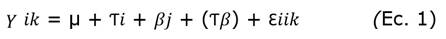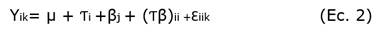INTRODUCTION
Gyneriun sagitatum Aubl. (Poaceae) is a perennial, giant, rhizomatous, reed grass with erectus stems basal clothed with bladeless sheaths while the upper part has the unfold leaf blades with an open fanshaped form. Leaves are bright green 160 to 230 cm long and 8 to 14 cm wide; a culm can form as much as 200 leaves during lifetime with 19 to 28 fresh blades at any time [1,2 ]. For centuries, in the flatlands of Córdoba and Sucre Departments (Colombia), indigenous Zenú communities have used arrow cane central nerve to make distinctive handicrafts worldwide known because of their beauty and tradition; one of the best known is the Sombrero Vueltiao established as Colombian cultural symbol; however, increasing demand of arrowcane products has negatively impacted arrow cane natural populations affecting not fiber availability but also associated wetland ecosystems. The growing handicraft market will require enough fiber supply to sustain increasing sales, to reduce the negative impact on the environment and to maintain the Zenú artisan legacy.
Arrow cane seeds produced in humid Caribbean are unviable; therefore, establishing an efficient clonal plant propagation system for large production of plant material has been a challenge. Cutting treatments such as source (aerial, underground), length, position (vertical, horizontal), substrate and hormone addition for rooting has been evaluated; however, most results showed the need for large cutting portions (<30 cm) making inefficient large-scale propagation using this method [3,4 ]. Micropropagation instead has been proved to be an efficient technique to produce large quantities of high quality plants from arrow cane Cv “Criolla” using explants with pre-existing meristems [4,5,6,7,8,9], but there are several other cultivars used by growers and artisans based on fiber softness, resistance and availability. In the present work, the micropropagation of arrow cane cultivars “Criolla”, “Costera” and “Martinera” were evaluated as a way for providing an efficient plant propagation system for establishing commercial arrow cane crops for fiber supply, for craftsmanship industry and to contribute to lower artisan activity impact on the environment.
METHOD
The study was done at the Plant Biotechnology Lab of the Biotechnology Institute of the Colombian Caribbean at the Agricultural Sciences School of the Universidad de Córdoba, Monteria, Colombia, located at Latitude 8° 31' North and Longitude75° 58' to Greenwich Meridian.
Plant material
Explants were isolated from in vitro established arrow cane cultivars “Criolla”, “Martinera” and “Costera” cultured in 250 cc flasks containing 30 ml of semisolid Murashige and Skoog (MS) medium [10 ] with (in mg L-1) myo inositol (100), sucrose (30.000), thiamine HCl (0,4) and TC agar (8.000) (Sigma Co.). Flasks were covered with two layers of heavy-duty aluminum foil, sealed with Nescofilm® and maintained at 20 °C with 12 h photoperiod (40 µmol m2 s2). Plants were maintained for more than a year with transfer to fresh medium of the same formulation every four weeks.
Shoot multiplication
Explants, consisting of clusters with three shoots isolated from in vitro growing “Criolla”, “Costera” and “Martinera” plants, were established into 200 cc flasks similar conditions to establishing stage except for addition of four benzylaminopurine (BAP) concentrations (0,0; 0,5; 1,0 and 1,5 mg L-1). The experiment was a 2-way factorial (3 cultivars x 4 BAP levels) with 12 treatments and 10 replicates per treatment; the experimental units (120) were distributed using a complete randomized design. After six weeks in culture, the variables number of new shoots per explant and the average shoot length were registered. Data were analyzed using an ANOVA based on the model:
where µ was the general mean, Ƭi was the effect of cultivar, βj was the effect of BAP level and Ɛ iik was the experimental error; means were separated with Tukey test (α = 0,05).
In vitro rooting
Three-shoots clusters isolated from in vitro multiplied shoots of “Criolla”, “Costera” and “Martinera” plants were transferred onto 30 ml semisolid MS with similar conditions to establishing stage except for addition of and independently supplied with four (0,0; 0,5; 1,0 and 1,5 mg L-1) naphthalene acetic acid (NAA). After four weeks in culture, the number of cultures with roots, the number of roots per culture and the average root length per culture were registered. Collected data were analyzed with ANOVA based on the model:
where µ was the general mean, Ƭi was the effect of cultivar, βj was the effect of NAA level and Ɛ iik was the experimental error.
