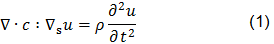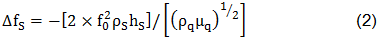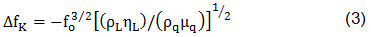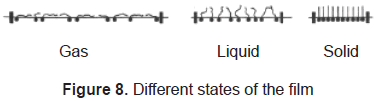Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista EIA
versão impressa ISSN 1794-1237
Rev.EIA.Esc.Ing.Antioq no.4 Envigado jul./dez. 2005
INTERFACE LAYER TO IMPROVE POLYSTYRENE ATTACHMENT ON A QUARTZ CRYSTAL RESONATOR
Doris Susana Llanes1, Ulrike Hempel2, Aquiles Ocampo3
1 Ingeniera Biomédica, EIA. Envigado, Colombia. dsllanes@hotmail.com.
2 Dipl. Wirtsch. Ing. Sensor & Measurement Technology Group. Otto-von-Guericke-University Magdeburg, Germany. ulrike.hempel@et.uni-magdeburg.de.
3 Ingeniero Químico Ph.D. Investigador EIA. Envigado, Colombia. investigar@eia.edu.co.
Artículo recibido 8-VIII-2005. Aprobado con revisión 20-X-2005
Discusión abierta hasta mayo 2006
ABSTRACT
Technologies for thin film deposition have been used to improve the functionalization of quartz crystal resonators (QCR); such technologies, for example, are spin coating and Langmuir-Blodgett (LB) film preparation. These experiments are required because the film uniformity and homogeneity over the quartz crystal resonator are fundamental for its applications as chemical and biological sensors.
Film deposition of polystyrene (PS) particles, as well as polyvinyl chloride (PVC), was performed in solvents such as cyclohexanone, tetrahydrofuran (THF), dimethylformamide and chloroform at different concentrations using the spin coating procedure on wafer targets. The film quality was determined by optical microscopy, ellipsometry, and profilometry.
Additionally, films on wafers or QCR with gold surface have been prepared by spreading the mentioned substances on an aqueous subphase and transferring it by dipping from the subphase surface onto the target.
For LB film preparation arachidic acid and PS particles have been used. Prepared mono- or multilayered films of these substances on wafer or gold quartzes have been controlled additionally by IR-spectrometry.
The resonant behavior of QCR before and after surface coating has been measured with a network analyzer. In combination with a graphical user interface the data could be easily recorded and visualized.
KEY WORDS: Piezoelectricity; quartz crystal microbalance; film deposition.
RESUMEN
Se describe el empleo de técnicas de formación de películas tendientes a mejorar el funcionamiento de un resonador de cristal de cuarzo (QCR); dichas tecnologías fueron, por ejemplo, preparaciones de recubrimiento mediante los métodos de spin y Langmuir-Blodgett (LB). La uniformidad y homogeneidad de la película sobre el cristal de cuarzo es fundamental para las aplicaciones que tiene un resonador como sensor químico y biológico.
Se formaron películas de poliestireno (PS) y de cloruro de polivinilo (PVC) en solventes como ciclohexanona, tetrahidrofurano (THF), dimetilformamida y cloroformo a diferentes concentraciones, usando el procedimiento de cubrimiento por spin en soportes corrugados tipo wafer. La calidad de las películas fue determinada usando microscopía óptica, elipsometría y perfilometría.
Además, se prepararon películas en soportes corrugados y en QRC con superficie de oro por irrigación de la mencionada sustancia en una subfase acuosa y transfiriéndola por impregnación de la superficie de subfase en el soporte.
Para la preparación de la película LB se empleó ácido araquídico y partículas de PS. Las preparaciones de películas de monocapa y multicapa de estas sustancias en soportes corrugados o en superficies de cuarzo doradas se controlaron adicionalmente por espectrometría IR.
El comportamiento resonante del QCR antes y después del recubrimiento de su superficie se midió con un analizador de red. En combinación con una interfaz gráfica los datos se pudieron fácilmente grabar y observar.
