Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Ciencias de la Salud
versión impresa ISSN 1692-7273versión On-line ISSN 2145-4507
Rev. Cienc. Salud v.9 n.3 Bogotá sep./dic. 2011
Expression of TRF2 and GAPDH in the aging of the in vitro
ovarian surface epithelial cells
Expresión de TRF2 y GAPDH en el envejecimiento de las células del epitelio superficial del ovario in vitro
Expressão de TRF2 e GAPDH no envelhecimento do epitélio superficial do ovário in vitro
Lilian Chuaire-Noack, PhD1, Pedro Monterrey-Gutiérrez, PhD2, César E. Payán-Gómez, PhD (a)3
1 Profesora titular, Facultad de Ciencias Naturales y Matemáticas, Universidad del Rosario, Bogotá, D.C. (Colombia). Correo electrónico: lilian.chuaire@urosario.edu.co
2 Profesor titular, Facultad de Ciencias Naturales y Matemáticas, Universidad del Rosario. E-mail: pedro.monterrey@urosario.edu.co
3 Profesor principal, Facultad de Ciencias Naturales y Matemáticas, Universidad del Rosario. E-mail: cesar.payan@urosario.edu.co
Recibido: Julio 28 de 2011 • Aceptado: Septiembre 12 de 2011
Para citar este artículo: Chuaire-Noack L, Monterrey-Gutiérrez P, Payán-Gómez CE. Expression of TRF2 and GAPDH in the aging of the in vitro ovarian surface epithelial cells. Revista Ciencias de la Salud 2011; 9(3): 219-228.
Abstract
GAPDH can bind single-strand telomere DNA both in vitro and in vivo. Thus, it was hypothesised that GAPDH has an important role in protecting the telomeres, role that could be shared with TRF2, a well-known telomeric protein involved in a myriad of functions related to telomere homeostasis. Objective: The aim of this study was to determine if there was a correlation between the expression of these genes in the in vitro ovarian surface epithelium. Materials and methods: The relative expression of each gene was established by qRT-PCR in primary cell cultures of the ovarian surface epithelium from 22 healthy mestizo Colombian donors. Results: The Kendall and Spearman non-parametric tests established a significant correlation between the levels of expression in subsequent passages of the cell line, in an age-independent way. Conclusion: Our findings suggest a synergistic effect between TRF2 and GAPDH that could counter telomere shortening in vitro.
Key words: gene expression, cell aging, replicative senescence.
Resumen
Se ha demostrado que la proteína GAPDH se puede unir al ADN telomérico de cadena sencilla, tanto in vitro como in vivo. Por lo tanto, se ha planteado la hipótesis de que la GAPDH juega un papel importante en la protección de los telómeros, papel que podría ser compartido con la TRF2, proteína que participa en una gran variedad de funciones relacionadas con la homeostasis telomérica. Objetivo: el objetivo de este estudio fue determinar si existe una correlación entre la expresión de ambos genes en el epitelio superficial del ovario in vitro. Materiales y métodos: la expresión relativa de cada gen fue establecida mediante qRT-PCR, en cultivos primarios de células del epitelio superficial del ovario provenientes de un grupo de 22 donantes colombianas mestizas sanas. Resultados: las pruebas no paramétricas de Kendall y Spearman permitieron establecer que existe una correlación significativa entre los niveles de expresión de GAPDH y TRF2 a lo largo de la historia replicativa de los cultivos, en forma independiente de la edad de las donantes. Conclusión: nuestros resultados sugieren un efecto sinérgico entre TRF2 y GAPDH, que podría estar orientado a contrarrestar la reducción de los telómeros in vitro.
Palabras clave: expresión génica, envejecimiento celular, senescencia replicativa.
