Introduction
Because of the complex physiopathology of spasticity, it is distinguished as one of the most significant positive clinical signs of upper motor neuron syndrome, constituting a clinical feature with great impact in the neurorehabilitation setting 1,2. Although the most commonly cited definition of spasticity is the one offered by Lance, other studies have proposed more modern concepts, with that formulated by Pandyan et al. referring to "a disordered sensory-motor control, resulting from an upper motor neuron lesion, which presents with involuntary, intermittent, or sustained activation of the muscles" 3,5.
The evidence suggests that poststroke spasticity (PSS) is caused by the loss of equilibrium between the supraspinal excitatory and inhibitory mechanisms, particularly of the dorsal reticulospinal tract, and abnormal intraspinal processing of the stretch reflex due to changes in the intrinsic properties of the spinal motor neuron, which is associated with modifications in the biomechanical properties of the muscle and surrounding soft tissue 6,8.
There is great variability in the beginning and evolution of PSS 9. This variation is related to changes in neural plasticity and mediated by different intrinsic and extrinsic factors after a brain injury 10,11. Studies have projected a prevalence of 24 % in the first week, 19 % at 3 months, 22 % to 43 % at 4 and 6 months, and 17 % to 38 % at 12 months poststroke 12,13. This high variability can be explained by the various assessment methods and multiple definitions used, for which there is currently no consensus 14.
Depending on the location and extent of the brain damage, the presentation of PSS can differ 15; however, the lesion does not predict the intensity nor impact it will have, as this can change within the same day or over longer periods as well as fluctuate in different physiological and psychological situations 6,16. The degree of PSS can vary from the effects of some antigravity muscle groups to global manifestations. In this way, three types of involvement are clear: focal, segmental, and generalized. Likewise, the spastic condition is typically manifested in the upper and lower extremities, through characteristic patterns, each with specific muscles involved, mainly affecting the elbow (79 %), wrist (66 %), and ankle (66 %) 9,17,19. The typical pattern of the spastic upper extremity (UE) is described as shoulder adduction with internal rotation, flexed elbow, flexed wrist, and closed fist; however, this is not the only pattern of spasticity that can manifest 17.
Although spasticity is a source of functional involvement, associated with reduced quality of life due to the generated disability, it is not always detrimental and does not always require treatment 20,22. In fact, some effects of spasticity may be beneficial, such as during the support phase in walking rehabilitation and when carrying weight through support on the UE 23. Lundstrõm et al. concluded that intervention is required when spasticity causes disability 24, which makes it necessary to consider the final impact of PSS before seeking therapeutic strategies 25.
Despite the broad scientific evidence on this topic, there remains no consensus on the moment when spasticity appears, its location, or its predictors 4,6. Therefore, new evidence could guide professionals in the investigation and handling of spasticity, supporting intervention planning and reducing functional limitations and rehabilitation costs 26. Thus, the purposes of the current study were to examine (I) the prevalence, (II) onset time, (III) evolution, and (IV) prediction of elbow and wrist spasticity during the first year poststroke.
Materials and methods
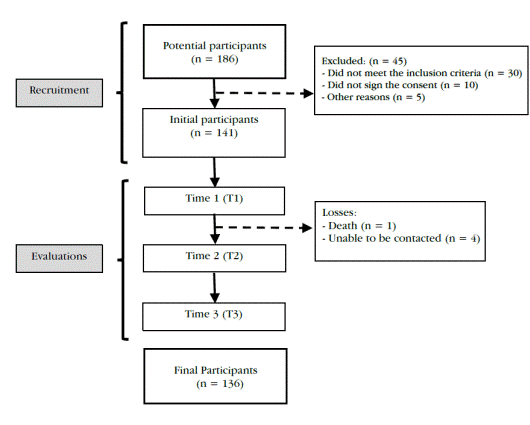
We used a correlational, longitudinal design for this study, with prospective follow-up, for descriptive and predictive purposes. We obtained the population and samples as described in Figure 1. In total, we recruited 186 patients, among whom 45 were excluded for not fulfilling the eligibility criteria, leaving 141 to participate in the first assessment period (T1). The final sample, with all three assessments, was composed of 136 patients, as 5 were lost between T1 and T2.