PALABRAS CLAVE: Piezoelectricidad; microbalanza de cristal de cuarzo; depositación de películas.
INTRODUCTION
The quartz crystal resonator (QCR), also known as thickness shear mode (TSM) sensor, is one of the most common acoustic-waves sensors. A TSM sensor consists in a thin circular AT-cut quartz disk, usually cut at 35,25° to the z axis, with circular electrodes, usually gold or silver, on both parallel surfaces of the disk. The quartz crystal microbalance (QCM) is an application of this kind of sensor. The simple gravimetric operation principle of the QCM can be described by the Sauerbrey equation that relates the mass of an adsorbed film with the shift of resonant frequency of the oscillating device.
Recently, there is an increased interest in the development of biological sensors for medical application using the principle of the known mass effect and non-gravimetric effects. These sensors get their biological sensitivity and selectivity from a chemical coating. The film uniformity and homogeneity over the quartz crystal resonator are fundamental for its applications as chemical and biological sensor.
An overview of established theoretical models describing the resonant behavior of a QCR under different load conditions is summarized in Lucklum-Hauptmann & Arnau.
Aim of a project at the Institute for Micro- and Sensor Systems at the University of Magdeburg (Germany) was to study the adsorption of large molecules and liposomes on a QCR surface. Monolayers of polystyrene (PS) particles act as a model substance for liposomes. For this it was used different thin film preparation methods: Langmuir-Blodgett film preparation, spin coating, and solvent evaporation. Parameters such as subphase temperature, concentration, and volume of the used substance were also important in the film deposition process. Some of the experiments described in this work were included for training purposes; others were revaluated for better result in parallel experiment. Additionally, the effect of a PVC interface layer was investigated to improve the PS particle attachment.
1. THE QCM
1.1 Piezoelectric resonator
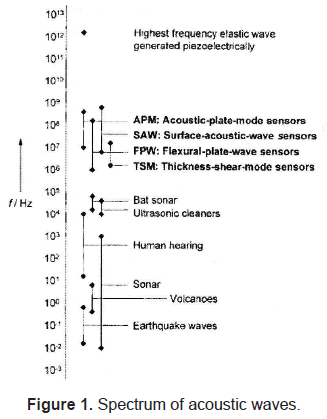
The spectrum of acoustic waves that have been detected covers a frequency range of fourteen orders of magnitude, from 10-2 Hz up to 1012 Hz (figure 1). Commonly used acoustic resonators operate in the range of 1MHz and 1GHz.
The elastic deformation (strain) travelling as a wave through a solid results from application of a periodic perturbation (stress). The structure of the crystal gives the type of wave and the phase of velocity. The equation 1 describes acoustic waves in solids.
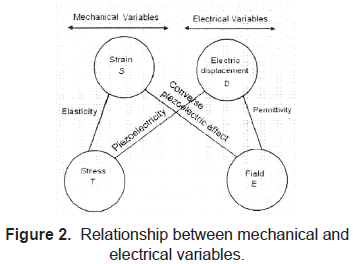
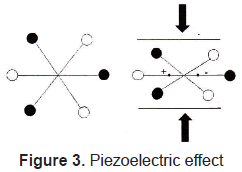
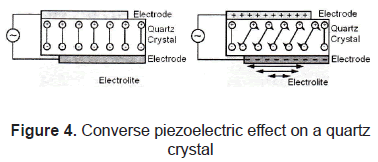
The piezoelectric effect, discovered by Curie brothers in 1880, is exhibited by certain crystals, e.g. quartz and Rochelle salt, and ceramic materials. The piezoelectricity effect can be defined as a generation of electrical charges on the surface of a solid after the pulling, pressing, or torsion of it. Piezoelectric crystal will build up charges on opposite faces, thus acting like a capacitor with an applied voltage. A current called piezoelectricity can then be generated between the faces (figure 2).