Resumo
Tem se demonstrado que GAPDH pode-se unir ao DNA telomérico de cadeia simples, tanto in vitro quanto in vivo. Portanto, tem se apresentado a hipótese de que GAPDH joga um papel importante na proteção dos telómeros, papel que poderia ser compartilhado com TRF2, proteína que participa em uma grande variedade de funções relacionadas com a homeostase telomérica. Objetivo. O objetivo deste estudo foi determinar se existe uma correlação entre a expressão de ambos os genes no epitélio superficial do ovário in vitro. Materiais e métodos: A expressão relativa de cada gene foi estabelecida mediante qRT-PCR em cultivos primários de células do epitélio superficial do ovário provenientes de um grupo de 22 doadoras colombianas mestiças sanas. Resultados. As provas não paramétricas de Kendall e Spearman permitiram estabelecer que existe uma correlação significativa entre os níveis de expressão de GAPDH e TRF2 ao longe da história replicativa dos cultivos, em forma independente da idade das doadoras. Conclusão. Nossos resultados sugerem um efeito sinérgico entre TRF2 e GAPDH que poderia estar orientado a contra-arrestar a redução dos telómeros in vitro.
Palavras chave: expressão gênica, envelhecimento celular, senescência replicativa.
TRF2 (TTAGGG repetition factor 1) is an important regulation factor of telomere length. Along with TRF1, RAP, T1N2 and TPP1, TRF2 comprises the shelterin complex, also known as the telosome. This complex interacts with DNA for a variety of functions including replication, repair and recombination and is also involved in the control of the cell cycle (1-3). TRF2 also regulates different enzymatic activities (4), promoting the formation of DNA structural conformations, such as T-loops and Holliday junctions, that regulate telomere homeostasis and prevent the formation of chromosomal defects at its ends (4, 5). The over expression of TRF2 results in telomere shortening and onset of premature aging (6). The loss of T-loops appears to have a fundamental role in both events (7-9).
On the other hand, GAPDH encodes an enzyme that is involved in the glycolytic and gluconeogenic pathways, catalysing the reversible oxidative phosphorylation of glyceraldehyde3-phosphate. Thus, it has a higher expression level in those tissues with high demand for energy, such as musculoskeletal or cardiac tissues (10). In the cell nucleus, GAPDH is involved in transcription (11), cell cycle regulation (12), DNA replication and repair (13), tRNA exportation (14), cell death (15-17) and several other processes. The discovery that GAPDH can bind single-strand telomere DNA both in vitro and in vivo has led to the hypothesis that GAPDH also has a role in protecting the telomeres, which is vital for cell viability (18, 19).
Because TRF2 and GAPDH are both involved in the regulation of telomere homeostasis, the aim of this study was to determine if there was a correlation between the expression of these genes in primary cell cultures of ovarian surface epithelium, using qRT-PCR.
Materials and methods
Donors
Ovarian tissue samples from 22 mestizo Colombian women were collected and cultured. These women did not have any history of cancer, and their ages ranged between 13 and 57 years. After agreeing to participate in the study and signing the consent forms, the donors underwent total or partial oophorectomy under benign conditions, in the obstetrics and gynaecology service of five Bogotá DC hospitals from 2007 to 2009.
Primary cell cultures
The ovarian epithelium and stroma were separated using dispase (Dispase II, Roche Applied Sciences) at 37°C and 5% CO2. The epithelial tissue fragments were seeded in culture vials with MCDB105 (Sigma)/M199 (Gib-Co) growth medium mix supplemented with 10% FBS and 0.05% of 10,000 U penicillin-streptomycin (Invitrogen). After reaching a confluence of 80-85%, the cells were dispersed with 0.25% trypsin-EDTA (Invitrogen), followed by the addition of extra growth medium to inactivate the enzyme. The cells were then centrifuged, and the resulting pellet was resuspended. The number of cells was counted with trypan blue in a haemocytometer prior to each passage, and they were seeded again in a 1:2 ratio.
Anticytokeratin 18-FITC (Sigma) was used to characterise the epithelial cells from three passages of each culture using fluorescence microscopy to record the signal. To prevent the growth of contaminating cells, a specific growth medium (described above) that does not allow the growth of epithelial cells when fibroblasts are present was used. The identity of the epithelial cells was established by cell morphology and the presence of anticytokeratin-18, which is absent in fibroblasts and leucocytes, during the first three passages of the culture.