Participant selection was controlled by strict compliance with the inclusion and exclusion criteria. Inclusion criteria were age between 18 and 90 years, without prior history of motor disability, being in the hospital phase diagnosed with an ischemic or hemorrhagic stroke, without severe cognitive impairment (Mini-Mental Scale score >> 11), hemodynamically stable, presenting a stroke confirmed by computed tomography (ct) and/or magnetic resonance imaging, and signing the informed consent. Exclusion criteria were presenting with sensitive aphasia, having a medical contraindication, and having had more than one stroke.
Table 1 presents the sociodemographic and clinical characteristics of the final sample, showing that 62.5 % of the participants were men, 49.3 % were aged 65 years or older, 76.5 °% lived in an urban area, and 32.4 °% self-classified as belonging to the Mapuche ethnic minority group. With regard to the extent and severity of the damaged brain area, 58.1 °% of the sample showed moderate to slight damage in the middle cerebral artery (mca; as measured by the Alberta Stroke Program Early ct Score [aspects]), and 56.6 °% showed a partial anterior circulation infarct (paci) according to the Oxfordshire Community Stroke Project (ocsp) classification.
Table 1 Sociodemographic characteristics of the sample
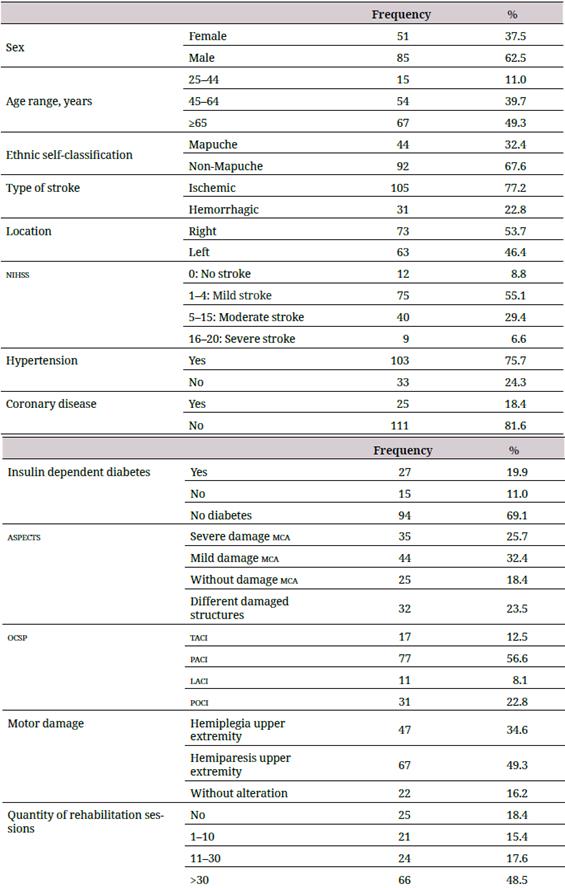
aspects: Alberta Stroke Program Early ct Score; laci: lacunar circulation infarcts; mca: middle cerebral artery; nihss: National Institute Health Stroke Scale; ocsp: Oxfordshire Community Stroke Project Classification; paci: partial anterior circulation infarcts; poci: posterior circulation infarcts; taci: total anterior circulation infarcts.
Initially, the potential participants were evaluated by two neurologists, who also confirmed compliance with the eligibility criteria, in each health center, and for the 10 hospitalization days. The sample was then recruited and identified. A previously trained physiotherapist belonging to each health center performed the evaluations of ue motor functions. Each participant was assessed on three occasions: during the first 10 hospitalization days (T1) and 3 months (T2) and 12 months (T3) after stroke. The length of the follow-up period was based on previous studies (27).
At T1, the neurologists registered sociodemographic data such as sex, age, residence, and ethnic self-classification (Mapuche, nonindigenous); clinical aspects referring to the type, extent, and severity of the stroke, assessment of the neurologic deficit (National Institute Health Stroke Scale), and motor damage; and comorbidities such as high blood pressure (hbp), cardiopathies, and diabetes. The physiotherapist assessed muscle tone at all time points.
At T3, the number of rehabilitation sessions of each patient was registered as one of four categories: none, 1 to 10, 11 to 30, and 31 sessions or more.