Means were separated with a Tukey test (α = 0,05).
Transfer to ex vitro conditions
In vitro rooted and rootless shoots from multiplication stage were transferred to ex vitro conditions in order to evaluate plant survival rate. In both cases, cultures were extracted from the flasks, washed with distilled water to remove medium remains, and planted in 72-plug trays filled with peat as substrate. A single culture was placed in every plug, sprayed with distilled water and the tray covered with a plastic transparent cover. Trays were placed in a shadehouse (50% light) at 28 C with fog irrigation every 4 h during 1 min. After 3 days, covers were removed and irrigation frequency was changed to one every 8 hours. After 40 days, the number of plants that survived was registered.
RESULTS
Shoot multiplication
New shoot growth from established explants was observed for all treatments. Growing shoots emerged from pre-existing meristems present in the original established explant. Cultures from all cultivars showed short stems with small leaves and dark green color as evidence of well adaptation to in vitro conditions (Figure 1).
The ANOVA allowed to detect statistical differences (Pr< 0,05) among mean results of new shoot formation rate as a result of the effect of cultivar, BAP concentrations in the medium and the interaction between both, cultivar versus BAP concentrations. The collected data showed that the highest multiplication rate (129 new shoots per explant) occurred when explants of “Martinera” were cultured in medium supplied with 1,0 mg L-1 BAP; in contrast, the lowest number of new shoots per explant (8,2) were observed in the same cultivar when explants were cultured in absence of BAP (Table 1). The Tukey test showed that for all three cultivars, the addition of BAP in the culture medium statistically increased, (>3x), the multiplication rate compared to non BAP-supplied media (Table 1). For cultivars “Criolla” and “Costera” multiplication rate were not different among BAP-supplied treatments; however for “Martinera”, 1,0 mg L -1 BAP in the medium induced a higher number of new shoots than other BAP treated explants (Table 1). For all cultivars, it was observed that a 1,5 mg L-1 BAP supply in the media lowered the formation of new shoots.
Table 1 Effects of different BAP levels on in vitro arrow cane shoot multiplication.
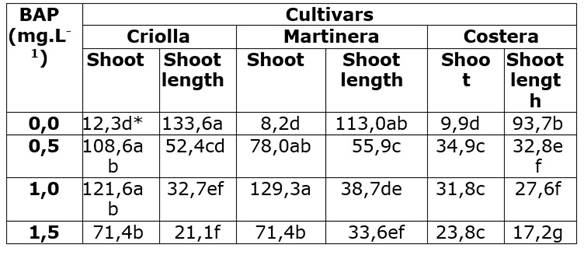
*Numbers with same letter are not different according to Tukey test (α 0,005)
For the variable shoot length, the ANOVA showed that length of shoots were statistically different (Pr< 0,05) as a result of the cultivars, BAP treatments and the interaction between both, BAP levels and cultivars. For all cultivars, the longest shoots grew in in media deprived of BAP while those cultured in the 1,5 mg L-1 BAP were the shortest (Table 1). There was an evidenced gradual reduction in shoot length as BAP levels were increased. The longest shoot average were registered in “Criolla” shoots cultured in medium with non-BAP supply while the shortest shoots developed from “Costera” explants cultured in medium supplied with 1,5 mg L-1 BAP (Table 1).
In vitro rooting
Adventitious root formation was observed in all cultures; however, statistical differences were detected (Pr<0,05) based on the ANOVA analysis with respect to the number and length of adventitious roots as a result of the effect of cultivar and NAA concentrations in the medium (Figure 2).
For cultivars “Criolla” and “Martinera”, shoots cultured in NAA-supplied media formed a significant higher number of roots than those cultured in absence of NAA (Table 2); while for “Costera”, the number of adventitious roots increased at least 4x in shoots cultured in NAA-supplied media compared to those cultured in simple media (Table 2). For shoots of all cultivars, the number of emerging adventitious roots increased as the NAA concentration in the medium was increased, registering the highest number of roots (73) for “Martinera” shoots cultured in medium supplied with 1,5 mg L-1 NAA and the lowest number (5,4) of adventitious roots in “Costera” shoots cultured in medium without NAA (Table 2).