Determination of cell aging
Senescence was determined by a visual inspection of the cultures. Specifically, cells were determined to have aged at the passage in which they acquired a flattened morphology that was reminiscent of mesenchymal cells and showed increased granularity. At this point, the cultures had difficulty reaching a confluent state. In addition, the senescence-associated β-galactosidase (SA-β-gal) activity at pH 6 was measured during each passage using chemiluminescence with the Beta-Glo Assay System kit (Promega Co, Madison WI, USA), which allows to determine the start of the senescence due to the notorious increase in the enzymatic activity.
qRT-PCR
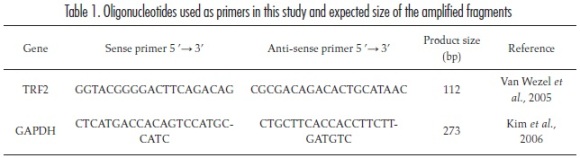
RNA from epithelial cells and stromal fibroblasts from passages 1-7 was extracted using Trizol® (Sigma). Its concentration and purity were evaluated using the NanoDrop 2000 (Thermo Scientific) spectrophotometer. The concentration was calculated using the absorbance at 260 nm. Sample purity was determined using the ratio of the absorbances at 260 and 280 nm, with a ratio between 1,8 and 2 indicating a pure sample. This ratio was also used to rule out contamination with proteins or DNA. In addition, integrity was evaluated using a denaturing agarose gel in the presence of ethid-ium bromide. Next, cDNA was synthesised using the SuperScript II First-Strand reverse transcription synthesis system for RT-PCR (Invitrogen). To minimise the possible sources of variation, each RT reaction used the same amount of RNA (500 ng). Real time PCR was carried out in a total volume of 20 μl containing the following reagents: Platinum® SYBR® Green qPCR SuperMix-UDG (InvitrogenTM) 1X, ROX (Invitrogen) 500 nM, positive sense DNA primer 500 nM, anti-sense DNA primer 500 nM (Table 1), 2 μl of cDNA and 3 μl of deionised water.
The resulting cDNA was amplified using the MJ Research® Opticon 2 thermocycler for a total of 40 cycles, each consisting of denaturation at 94°C for 15 seconds, primer annealing at 59°C for 30 seconds and elongation at 72°C for 30 seconds.
The primer sequences were analysed with the Primer Blast software program (Primer-Blast in: <http://www.ncbi.nlm.nih.gov/tools/primerblast/index.cgi?LINK_LOC=BlastHome>) to confirm their specificity and to determine the size of the amplified product.
Because TRF2 and GAPDH had the same thermal profile, the corresponding cDNA samples were amplified simultaneously and in duplicate for each experiment. In addition, efficiency curves were constructed using a dilution series of reference cDNA samples ranging from 3.125-100 ng/μl. Melting curves were obtained to ensure the exclusive amplification of TRF2 and GAPDH.
Normalisation and data analysis
The results were normalised based on the amplification efficiency of each gene raised to the difference between the ct of the passage analysed and the CT of the first passage (calibration value) according to the formula given by Livak & Schmittgen 2001 (20). After applying the Kolmogorov-Smirnov and Shapiro-Wilk tests, the results were processed with covariance analysis (Ancova), which used a regression model to analyse the effect of the culture passages and the age of the donors, as well as with the Kendall and Spearman non-parametric correlation tests. P values less than 0.05 were considered statistically significant.
Results
The amplification efficiency was calculated with the formula E = 10(-1/-S), where S corresponds to the slope of the efficiency curves. The efficiency for TRF2 and GAPDH was 1.94 (94%) and 1.93 (93%) respectively, and these values were used to normalise the results.
