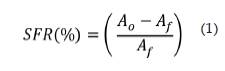1.INTRODUCTION
Reactive oxygen species (ROS) have the ability to react with a large variety of biological components (e.g., lipids, sterols, proteins, DNA, and RNA) for inducing deterioration of biological systems; among ROS, hydroxyl free radical (•OH) exhibits strongest oxidative activity [1-3]. In recent years, to study the antioxidant ability of different natural compounds is an important subject of research in the field; natural compounds have possible applications in the pharmaceutical, cosmetic and food industries. Nowadays, research about •OH has been focused on specific chemical species in the medicine sciences with regard to disease factors and health maintenance [4].
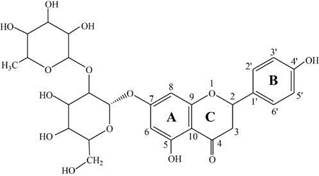
The antioxidant activity is the ability of a substance to inhibit the oxidative degradation of different compounds; the antioxidant capacity relies on ability of specific compounds to react to radical and non-radical species (e.g., •OH) present in the chemical or biological environment. As a result, development of a practical assay for hydroxyl radical scavenging measurement is important, and it is a challenging task owing to the high activity, short lifetime, and low concentration of •OH [5-7]. Different natural antioxidants from fruits and vegetables (e.g., carotenoids, vitamins and flavonoids) "scavenging" to •OH. Among these, plant-derived flavonoids have been shown a wide range of biological activities, actually, flavonoids have demonstrated higher potential antioxidant and antibacterial activity against differents ROS species, research about new natural sources of both cheap and high activity is an important research topic [8 -10]. Naringin (4',5,7-trihydroxyflavanone-7-p-L- rhamnoglucoside-(1,2)- a-D-glucopyranoside) is one flavanone found in the peel and juice of grapefruit and certain types of orange like Citrus aurantium (see figure 1); this compound has showed biological properties such as anti-inflammatory, anti-carcinogenicity and neuro-protective effect [11-13].
It is known that flavonoids from fruits extracts are scavenger to •OH in solution and their possible applications as antioxidant to protect different biological systems is an important research issue, however physical-chemistry reports about the scavenger to •OH that permits so study and to compare kinetical parameter to develop possible application are limited. The aim of this work was studied antioxidant capacity of the naringin obtained from inexpensive and abundant source orange peel, and besides, we tried to give relevant information about its potential natural antioxidant.
2. EXPERIMENTAL
2.1 Reagents and equipment
All reagents used in this work were analytical grade. The properties of the compound were studied by measurements of UV-Vis and Fourier transform infrared spectroscopy (FT-IR). The UV-Vis spectrum was measured in a Hewlett-Packard 8453 spectrophotometer, and the FT-IR spectra (KBr) of the compounds were recorded on a Bruker Tensor 27 spectrometer in the spectrum region between 4000 and 500 cm-1.
2.2 Isolation of naringin from Citrus aurantium
Sour orange (Citrus aurantium) fruits, in fresh and ripe state, were collected from Baranoa (latitude 10° 73' 33", longitude 74° 91' 66" - Colombia) between April and May 2013. After washing the fruits, the flavedo was mechanically separated of the albedo. Naringin was isolated from peel fruits according to the procedure described by Ikan [14]. After isolation; the solid was chemically characterized by UV-Vis and IR-FT techniques.
2.3 ABTS∙+ radical scavenging capacity
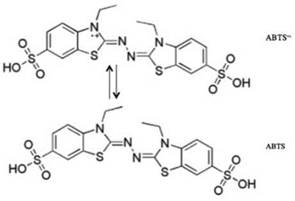
The scavenging the ABTS*+ radical scavenging assay was developed follow procedure described by Miller et. al. This method relies on the ability of an antioxidant to stabilize the radical cation ABTS*+, which is previously formed by the oxidation of ABTS (2,2'-azinobis (3-symphonic ethylbenzothiazoline-6 acid) and hydrogen peroxide meta-myoglobin; the results are expressed as Trolox equivalents or TEAC (Trolox Equivalent Antioxidant Capacity); figure 2 shows structures of ABTS and cation radical ABTS*+ [15, 16]. Scavenging free radical of naringin was evaluated by monitoring radical cation bleaching of ABTS*+ by visible spectroscopy at 734 nm; a solution 14 mg ABTS and 3.3 mg of potassium persulfate was prepared in distilled water and reacted in the dark (conditions t: 16-24 h, T: 25 °C). After that, stock solutions of the extracts using ethanol as solvent were prepared. While performing tests each 20 uL of stock solution and 2 mL of ABTS*+ solution were added. The scavenging free radical (SFR %) of radical cation ABTS*+ was calculated according to the following equation:
Where: A0 is the absorbance of uninhibited radical cation, Af is the absorbance measured at 30 min after addition of antioxidant potential. All assays were in triplicate director.