Table 2 Rooting of three arrow cane cultivars in media with different levels of NAA.
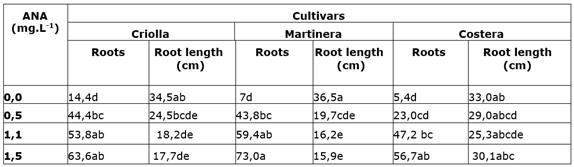
*Numbers with same letter are not different according to Tukey test (α 0,005)
Average length of adventitious root formed in vitro showed statistical differences (Pr< 0,05) as a result of the effect of cultivar, NAA concentrations and the interaction of both factors. Although, for all cultivars, the longest roots grew in media without NAA, only roots produced from “Martinera” shoots were statistically larger than roots grown from shoots cultured in any NAA supplied media (Table 2). “Criolla” and “Martinera” shoots developed roots that were shorter as NAA concentration in the culture medium was increased, while for “Costera” shoots, root length showed no statistical different at any NAA treatment (Table 2); “Martinera” adventitious roots registered the longest (36,5 cm) and shortest (15,9 cm) average root length.
Plant adaptation to ex vitro conditions
Transplanted in vitro rooted plants from all cultivars, as well as rootless shoots from multiplication stage, showed a complete (100%) survival and full adaptation to ex vitro conditions. Four weeks after transplant, plants were healthy, green colored and actively growing with no dead tissues, necrotic areas or adventitious growth (Figure 3). Performance of both, rooted and rootless shoots, on field conditions evidenced that in vitro rooting is not necessary for arrowcane micropropagated plants to adapt to ex vitro conditions; this shows that ex vitro transfer from multiplication stage can be performed with no implications for plant survival, but instead time for plant production can be shortened and costs reduced (Figure 3 ).

Figure 3 Ex vitro adaptation of micropropagated arrow cane plants cultivars “Criolla and “Martinera”.
Micropropagation is a clonal plant propagation technique to produce plants in aseptically closed recipients with artificial media and stored under controlled environmental factors, that has proved to be very efficient for producing large quantities of high quality plants in comparative short periods of time and reduced space [11,12,13]. Arrow cane micropropagation as well as other biotechnological techniques has been performed on “Criolla” cultivar [9 ]. Artisans prefer Cv “Criolla” flexibility and resistance fiber to make hats; however, fiber from other cultivars such as “Martinera” and “Costera” are precious for their softness and plant availability to make shoes, necklace, bracelets, rings and purses. However, in the present research in vitro multiplication, rooting and ex vitro adaptation of “Martinera” and “Costera” to “Criolla” plants were evaluated.
In vitro multiplication based on culture of pre-existing meristems is promoted by adding cytokines to the culture medium in order to disrupt apical dominance and induce a repetitive axillary shoot growth [14,15,16,17,18,19,20,21,22]. The results of the multiplication stage evaluation showed that supplying BAP in the culture medium induced an increase in shoot multiplication rate for all cultivars compared to non-supplied treatments; effects previously reported in different plant species [22, 23 ]. BAP supply association to increased shoot multiplication for arrow cane micropropagation has been reported for different cultivars and alternative media formulation. A previous study reported significant increase in new shoot formation for cultivars “Criolla”, “Criolla 1” and “Martinera” when cultured with different BAP concentrations in double phase (liquid/semisolid) medium system; indicating that cytokine effects on arrow cane in vitro multiplication are independent o cultivar and culture system Previous reports on arrow cane micropropagation using semisolid system [4].
Despite the significant increase in BAP presence, shoots of cultivars “Criolla” and “Martinera” multiplied at least 4x more than “Costera” shoots when cultured in a medium supplied with 1,0 mg L -1 BAP. This indicates significant genotype dependent response to culture conditions and BAP supply; however, even for “Costera” shoots, a multiplication rate of >30 shoots per multiplication round means potentially producing >2 million plants in less than a year.