The extent and severity of the damage were recorded using the aspects, a quantitative topographic scale (0 to 10 points) that assesses acute ischemic changes in patients with a recent stroke in the territory of the mca at the level of the basal ganglia and above the basal ganglia through cranial ct (28). In the present study, we classified the scores derived from the ct according to four mca damage categories: severe (0-4), moderate (5-7), slight (8-9), and no damage (10). (29).
The damaged area of the brain was identified using the four subtypes included in the ocsp classification: total anterior circulation infarct (taci), paci, lacunar circulation infarct (laci), and posterior circulation infarct (poci). This classification provides fast, simple, and prognostically valuable information, as well as predicts the size and location of the lesion in 75 % of patients (30,31).
The elbow and wrist muscle tone were evaluated as flaccid, normal, and spastic. We used the Modified Ashworth Scale (mas) to differentiate the degree of spasticity, given its utility when applied to the ue and its high interobserver reliability (32-34). The mas classifies the tone according to six categories, which, in this study, were regrouped into the following four categories: normal (value 0), slight (values 1 and +1), moderate (values 2 and 3), and severe spasticity (value 4). Other studies have also used this abbreviated classification (35). We added a fifth category, flaccidity, to the classification. Although flaccidity is not part of the mas, its inclusion allows a more comprehensive view of the different alterations in muscle tone after stroke.
To evaluate the presence of motor damage in the affected ue, the patient was asked to place their affected hand on their head and was categorized as normal if they achieved the complete movement, hemiparesis if they began the movement but did not complete it or hemiplegia if they did not perform the movement.
We used the statistical package spss 25. The prevalence of spasticity was calculated as the percentage of patients with at least slight muscle tone according to the mas, thus excluding those with normal tone and flaccidity. We used Cohen's kappa coefficient to evaluate the degree of agreement in muscle tone between the elbow and wrist at each evaluation time. The evolutionary pattern of the elbow and wrist tone was examined by comparing T1 and T2 and then T2 and T3, using the marginal homogeneity test and reporting its standard Mantel-Haenszel statistic. This statistical test evaluates changes in repeated measures of a variable composed of three or more categories. Finally, to predict muscle tone, we used Cramer's V coefficient when the predictor was nominal and Goodman and Kruskal's gamma (G) coefficient when the predictor was ordinal. Obtaining significant V values was followed by a post hoc analysis of the contingency table using the z test, with the Bonferroni correction for pairwise comparisons of the categories (percentages) of the predictor variable.
Prevalence and onset of spasticity in the elbow and wrist
The prevalence of pss in the elbow was 37.5 °% at T1 and 57.4 °% at T2 and T3. We observed similar or identical percentages in the wrist: 36.8 % at T1 and 57.4 % at T2 and T3. These results indicate that, for both joints, the prevalence of spasticity increased from T1 to T2 but remained the same from T2 to T3. Among the 136 patients with motor damage, elbow spasticity set in at T1 (i.e., during the first 10 days), between T1 and T2, and between T2 and T3 in 44.7 °%, 23.7 °%, and 0.9 °% of the cases, respectively. Almost one-third (30.7 °%) of these patients lacked spasticity in the elbow at all three evaluation times. Similar results occurred with wrist spasticity: for 43.9 °% of patients, it began at T1; for 24.6 °%, between T1 and T2; and for 0.9 °%, between T2 and T3. In addition, 30.7 °% of these patients did not present spasticity in the wrist.
Evolution of muscle tone
The kappa coefficients were 0.97 at T1 and 0.84 at T2 and T3, with p < 0.001 for all, which indicates a high degree of agreement between the muscle tone of the elbow and wrist at every measurement time. Taking into consideration this high convergence and the high similarity between these two joints in terms of prevalence and onset of spasticity, a decision was made to continue examining only the muscle tone of the elbow in the subsequent analysis. In fact, the evolution pattern of muscle tone in the elbow was nearly identical to that in the wrist. Equally, we observed high similarity between these two joints in terms of the muscular tone prediction patterns. Another reason for this decision was that the literature reveals a higher prevalence of spasticity in the elbow (79 %) compared with the wrist (66 %) (19).