This effect is used for detection of acoustic waves by transforming mechanical deformation into electrical signal. Figures 3 and 4.
Piezoelectricity is present in crystal with no symmetry centre. For piezoelectric crystal two kinds of acoustic waves are present. Bulk acoustic waves (BAW) travel through the interior of the substrate. In this case of surface acoustic waves (SAW), the waves propagate on the surface of the substrate.
Two types of BAW can be propagated, longitudinal waves (compressional/extensional) and transverse (shear) waves. In the last case the motion of the particles is parallel or perpendicular to the direction of wave propagation.
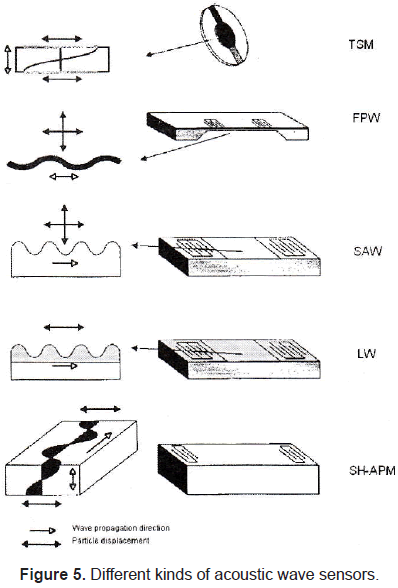
There are different kinds of acoustic wave sensors (figure 5) depending on wave propagation. The most commonly used are: thickness shear mode (TSM), flexure plate wave (FPW), surface acoustic wave (SAW), Love wave (LW), and shear horizontal acoustic wave mode resonator.
1.2 Thickness shear mode (quartz crystal microbalance)
The quartz crystal microbalance (QCM) is a thickness shear mode type resonator. This kind of resonators consist in a thin disk of a AT-cut quartz (cut with an 35,25° to the z axis from the mother crystal) with circular electrodes on both sides. An application of voltage between these electrodes causes a shear deformation of the quartz. The sensor resonant frequencies are inversely proportional to the crystal thickness. The advantage of the AT-cut quartz crystal is that it has nearly zero frequency drift with temperature around room temperature.
A film addition on the crystal surface modifies the resonance frequency of the resonator. Sauerbrey affirmed that a thin solid film over a crystal generates a shift in the resonant frequency. This is described by his equation:
where ΔfS is the measured frequency shift in the gravimetric regime, fo is the resonant frequency of the fundamental mode of the crystal, ρs is the density of the substance, hs is the thickness of the film, ρq is the density of quartz (2,65 g/cm3), and µq is the shear modulus of quartz (2,947×1010 N/m2). But in the case of liquids, Sauerbrey equation is not valid. For the behavior of the QCM in Newtonian liquid, Kanazawa showed that the change in resonant frequency of a QCM taken from air into a liquid is proportional to the square root of the liquid's densityviscosity product:
where ΔfK is the measured frequency shift in this non-gravimetric regime, ρL is the density of liquid in contact with the crystal and ηL is the viscosity of the liquid in contact with the crystal. Besides, there is a non-negligible damping of the shear wave in liquid expressed by a resistance RK (see Kanazawa-Gordon for further information).
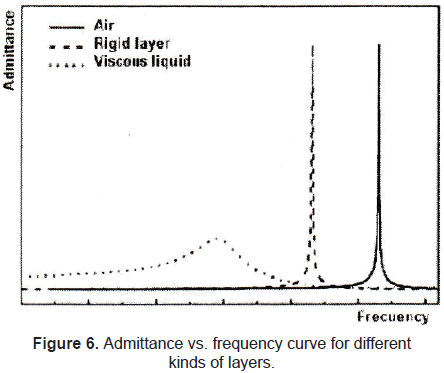
A typical resonance curve of admittance vs. frequency of a QCM with different kinds of layers (gravimetric regime presented by a rigid layer, nongravimetric regime presented by a viscous liquid) is shown in figure 6.