The melting curves verified the existence of a single fluorescence peak at each concentration, confirming the specificity of the primers and therefore the exclusive amplification of the TRF2 and GAPDH gene products.
Real-time PCR
The relative expression of each gene was calculated according to the formula from Livak & Schmittgen, 2001 (20), where Relative Expression = Amplification Efficiency(CT passage x - CT passage 1).
TRF2 and GAPDH descriptive statistics
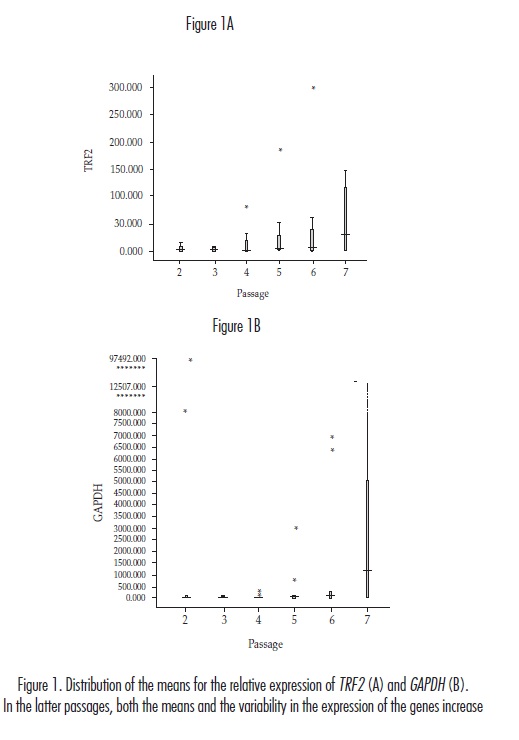
For TRF2 the mean values of its relative expression displayed variations throughout the sampled passages: they decreased from passage two to three and increased beginning with passage four, which was the point at which the cell cultures began to age. Because the corresponding standard deviations increased as the cell cultures aged, the difference between the mean values became irrelevant (figure 1A). Similar behaviour was observed for GAPDH; although there were differences between the mean values, the standard deviations were so great that the effect of these variations was minimised (figure 1B).
According to the Kolmogorov-Smirnov and Shapiro-Wilk tests, the results of the relative expression of both genes did not fit a normal distribution. The Ancova showed that neither the chronological age of the donor nor the age of the cell culture affected the relative expression of TRF2 (p = 0.1280 and p = 0.1608, respectively), which was corroborated with the value (p = 0.1013) obtained using the general F test for the united significance of the regression coefficients applied in Ancova. In contrast, although there were no significant differences in the relative expression of GAPDH with respect to the age of the cell culture (p = 0.5394), the chronological age of the donors had an apparently significant result (p = 0.0069). The p value calculated using the F test, however, indicated that the result was not significant (p = 0.0895). In addition, the 95% confidence intervals used to estimate the effect of chronological age on the expression of GAPDH were so small (-1071.4 ~ -179.5), that they were not taken into account.
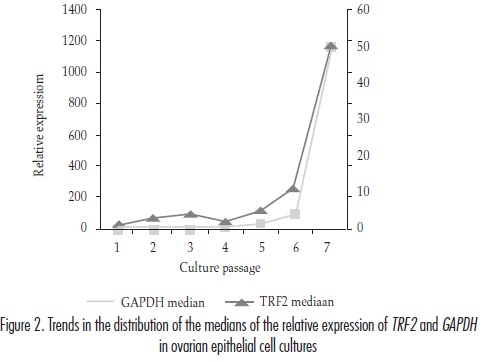
When the distribution trends of the median values for the expression of TRF2 and GAPDH throughout the multiple cell passages were analysed, an apparently similar behaviour was observed (figure 2). In order to verify this fact, we applied Kendall and Spearman correlation tests that yielded coefficients of 0.810 (p < 0.05) and 0.893 (p < 0.01), respectively.