Table 2 presents the crossed distributions of muscle tone categories in the elbow, comparing T1 and T2 and comparing T2 and T3. For correct interpretation, it must be considered that although the cells of the downward diagonal (indicated with a border) include the patients who demonstrated maintained tone between assessment times, the cells above and below the diagonal indicate the patients who presented increased or reduced tone, respectively.
Table 2 Evolution of muscle tone in the elbow: T1 x T2 and T2 x T3
| T2 | |||||
|---|---|---|---|---|---|
| Spasticity | |||||
| T1 | Flaccidity | Normal | Mild | Moderate | Severe |
| Flaccidity | 4 | 13 | 21 | 4 | 0 |
| Normal | 0 | 41 | 1 | 1 | 0 |
| Mild | 0 | 0 | 32 | 14 | 1 |
| Moderate | 0 | 0 | 0 | 1 | 3 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| T3 | |||||
| Spasticity | |||||
| T2 | Flaccidity | Normal | Mild | Moderate | Severe |
| Flaccidity | 1 | 2 | 1 | 0 | 0 |
| Normal | 0 | 54 | 0 | 0 | 0 |
| Mild | 0 | 1 | 53 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 20 | 0 |
| Severe | 0 | 0 | 0 | 0 | 4 |
Significant changes in elbow tone between T1 and T2 were shown in the test of marginal homogeneity, hm = -6.98, p < 0.001. As noted in Table 2 (section T1 x T2), no patients presented reduced tone, whereas 58 patients (42.6 %%) presented increased tone. Of the 42 patients with flaccidity at T1, 13 changed to normal tone, 21 to slight spasticity, and 4 to moderate spasticity. Of the 43 patients with normal tone at T1, 1 changed to slight spasticity and 1 to moderate spasticity. Of the 47 who exhibited slight spasticity at T1, 14 moved to moderate and 1 to severe spasticity. Finally, of the 4 patients with moderate spasticity at T1, 3 changed to severe spasticity. It is relevant to note that, of the 41 patients who maintained normal tone between T1 and T2, 21 presented no motor damage. We noted no significant changes in elbow tone between T2 and T3 (hm = -1.41; p = 0.157). Indeed, at T3, 132 patients (97.1 %%) showed conservation of the tone they presented at T2 (see Table 2, section T2 x T3).
Prediction of muscle tone in the elbow
Table 3 presents the results of three sociodemographic and five clinical variables as predictors of muscle tone in the elbow at each evaluation time. Sex, age, type of stroke, and hypertension did not significantly predict elbow muscle tone at any of the measurement times.
Table 3 Prediction of muscle tone in the elbow from sociodemographic and clinical variables
| Predictor | Test | T1 | T2 | T3 |
|---|---|---|---|---|
| Sex | V | 0.14 | 0.15 | 0.12 |
| Age range | G | 0.16 | 0.13 | 0.12 |
| Ethnic self-classification | V | 0.19 | 0.31* | 0.28* |
| Type of stroke | V | 0.31** | 0.27* | 0.26 |
| Hypertension | V | 0.21 | 0.18 | 0.17 |
| Brain damaged area (ocsp) | V | 0.34*** | 0.37*** | 0.39*** |
| Extent of damage mca (aspects) | G | 0.17 | 0.52*** | 0.50*** |
| Quantity of rehabilitation sessions | G | 0.16 | 0.69*** | 0.70*** |
aspects: Alberta Stroke Program Early ct Score; G: Goodman and Kruskal's gamma coefficient; mca: middle cerebral artery; ocsp: Oxfordshire Community Stroke Project Classification; V: Cramer's V coefficient. *p > 0.05. **p > 0.01. ***p > 0.001.
Ethnic self-classification was the only sociodemographic variable that significantly predicted tone, although only at T2 and T3. As shown in Table 4, post hoc analyses at T2 revealed significant differences in the categories of normal tone (46.7 %% in nonindigenous people vs. 25.0 %% in Mapuches) and slight spasticity (33.7 %% in nonindigenous people vs. 52.3 % in Mapuches). The significant difference observed in the normal category at T2 persisted at T3 (48.9 %% in nonindigenous people vs. 27.3 %% in Mapuches). These results suggest that, comparatively, the nonindigenous patients tended to present normal tone and the Mapuche patients tended to present slight spasticity.