The quartz crystal resonator is basically a mass sensing device with the ability to measure mass changes, even as small as a fraction of a monolayer or single layer of atoms. This makes QCM a very attractive technique for a large range of applications. Major advantage of the QCM technique used for liquid systems is that it allows a label-free detection of molecules.
2. PREPARATION AND METHODS
Several technologies have been used to obtain chemically modified electrodes. Three of them were used to prepare the QCM coverage.
2.1 Solvent evaporation
In this process the suspension of the substance in a specific solvent is spread onto an air-liquid interface with a special subphase temperature. The particles are distributed on the subphase while the solvent is evaporating. After that the film on the interface is transferred onto a target by dipping it.
2.1.1 Polyvinyl chloride in dimethylformamide
Different experiments of spreading solutions of polyvinyl chloride in concentration of 1% to 4% in dimethylformamide onto an air-liquid interface at room temperature have been made to determine whether or not this solvent was useful for PVC film deposition. The films have been transferred onto a silicon substrate. The film deposition quality has been controlled by an optical microscope.
2.1.2 Polyvinyl chloride in tetrahydrofuran
Different experiments of spreading solutions of polyvinyl chloride in concentration of 1% to 5% in tetrahydrofuran onto an air-liquid interface at room temperature have been made to determine whether or not this solvent was useful for PVC film deposition. The films have been transferred onto a silicon substrate. The film deposition quality has been controlled by an optical microscope and profilometry.
2.1.3 Polyvinyl chloride in cyclohexanone
Different experiments of spreading solutions of polyvinyl chloride in concentration of 1% to 3% in cyclohexanone onto an air-liquid interface at room temperature have been made to determine whether or not this solvent was useful for PVC film deposition. The films have been transferred onto a silicon substrate. The film deposition quality has been controlled by an optical microscope.
2.1.4 Polystyrene in cyclohexanone
Monodisperse particles of polystyrene (PS) with a diameter of 300 nm dissolved in cyclohexanone have been used in this experiment. Two kinds of cross linked particles have been studied in this experiment: hydrophobic particles and not hydrophobic particles.
Different experiments of spreading these two dispersions of polystyrene in a concentration of 10mg/ml in cyclohexanone onto an air-liquid interface varying the temperature in a range of 7°C to 30°C have been made to determine the best temperature of the subphase for a better film deposition. The films have been transferred onto a silicon substrate. The film deposition quality has been controlled by an optical and a scanning electron microscope (SEM).
2.2 Spin coating
In the spin coated process the substance is dropped onto a target that is spinning at a specific speed. The centrifugal forces cause the spreading of fluids against viscous resistance resulting in the formation of homogeneous film on the target surface. Spin coating parameters are volume of the substance and speed of the disk.
2.2.1 Polyvinyl chloride in dimethylformamide
Different experiments of spinning polyvinyl chloride in concentration of 1% to 3% in dimethylformamide onto a silicon wafer at 3500 rpm were performed.
2.2.2 Polyvinyl chloride in tetrahydrofuran
Different experiments of spinning polyvinyl chloride in concentration of 0,5% to 3% in tetrahydrofuran onto a silicon wafer at 3.500 rpm were performed. The film deposition has been studied using optical microscopy and ellipsometry.
2.2.3 Polyvinyl chloride in cyclohexanone
Polyvinyl chloride in concentration of 0,5% to 3% in cyclohexanone onto a silicon wafer at 3.500 rpm has been spin coated. The film deposition has been studied using optical microscopy and ellipsometry.
2.2.4 Polystyrene and polyvinyl chloride in cyclohexanone
A mixture of 4 mg of PS in 1% PVC in cyclohexanone has been spinned onto a silicon wafer at different speed in order to get a homogeneous distribution of the PS in the PVC film. The film deposition was analyzed by using an optical microscope.
2.2.5 Polystyrene in chloroform
A solution of 3% of PS in chloroform has been spinned onto quartzes with silver electrodes as a sealing layer. The frequency of resonance of the silver quartzes is 10 MHz. The quartzes resonance frequency has been measured with the network analyzer before and after the spin coating. The density of PS is 1,05 g/cm3.