High quality shoot multiplication stage must complain not only for high multiplication rate but also for good size shoots that are able to develop new organs specially when transferred to ex vitro conditions [24,25,26 ]. In the present research, shoots reduced >50% their length when cultured in media supplied with BAP and decreasing as BAP levels increased; however, the shortest shoots were >10 mm. Usually, micropropagated shoots shorter than 10 mm are prone to die when transferring to ex vitro conditions due to shortage in energetic reserve supply [27,28]. The collected data showed that when shoots were cultured in the presence of 0,5 mg L-1 long was at least 33 mm, maintaining a larger standard for plant survival after transferring to ex vitro conditions. Root formation is a critical step for micropropagated plant to be able to take water and minerals [29,11,30 ]. Usually, in vitro cultured plants are able to grow without roots since media formulation and environmental conditions protect plants from dehydration and minerals diluted in the medium are easily adsorbed by plants tissues [31 ]. In contrast, when transferred to ex vitro conditions, roots are necessary for both, water and nutrient uptake [32,33]. Adventitious root growth from in vitro cultured shoots is induced by supplying auxins in the culture medium [34,35]. In the present research, micropropagated shoots cultured in absence or presence of ANA did form adventitious roots independently of the cultivar, indicating that arrow cane is an easy to root plant species [36]. In vitro rooting evaluation is based on both quantity and quality of roots formed.
For non-NAA supplied treatments, “Criolla” shoots formed >10 roots per explant; however, “Martinera” and “Costera” resulted in low root formation. As observed in previous studies, in the present research, shoots cultured in NAA supplied media formed 4X more roots than those ransferred to simple media with at least 20 adventitious shoots per explant, and increasing the number of roots as NAA level in the medium were augmented [37,38,39,7,8]. These results showed that the quantity of adventitious roots formed in vitro by arrow cane micropropagated shoots increases when shoots are cultured in the presence of NAA in the medium, independently of the genotype. However, with respect to root quality, length and width of roots considered as indicators[40,41,42], Long-thin roots are usually considered inconvenient since they are fragile and transfer to ex vitro may require trimming that opens wounds for microbial contamination under ex vitro conditions. Non-supplied NAA treatments not only induced a lower number of roots but also larger roots than those produced by shoots cultured with NNA-supplied media. The in vitro rooting data collected in the present research suggests that NAA supply not only increases the number of adventitious roots but also improves quality of root formation.
Ex vitro adaptation is the stage where micropropagated plants are adapted to regular environmental conditions. Two are the major problems for micropropagated plants to survive ex vitro conditions: low control of water loss and low photosynthetic competence [43,44 ]. In vitro micropropagated plants are cultured in culture media with >90% water inside sealed containers that produce a highly saturated humid environment. Under these conditions, plants do not develop mechanism to control water loss, such as stomata closure and cuticle accumulation; therefore, if transferred directly to outside conditions, tissues will rapidly dehydrate to death. To avoid this situation, in vitro maintained plants must be transferred initially to a water saturated environment, usually modified with fog irrigation systems to protect plants from high water loss. On the other hand, in vitro cultivated plants grow under low light intensity that results in a deficient chloroplast development and incompetence for photosynthesis activity; therefore, during adaptation to ex vitro conditions sunlight should be increased gradually to avoid plant loss and induce new leaf formation to acquire full photosynthetic competence. Energy required for new leaf development is obtained from energetic reserves store in Shoots organs such as stem and leaves, developed in vitro [45,46,47,48,49,50 ]. In the present research, micropropagated shoots, with and without adventitious roots, were transferred to ex vitro conditions in a shadehouse with fog irrigation. Both conditions were gradually reduced until plants were established under complete sunlight with a single daily irrigation. Independently from cultivar, all plants transferred to ex vitro conditions fully adapted and survived (100% survival). These results confirm once again that direct transfer of arrow cane shoots from propagule multiplication stage to ex vitro adaptation do not have any effect on plant survival rates compared to transferring of in vitro rooted shoots but instead reduces costs and time.
CONCLUSIONS
BAP supply in the medium increased in vitro shoot multiplication rates and reduces shoot length for arrow cane micropropagated plants cultivars “Criolla”, “Martinera” and “Costera”. Addition of NAA in the culture medium for root formation increased the number of adventitious roots formation for arrow cane micropropagated plants cultivars “Criolla”, “Martinera” and “Costera”. In vitro rooting did not have effect on survival rates of for arrow cane micropropagated plants cultivars “Criolla”, “Martinera” and “Costera” when transferred to ex vitro conditions. The present micropropagation protocol offers the opportunity for large quantity of arrow cane plant production of “Criolla”, “Martinera” and “Costera” cultivars.