Table 4 Prediction of muscular tone in the elbow according to ethnic self classification at T2 and T3 (%)
| T2 | |||||
|---|---|---|---|---|---|
| Spasticity | |||||
| Ethnic self-classification | Flaccidity | Normal | Mild | Moderate | Severe |
| Mapuche | 0.0 | 25.0a | 52.3a | 22.7 | 0.0 |
| Non-Mapuche | 4.3 | 46.7b | 33.7b | 10.9 | 4.3 |
| T3 | |||||
| Mapuche | 0.0 | 27.3a | 50.0 | 22.7 | 0.0 |
| Non-Mapuche | 1.1 | 48.9b | 34.8 | 10.9 | 4.3 |
Note: At each time, percentages in a column with different superscripts differ significantly (p < 0.05) according to paired comparisons using the Bonferroni correction z test.
With the exception of hbp, all clinical variables significantly predicted elbow tone for at least two evaluation times (see Table 3). In addition, the damaged brain area (according to the ocsp) significantly predicted the muscle tone of the elbow (see Table 3). We also observed greater ischemic damage (according to ASPECT) at T2 and T3. Finally, a higher number of rehabilitation sessions predicted increased elbow tone (see Table 3).
Discussion
his study is the first in Chile to longitudinally examine pss and its predictors. Currently, the evidence concerning the onset and predictions of spasticity is controversial (36). It would be advantageous for professionals in the area of rehabilitation to have guiding scientific evidence during the acute period after a stroke that identifies which patients are at risk of developing spasticity and which motor functions could be affected, as well as evidence that provides details of the prevalence and onset of spasticity in the elbow and wrist.
There is disparate scientific evidence regarding the evolution time of spasticity, stimulating the search for new knowledge. In addition, although not all patients with spasticity require therapeutic intervention, early attention could reduce disability levels in those who need it (38), making early investigation fundamental (37). The reported prevalence of spasticity varies from 4 %% to 27 %% during the first 6 weeks after stroke, with the peak between 1 and 3 months after the damage, results that are similar to those found in the present study (9,13,39).
Among all patients assessed, the prevalence of spasticity in the elbow was 57.4 %% at 3 months, which remained steady at 12 months poststroke. These findings are comparable with other studies in which the prevalence varied between 16 %% and 42.6 %% in at least one joint in cohorts of 83 to 328 patients (19,24,40). The increase between T1 and T2 can be explained through studies with electromyography, which showed that muscle tone reached its maximum at 3 months poststroke, implying that spasticity may be caused not only by damage in the neural structures but also by adaptive characteristics, such as intrinsic changes in the muscle (41-43). The ue adopts specific patterns causing immobilization (especially in short lengths), which triggers muscle contracture (8,17,44). The contracture and subsequent muscle fibrosis could even increase spasticity via the hyperactivation of the afferent pathways of the spindle during muscle lengthening (8). At the same time, muscle immobilization reduces postactivation depression, which is a fundamental mechanism in spasticity development (45).
Of the patients who had motor damage at T1, 47 presented with hemiparesis and 67 with hemiplegia in the ue, and for almost half of these individuals, spasticity set in during the first 10 days of evolution, results similar to those presented by Dorñák (14). With regard to the evolution of muscle tone, between T1 and T2, no patients presented reduced tone, whereas 58 patients demonstrated increased tone. This finding is in line with the results of Wissel et al., who suggested that patients with no initial signs of increase in tone can develop spasticity during a longer follow-up (19). The accumulated evidence suggests that the identification of predictors of spasticity is limited, and the present study contributes to expanding this knowledge (9,38,46).
Ethnic self-classification (Mapuche or nonindigenous people) was the only sociodemographic variable that significantly predicted elbow tone at T2 and T3, indicating that, comparatively, nonindigenous patients tended to exhibit normal tone and Mapuche patients increased tone-specifically, slight spasticity. Although this finding is highly relevant, because it contributes new information in this area of study, possible explanations should be considered.
Some evidence has revealed a greater incidence of stroke and worse outcomes in native populations and ethnic minorities (47,48). The largest Mapuche indigenous population in the country is concentrated in the Chilean region of La Araucanía, where this study was conducted (49). In this region, the Mapuches have a mortality rate from stroke that is twice the rate observed in the rest of the country, as they also have a high prevalence of poverty, hbp, diabetes, sedentary lifestyle, and overweight, all risk factors for stroke (50-53). However, there is no evidence that specifically associates this ethnic group with the occurrence of stroke (54).