2.3 Langmuir-Blodgett film
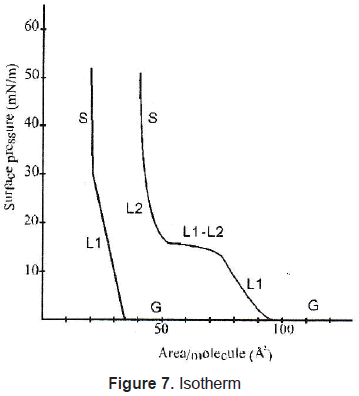
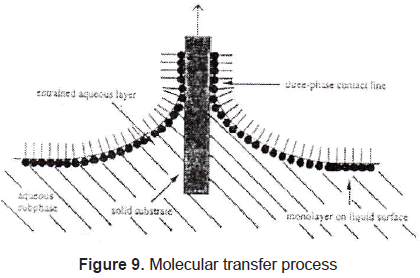
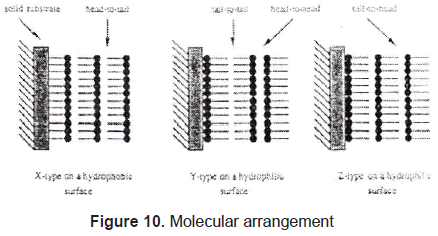
In Langmuir-Blodgett films the substance is spread onto a liquid-air interface. The solutions spread fast to cover the whole available area. The interaction between the molecules is very weak. Therefore, the monolayer is not a compact film. Closing the area with a constant pressure decreases the distance between the molecules. In this way the particles get organized in a compact solid film. The area is closed when the molecules reach the solid phase; that is shown in figures 7 and 8. Once the molecules are organized, the film can be transferred on a solid target, by dipping it in or out of the liquid (figure 9) in a specific speed. To get a multilayer film, the target must be dipped the number of times as the desired number of layers. A multilayer can have different arrangement of the molecules as it can be seen in figure 10.
2.3.1 Arachidic acid
A solution of 2,25 mg/ml of arachidic acid in chloroform has been spread onto subphase of 100 µmoles/ml of NaCl in deionized water at 20°C of temperature. The mono- and multilayers were deposited onto a hydrophobic wafer using a KSV LB film balance. The wafer was hydrophobically functionalized to enhance the attachment of the hydrophobic tail of the molecules. The surface pressure was 35 mNm-2 and a dipping rate of 5 mm/min.
2.3.2 Polystyrene
A suspension of 10 mg/ml of polystyrene particles in cyclohexanone has been spread onto water at 15 °C of temperature. The monolayer was deposited onto a homemade square shaped QCM (figure 11) with 12 MHz of resonance frequency in a KSV LB film balance. The surface pressure was 30 mNm-2 and a dipping rate of 5 mm/min. The film has been analyzed with the network analyzer.
3. MEASUREMENT METHODS
There are several techniques to characterize the topography of materials. Some of them were used in this work.
3.1 Optical microscopy
A Zeiss optical microscope with four lenses with an amplification of 5x, 10x, 20x, and 50x has been used for film characterization. This microscope has a dark and a light field option for a better viewing of the samples. Most of the samples were analyzed in the dark field.
3.2 Ellipsometry
Ellipsometry is an optical technique that uses polarized light to probe the dielectric properties of a sample and is used for analysis of very thin films. For a study of the polarization of the light reflected from the sample, ellipsometry can give information about layers that are thinner than the wavelength of the light itself, as small as a single atomic layer. Depending on what is already known about the sample, the technique can probe a range of properties including the layer thickness, morphology, or chemical composition.
Due to the state of polarization is mostly elliptic this technique received its name. The polarization of light reflected perpendicular and parallel to the plane of incidence is measured. It allows the relative phase change and relative amplitude change from the reflected surface to be determined.
