South African Journal of Botany, 90(1), 2014, p. 128-130. [ Links ]
[14] SHOKO, T., APOSTOLIDES, Z., MONJEREZI, M. and SAKA, J. Volatile constituents of fruit pulp of Strychnos cocculoides (Baker) growing in Malawi using solid phase microextraction. South African journal of botany, 84(1), 2013, p. 11-12. [ Links ]
[15] QIN, G. et al. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food chemistry, 134(4), 2012, p. 2367- 2382. [ Links ]
[16] OLIVEIRA, I. et al. Volatile profile of Arbutus unedo L. fruits through ripening stage. Food chemistry, 128(3), 2011, p. 667-673. [ Links ]
[17] CONDURSO, C. et al. Effects of different rootstocks on aroma volatile compounds and carotenoid content of melon fruits. Scientia horticulturae, 148(1), 2012, p. 9-16. [ Links ]
[18] OBENLAND, D., COLLIN, S., SIEVERT, J., NEGM, F. et al. Influence of maturity and ripening on aroma volatiles and flavor in 'Hass' avocado. Postharvest biology and technology, 71(1), 2012, p. 41-50. [ Links ]
[19] PONTES, M., MARQUES, J. and CÂMARA, J. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchemical Journal, 93(1), 2009, p. 1-11. [ Links ]
[20] NARDINI, G. et al. Determination of volatile profile of citrus fruit by HS-SPME/GC-MS with oxidized NiTi fibers using two temperatures in the same extraction procedure. Microchemical journal, 109(1), 2013, p. 128-133. [ Links ]
[21] IBÁÑEZ, E. et al. Analysis of volatile fruit components by headspace solid-phase microextraction. Food chemistry, 63(2), 1998, p. 281-286. [ Links ]
[22] SPADAFORA, N. et al. Detection of Listeria monocytogenes in cut melon fruit using analysis of volatile organic compounds. Food Microbiology, 54(1), 2016, p. 52-59. [ Links ]