Discussion
Normalisation
The results of TRF2 and GAPDH expression were normalised according to the ACT model (20) because the conditions of this study are consistent with those required for the application of this model: identical culture conditions and an identical amount of RNA used in the reverse transcription assays in all the experiments. As a result, the amplification efficiency values (1.94 for TRF2 and 1.93 for GAPDH) were raised to the difference between CTs (CT of the analysed passage minus CT of the first passage). Although quantification of gene expression at time zero (0) is generally desirable, this calculation was not possible due to the small amount of tissue collected. Furthermore, the first cell passage should be noted as being the most similar to the condition seen in cells when the sample WAS TAKEN.
Relative expression of TRF2 and GAPDH
In this study, there were no significant differences in the levels of TRF2 expression with either the chronological age of donors or the age of cell cultures. By analysing the trends in the expression means, however, we found a decrease in the TRF2 expression from passage two to passage three, while in the GAPDH expression, we also found a decrease even to passage four, followed in both cases by a continuous increase until the cells could no longer be passaged. On the other hand, an analysis of the trends of medians revealed fluctuations - both increases and decreases - up to passage number seven, followed by a marked increase that cannot be considered as significant, probably due to the small amount of sample available in this passage. Thus, as the cell cultures aged, the ability of the epithelial cells to maintain the expression of TRF2 and GAPDH at stable levels began to diminish, causing the increased variability in their expression. Furthermore, the inhibition of TRF2 appears to damage the telomere cap, resulting in the activation of ATM (ataxia telangiectasia mutated)-dependent signal pathways (21) along with the appearance of nucleation sites for proteins as a response to DNA damage (22, 23). When TRF2 is activated, it binds to ATM and prevents its activation (21). When TRF2 is inhibited, ATM can detect the double-stranded DNA denaturation and then auto phosphorylates the serine residue at position 1981. Subsequently, several downstream substrates, such as P53 and ChK2, and DNA repair factors, such as H2AX and NBS1, are activated (24). In mitotic cells, otherwise, the inhibition of TRF2 causes telomere breakdown. This event then activates pathways involved in the double-stranded DNA repair and an entire series of associated events, such as p53 and/or rb induction as well as increases the activity of the aging biomarker SA-β-gal (8, 25). These observations, in conjunction with our results, suggest that alterations of TRF2 at telomere level can induce aging in proliferating cells, as shown by Zhang et al. (23). Based on these studies, the breakdown of TRF2 is "detected" by ATM or by associated proteins, which then activates those signal pathways that respond to DNA damage. In contrast, the over expression of TRF2 has been associated with telomere shortening (8), which can lead to aging or malignant transformation; both of these events are accompanied by genomic instability. Taken together, these data suggest that any alteration in the expression levels of TRF2 impacts the fate of the affected cells, such that they eventually start to age and become either apoptotic or, in the worst case scenario, cancerous (26). Both oxidative and mechanical stresses have been reported to trigger a breakdown of stable TRF2 expression (27). Thus, the stress generated by the cell culture environment likely causes a breakdown in the regulation of TRF2 that leads to fluctuations in its expression.
The heightened variability in the expression of GAPDH is well known. This variability may be due to the participation of the product encoded by this gene in cell functions different than the classically described metabolic ones. Therefore, GAPDH has been assigned an antia-poptotic role in cells with a permeated external mitochondrial membrane, which should lead to caspase activation and apoptosis. The lack of target proteins downstream of the mitochondrial pathway, such as APAF-1, allows the increase of GAPDH levels in order to maintain the AYm via an increase of ATP, which interrupts the caspases activation and, in turn, enables cells to progress toward malignant transformation (28, 29).
Furthermore, Colell et al. (30), showed that nuclear GAPDH protects cells against caspase independent death, when the external mitochondrial membrane is permeated, thus promoting survival due to the induction of atg12 (autophagy-related protein 12), a protein that can facilitate autophagy. This mechanism irreversibly eliminates the damaged mitochondria, while at the same time, the elevated levels of GAPDH increase glycolysis to produce the ATP required for cell survival (30).