3.3 Profilometry
Profilometry is a technique that optically determines the height profile of a sample. In the profilometer the sample is placed under the sensor; this allows a perpendicular incidence of light. The sensor is moved by the stages while the sensor transmits the height data to the measurement control unit. In order to improve lateral accuracy, the sensors should be synchronized to the stage movement. This method avoids inaccuracies caused by acceleration and deceleration of the stages in the moment of the data acquisition. The control unit shows by software a 3D picture of the sample that has been scanned.
3.4 Network analyzer
The network analyzer is used to characterize electrically passive and active components, such as amplifiers and filters. The vector network analyzer measures frequencies from 5 Hz to 100 GHz. It also displays the complete amplitude and phase characteristics of an electrical network. S-parameters, amplitude and phase, standing wave ratios (SWR), insertion loss or gain, attenuation, group delay, return loss, reflection coefficient, and gain compression are included in the characteristics that are measured with this device.
3.5 Scanning electron microscopy
The use of electron beams requires that the sample be placed in a vacuum chamber for analysis. An electron beam is produced by applying a high voltage to a hot tungsten filament, and accelerating the emitted electrons through a high electric field, typically 10-100 keV. The electron beam is then focused with magnetic field lenses to a typical spot diameter of 1-100 nm on the sample.
Scanning electron microscopy (SEM) uses the secondary electrons that are ejected from a sample to image a surface. These images are useful for studying surface morphology or measuring particle sizes. The electron beam is rastered across the sample by ramping the voltages on x- and y-deflection plates through which the electron beam passes (the z axis is the electron-beam direction). A detector above the sample detects the secondary electrons to produce an intensity map as a function of electron-beam position, which is displayed on a video or computer screen. Nonconductive samples require an evaporated gold or graphite coating over the sample to prevent charging effects that would distort the electric fields in the electron microscope.
3.6 IR spectroscopy
The infrared (IR) absorption spectroscopy measures the wavelength and intensity of the absorption of mid-infrared light by a sample. Mid-infrared light excites molecular vibrations to higher energy levels.
The wavelengths of many IR absorption bands are characteristic of specific types of chemical bonds. IR spectroscopy is used to confirm the identification of a particular substance and is also used to determine the structure of a new substance.
4. RESULTS
4.1 Solvent evaporation results
4.1.1 Polyvinyl chloride in dimethylformamide
Polyvinyl chloride in dimethylformamide has not shown a homogeneous film deposition. These solutions have presented an incomplete film deposition and agglomerations of PVC in form of island, as it is shown in figure 12. Apparently most of the film has been formed as multilayers.
4.1.2 Polyvinyl chloride in tetrahydrofuran
Polyvinyl chloride in tetrahydrofuran has shown a quite homogeneous film deposition. Best film depositions have been achieved at 2% concentration (films of 150 nm to 200 nm). For the naked eye, it has been a homogeneous film deposition, but optical microscope has shown film irregularities. Evaporation of tetrahydrofuran has made a not completely homogeneous and compact film deposition. It is shown in figure 13.
4.1.3 Polyvinyl chloride in cyclohexanone
Polyvinyil chloride in cyclohexanone has shown a homogeneous thin film deposition. A good film deposition has been achieved at 2% concentration (films of 50 nm to 150 nm). After cyclohexanone evaporation, a compact and homogeneous film can be seen, as it is shown in figure 14.
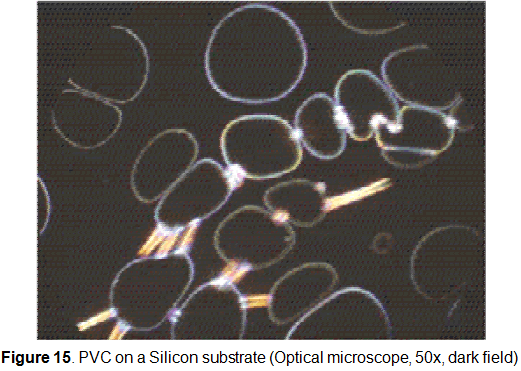
In some cases the film has presented an unusual behavior. The film has been broken in a quite homogeneous way as shown in figure 15. This is not completely understood.