3. RESULTS AND DISCUSSION
3.1. Characterization of isolated naringin
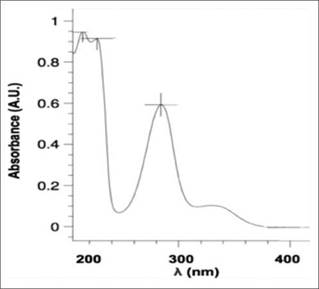
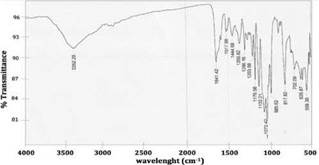
The UV-vis spectrum of naringin in ethanol is shown in figure 3. Naringin exhibits two major absorption bands in the UV region, at 284 nm (band I) representing B-ring absorption (cinnamoyl system), and at 229 nm (band II), considered to be associated with the absorption involving the A ring benzoyl system, this is a typical result for flavones and flavonols. Furthermore, the FT-IR spectrum of naringin is shown in figure 4. This spectrum is characterized by vibrational bands mainly due to the OH, C-H, C=O, and C=C functional groups. The u (OH), u (C-H), u (C=O), u (C=C) for naringin appear at 3425 cm-1, 2957 2859 cm-1, 1645 cm-1 respectively [17].
3.2 ABTS∙+ radical scavenging capacity
The scavenging activity was evaluated by monitoring ABTS*+. Assays were compared with reference antioxidants BHT, a-tocopherol and Trolox.
Table 1 shows the values obtained from statistical analysis of antioxidant references and naringin (5.0x103M). Results indicate naringin has lower scavenging free radical activity that reference antioxidant references, however, values are very close, this is an important result due to Citrus aurantium peel (source of naringin) is an inexpensive and abundant resource, results indicate that Citrus aurantium peel has high potential practical application to orange peel waste. The antioxidant capacity of extracts of citrus fruits is due to the presence of phenolic acids, flavonoids and other phenolic compounds, as generally reported in the literature [18-20]. The results for antioxidant activity of naringin against hydroxyl radicals by ABTS*+ verified that Citrus aurantium peel has potential antioxidant activity against free radical; this an important result due to Citrus aurantium peel is cheap and abundant natural resource in our Caribbean region.
Table 1 Scavenging free radicals of naringin and references patterns.
| Sample | Absorbance* | Standard Deviation | cv** | SFR (%) |
| TROLOX 3.5x10 2 ppm | 0,33 | 0,02 | 5 | 50,4 |
| BHT 3.5x10 2 ppm | 0,34 | 0,02 | 6 | 49,4 |
| VIT E 5.5x10 2 ppm | 0,31 | 0,02 | 8 | 52,9 |
| Narigin 5x10 ppm | 0,312 | 0,007 | 2 | 43,1 |
*After 30 min
**Variation Coefficient, %
4. CONCLUSIONS
In this work we isolated naringin from Citrus aurantium, the analysis through UV-vis and IR-FT confirmed the identity of compound. Furthermore, we showed that naringin is scavenging free radicals. The naringin stabilizes the radical-cation and it had a scavenging free radicals of 43.1 %, this result corresponds to 85.5 % of scavenging free radical of Trolox; Our results indicated that orange peel waste could be used as source of scavenging free radicals.
5. ACKNOWLEDGMENTS
W. Vallejo and C. Diaz-Uribe thanks to Universidad del Atlántico for financial support through project of IMPACTO CARIBE 2014 (Resolución 002627 - 3 / Mar/2015).
A. Muñoz thanks to Universidad del Norte for the financial support through of the Strategic Area "Biodiversidad, Servicios Ecosistémicos y Bienestar Humano" (Code Project 2013-DI0024).