In our study, the largest fluctuations in both the mean and the median values for GAPDH expression occurred after the fourth passage. The results indicate that the aging onset in the epithelial cells began at this time (passages 3-4). Thus, cell aging may be responsible for the increased variability of GAPDH expression. One possible explanation is that the fluctuations and the overall increasing trend in the GAPDH levels are related to the survival attempts of the cultured cells, which may activate the autophagic pathway on one hand or the glycolytic pathway on the other hand, in order to avoid cell death and produce the energy required for cell survival.
In addition, the correlation between TRF2 and GAPDH expression levels suggests an interaction at nuclear level between either genes or their products. This hypothesis is supported by the overall trend of increased expression, once the aging onset occurs in the cell culture, a state characterised by telomere shortening. Interestingly, stress conditions, such as the events that lead to apoptosis or oncogenesis, result in the translocation of GAPDH into the nucleus (31-33). A similar outcome may occur during the onset of cell aging, which can be a cellular response to many of the same stresses that lead to apoptosis. Once translocated to the nucleus, GAPDH binds to DNA with no apparent discrimination between normal and cancerous cells (19). In the latter, the over expression of the GAPDH enzyme protects against telomere erosion in response to certain antitumor agents (18, 19). The over expression of TRF2 has been related to the aging state and telomere shortening (6). Reinforcing our hypothesis, the binding affinity of both proteins to DNA is similar, despite the affinity of TRF2 for double-stranded telomere DNA (34) and that of GAPDH for single-stranded telomere DNA (19). In addition, the binding sites of GAPDH and other members of the shelterin complex, such as Pot1, are adjacent (35).
In conclusion, neither the chronological age of the donors nor the age of the cell cultures seemed to correlate with the expression of TRF2 and GAPDH. The heightened variability in the expression levels of both genes could indicate the occurrence of alterations affecting the control of their expression when the cells have begun to age. Any interpretation of these data, however, should consider the diversity of functions that these gene products perform at cellular level. Because TRF2 and GAPDH both play roles in the protection of telomeres, the correlation between their expression levels during the consecutive cell passages suggests that they could cooperate to counter telomere shortening, which is a characteristic of aging.
Acknowledgements
To Dr. Sandra Ramírez and Dr. Milcíades Ibáñez for their invaluable support and contributions throughout this study.
References
1. De Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19:2100-10. [ Links ]
2. Verdun RE, Karlseder J. Replication and protection of telomeres. Nature 2007; 447:924-31. [ Links ]
3. Kim H, Lee OH, Xin H, Chen LY, Qin J, Chae HK et al. TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs. Nat Struct Mol Biol 2009; 16:372-9. [ Links ]
4. Gilson E, Géli V. How telomeres are replicated. Mol Cell Biol 2007; 8:825-38. [ Links ]
5. Bae NS, Baumann P. A RAP1/TRF2 complex inhibits nonhomologous end-joining at human telomeric DNA ends. Mol Cell 2007; 26:323-34. [ Links ]
6. Blasco MA. Telomeres and human disease: cancer, ageing and beyond. Nat Rev Genetics 2005; 6:611-22. [ Links ]
7. Ancelin K, Brunori M, Bauwens S, Koering CE, Brun C, Ricoul M et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol Cell Biol 2002; 22:3474-87. [ Links ]
8. Karlseder J, Smogorzewska A, De Lange T. Senescence induced by altered telomere state not telomere lost. Science 2002; 295:2446-9. [ Links ]
9. Wang RC, Smogorzewska A, De Lange T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 2004; 119:355-68. [ Links ]
10. Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 2005; 21:389-95. [ Links ]
11. Zheng L, Roeder RG, Luo Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 2003; 114:255-66. [ Links ]
12. Carujo S, Estanyol JM, Ejarque A, Agell N, Bachs O, Pujol MJ. Glyceraldehyde 3-phosphate dehydrogenase is a SETbinding protein and regulates cyclin B-cdk1 activity. Oncogene 2006; 25:4033-42. [ Links ]
13. Meyer-Siegler K, Mauro DJ, Seal G, Wurzer J, deRiel JK, Sirover MA. A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA 1991; 88:8460-4. [ Links ]
14. Singh R, Green M. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science 1993; 259:365-8. [ Links ]
15. Ishitani R, Sunaga K, Hirano A, Saunders P, Katsube N, Chuang DM. Evidence that glyceraldehyde-3-phosphate dehydrogenase is involved in age-induced apoptosis in mature cerebellar neurons in culture. J Neurochem 1996; 66:928-35. [ Links ]
16. Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL et al. Neuroprotection by pharmaco-logic blockade of the GAPDH death cascade. Proc Natl Acad Sci USA 2006; 103:3887-9. [ Links ]
17. Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol 2008; 10:866-73. [ Links ]
18. Sundararaj KP, Wood RE, Ponnusamy S, Salas AM, Szulc Z, Bielawska A et al. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 2004; 279:6152-62. [ Links ]
19. Demarse NA, Ponnusamy S, Spicer EK, Apohan E, Baatz JE, Ogretmen B, Direct Binding of Glyceral-dehyde 3-Phosphate Dehydrogenase to Telomeric DNA Protects Telomeres against Chemotherapy-Induced Rapid Degradation. J Mol Biol 2009; 394:789-803. [ Links ]
20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-AACT method. Methods 2001;25:402-8. [ Links ]
21. Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH et al. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol 2004; 2:E240. [ Links ]
22. D'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003; 426:194-8. [ Links ]
23. Zhang P, Furukawa K, Opresko PL, Xu X, Bohr VA, Mattson MP. TRF2 dysfunction elicits DNA damage responses associated with senescence in proliferating neural cells and differentiation of neurons. J Neurochem 2006; 97:567-81. [ Links ]
24. Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276:462-7. [ Links ]
25. Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21 (C1P1), but not p16 (INK4A). Mol Cell 2004; 14:501-13. [ Links ]
26. Muñoz P, Blanco R, Blasco MA. Role of the TRF2 telomeric protein in cancer and ageing. Cell Cycle 2006; 5:718-21. [ Links ]
27. Oh H, Wang SC, Prahash A, Sano M, Moravec CS, Taffet GE et al. Telomere attrition and Chk2 activation in human heart failure. Proc Natl Acad Sci USA 2003; 100:5378-83. [ Links ]
28. Ferraro E, Corvaro M, Cecconi F. Physiological and pathological roles of Apaf1 and the apoptosome. J Cell Mol Med 2003; 7:21-34. [ Links ]
29. Revillion F, Pawlowski V, Hornez L, Peyrat JP. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur J Cancer 2000; 36:1038-42. [ Links ]
30. Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 2007; 129:983-97. [ Links ]
31. Ishitani R, Tanaka M, Sunaga K, Katsube N, Chuang DM. Nuclear localization of over expressed glyceraldehyde-3-phosphate dehydrogenase in cultured cerebellar neurons undergoing apoptosis. Mol Pharmacol 1998; 53:701-7. [ Links ]
32. Krynetski EY, Krynetskaia NF, Gallo AE, Murti KG, Evans WE. A novel protein complex distinct from mismatch repair binds thioguanylated DNA. Mol Pharmacol 2001; 59:367-74. [ Links ]
33. Xing C, LaPorte JR, Barbay JK, Myers AG. Identification of GAPDH as a protein target of the saframycin antiproliferative agents. Proc Natl Acad Sci USA 2004; 101:5862-6. [ Links ]
34. Poulet A, Buisson R, Faivre-Moskalenko C, Koelblen M, Amiard S, Montel F et al. trf2 promotes, remodels and protects telomeric Holliday junctions. EMBO J 2009; 28:641-51. [ Links ]
35. Lei M, Podell ER, Cech TR. Structure of human pot1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol 2004; 11:1223-9. [ Links ]