4.1.4 Polystyrene in cyclohexanone
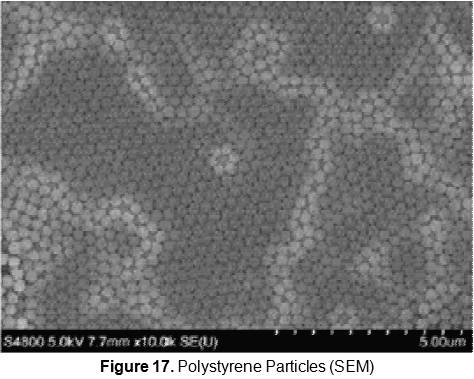
Both kinds of particles (hydrophobic and non hydrophobic) have presented almost the same behavior in relation to film deposition in the different temperature of the subphase. The spreading of the substance in different subphase temperature has shown an influence on the evaporation of the solvent. The best distribution of the PS particles has been obtained at temperatures between 10 °C to 15 °C. In this range the PS particles form a compact and well ordered monolayer. Optical and SEM microscopes have been used to study the film deposition onto the substrate as it is shown in figures 16 and 17.
4.2 Spin coating results
4.2.1 Polyvinyl chloride in dimethylformamide
PVC in this solvent has not shown a homogeneous spread behavior.
4.2.2 Polyvinyl chloride in tetrahydrofuran
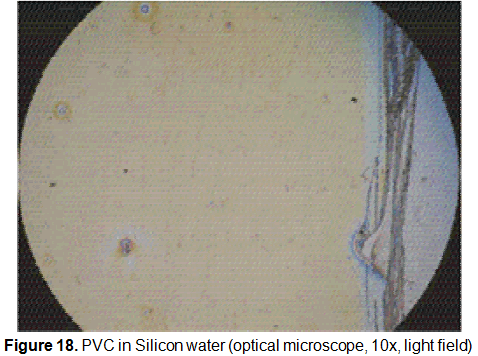
This mixture at the concentration of 1% of PVC in THF has shown the best result to get homogeneous thin films. Ellipsometry measurement showed an average thickness of 13,2 nm (figure 18).
4.2.3 Polyvinyl chloride in cyclohexanone
A concentration of 0,5% PVC in cyclohexanone has turned out to be the best mixture to obtain thin films. Ellipsometry measurement showed an average thickness of 16,58 nm (figure 19). Increasing the concentration has shown an increment in the thickness of the film. Lowering the concentration did not allow a film deposition. The behavior of PVC in cyclohexanone and THF has been quite similar.
4.2.4 Polystyrene and polyvinyl chloride in cyclohexanone
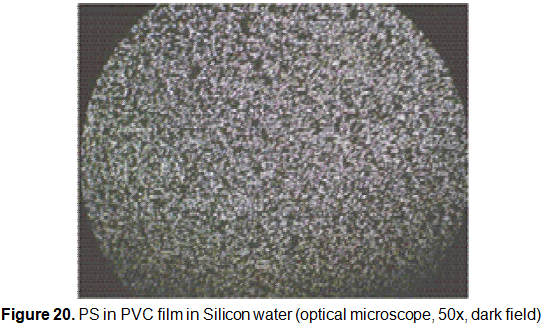
The PS particles show a well distributed arrangement within the spin coated PVC film. The PS particles have shown that, even increasing the PS volume in the suspension, they did not agglomerate (figure 20).
4.2.5 Polystyrene in chlorform
Several quartz samples with 4 mm of diameter electrodes were measured in fundamental mode with a network analyzer. The mean decreased value of the resonance frequency of the silver quartzes was 18,111 kHz and the change of the resistance was almost 0 ohms. With the reference that the density of PS is 1,05 g/cm3, the Sauerbrey equation has been used to calculate the thickness of this sealing layers. An average value of 762 nm of thickness has been found on these quartzes.