It is possible that the increased tone in the Mapuche population, in contrast to nonindigenous people, is due to the stroke being more serious in the indigenous group, generating greater spasticity. Two additional findings in the present study seem to support this possible explanation. On one hand, there was a marginally significant association between ethnic group and motor damage (p = 0.08), indicating that 52.6 %% of Mapuches presented with hemiplegia (vs. 35.5 %% of nonindigenous people) and 64.5 %% of nonindigenous people presented with hemiparesis (vs. 47.4 %% of Mapuches). These results suggest a tendency for Mapuches to present with hemiplegia and nonindigenous people to present with hemiparesis. From the point of view of motor function, hemiplegia is known to involve greater disability than hemiparesis does. On the other hand, when examining the relation between ethnic group and number of rehabilitation sessions, we found that a significantly higher percentage of Mapuches required more than 30 sessions (p = 0.04). If strokes were more serious in Mapuches, their spasticity would also be greater, and they would consequently require more rehabilitation sessions.
From the clinical point of view, three variables significantly predicted elbow tone for at least two evaluation times.
1. The type of stroke predicted muscle tone at T1 and T2; in relative terms, patients with ischemic stroke tend to have normal tone, and patients with hemorrhagic stroke have slight spasticity. These results agree with studies indicating that after a 3-month follow-up period, patients who developed spasticity had a previous diagnosis of hemorrhagic stroke (55). In addition, Lundstrõm et al. found that hemorrhagic stroke was a predictor of spasticity at both the first month and 6 months after the damage, with a similar statistical significance to this study (13). Katoozian et al. suggested that the risk of presenting spasticity due to hemorrhagic stroke is 2.5 times, with no differences regarding anterior or posterior circulation (56).
2. The damaged brain area (according to the ocsp) significantly predicted muscle tone in the elbow at the three assessment times, identifying a significantly greater presence of patients with laci and poci in the normal tone category and more patients with taci and paci in the slight spasticity category. Previous research remains controversial regarding this issue. In their study, Kong et al. found that the lesion site, evaluated through the ocsp, was not a prognostic factor for spasticity (35). However, in the salgot study, in which several predictive models were evaluated, the ocsp was an important predictor of severe spasticity (57). However, these disparities could be due to the distribution by location; for example, in the two cited studies, patients with taci or paci comprised fewer than 50 %% of the sample, whereas in the present study, they represent 69.1 %% indicating that, clinically, patients with greater damage have higher chances of presenting with pss.
3. The association between pss and the extent of the lesion is not well known (58). The scientific evidence presents some software-support imaging studies. In our study, the extent of the damage assessed through aspects significantly predicted elbow tone at T2 and T3. In line with this result, Picelli et al. conducted a retrospective analysis of patients with chronic stroke who had lesions in the insula, basal ganglia, and thalamus as well as in the internal and external capsule, corona radiata, and superior longitudinal bundle, concluding that these patients can suffer severe pss in both the ue and the lower extremity (59). Cheung et al. observed a correlation between spasticity and lesions occurring in the putamen (60). This association was not unexpected, as these structures are irrigated by the mca and its lenticulostriate branches, encompassing broad areas of the brain that receive primary sensory input, the motor and premotor cortices, and the extrapyramidal cortex.
Among its strengths, our study is the first conducted in Latin America to include a longitudinal evaluation of the prevalence, onset, evolution, and prediction of elbow pss. This study also included ethnic self-identification, a variable that seems to be a relevant predictor of muscle tone and a finding that should be considered when studying ethnically heterogeneous populations. Another aspect to emphasize is the low percentage of follow-up loss over the three measurement times. In addition, we assessed all patients clinically and through neuroimaging, using scales widely validated in vascular neurology.
The limitations of this study include the recruitment of the sample, as only two health centers in the city were included. Although these centers attend 80 %% of the stroke admissions in the geographic sector of reference, they do not necessarily represent the reality of other centers in the geographical study area. Another limitation is the inclusion of a low percentage of patients with severe stroke, which could lead to underestimation of spasticity prevalence; however, this was a characteristic of the subjects who comprised the study sample.