4.3 Langmuir-Blodgett film results
4.3.1 Arachidic acid
Mono- and multilayers (23-40 layers) of arachidic acid have been analyzed by optical microscopy (figure 21). It resulted in a rough layer. It also has been analyzed with IR spectroscopy, but the low thickness or the irregular surface of the layers did not allow a quantitative measurement.
4.3.2 Polystyrene
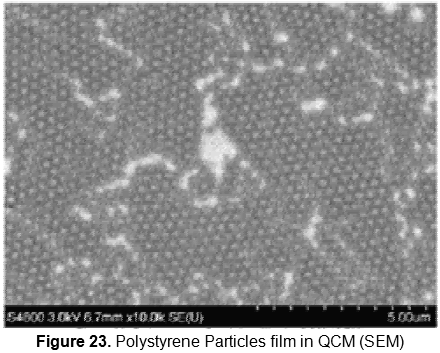
A partial coverage of the electrode has been obtained by these experiments; 85% coverage has been obtained in the electrode surface. The samples were analyzed by optical and SEM microscopy (figures 22 and 23). SEM has showed a monolayer arrangement of the particles. The problem was that the cables of the QCM interferred the film deposition. Cable reposition is in process for further experiment with this kind of sensor.
5. CONCLUSION
Some techniques for the preparation of thin films of PS particles have been developed by using several methods to get an interface layer to improve the PS attachment to the silicon substrate wafer.
Using the solvent evaporation method, the best quality of the layer was obtained with PVC dissolved in cyclohexanone at 2% concentration. The spin coated method gave the best result at a concentration of 1% of PVC in tetrahydrofuran obtaining an average thickness of 13,2 nm; however, a concentration of 0,5% of PVC in cyclohexanone gave similar good results. The Langmuir-Blodgett method did not give satisfactory results and requires further experiments.
AKNOWLEDGES
The authors are very grateful with the Petra II, subprogram of the Alfa II program for the financial support. Also they are grateful for the support given by the Otto-von-Guericke-University Magdeburg and Escuela de Ingeniería de Antioquia.
REFERENCES
D. S. Ballatine, R. M. White, S. J. Martin, A. J. Ricco, E. T. Zeller, G. C. Freye, H. Wohltjen. Acoustic wave sensors, Academic Press, San Diego, 1988. [ Links ]
G. Sauerbrey, Z. Physics. 1959, 155, 206. [ Links ]
R. Lucklum, P. Hauptmann, Transduction mechanism of acoustic-wave based chemical and biochemical sensors, Measurement Science and Technology 14, 2003, 1854-1864. [ Links ]
A. Arnau (Ed), Piezoelectric transducers and applications, 1 ed., Springer Verlag Berlin, Heidelberg, 2004. [ Links ]
B. Schlatt-Masuth, U. Hempel, R. Lucklum, P. Hauptmann; QCR response to attachment processes of particles, IEEE Sensors 2004 Proceeding, pp. 790-793. [ Links ]
C. Behling, The non-gravimetric response of thickness shear mode resonators for sensors applications. Shaker Verlag, Aachen, 1999. [ Links ]
K. K. Kanazawa and J. G. Gordon. Frequency of quartz microbalance in contact with liquid, Anal. Chem., 57, 1985, 1770-1771. [ Links ]
R. A. Durst, A. J. Baumner, R. W. Murray, R. P. Buck, and C. P. Andrieux. Chemically modified electrodes: recommended terminology and definitions. Pure and App/. Chem. Vol. 69, No. 6, pp. 1317-1323, 1997. Printed in Great Britain. [ Links ]
J. Velho, N. Santos. Methods for the analysis of surface topography at different scales: the case of coated papers. Materiais 2005: III International Materials Symposium and XII Portuguese Materials Society Meeting. University of Aveiro, Aveiro, Portugal. [ Links ]