Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista EIA
versión impresa ISSN 1794-1237
Rev.EIA.Esc.Ing.Antioq no.4 Envigado jul./dic. 2005
ENGINEERING MATERIALS SURFACES BY MICROFABRICATION TECHNIQUES, TO REGULATE IN VITRO CELL FUNCTIONS: REVIEW
Daniel Gallego*, Derek Hansford**
* Ingeniero Biomédico EIA-CES. Grupo de Investigación en Ingeniería Biomédica EIA-CES, Línea de Biomateriales y Biotecnología en Salud. Envigado, Colombia. bmdagal@eia.edu.co.
** PhD Materials Science and Mineral Engineering (UC Berkeley). Biomedical Engineering Center, The Ohio State University, The Ohio MicroMD Laboratory. Columbus, United States. hansford.4@osu.edu.
Artículo recibido 9-IX-2005. Aprobado con revisión 9-XI-2005
Discusión abierta hasta mayo 2006
ABSTRACT
It has been clear that the surface properties of the materials used as substrates for cell culture can influence the cell behavior affecting different factors such as cell orientation, adhesion, growth, differentiation, and apoptosis. In the last few years, several micro and nanofabrication techniques have emerged as important tools used to modify the surface properties of those materials at the micro or nano scale, topologically or chemically. This paper reviews the most used microfabrication techniques to introduce modifications on the materials surfaces and its implications on cell behavior. This review ends up showing the possible medical applications derived as a source of future work in the field.
KEY WORDS: Cell adhesion; cell proliferation; materials surfaces; microfabrication; surface topography; surface chemistry.
RESUMEN
Ha quedado claro que las propiedades superficiales de los materiales utilizados como sustratos para cultivo celular pueden influir en el comportamiento de las células afectando diferentes factores tales como la orientación celular, adhesión, crecimiento, diferenciación y apoptosis. En los últimos años, varias técnicas de microfabricación y nanofabricación han emergido como herramientas importantes usadas para modificar las propiedades superficiales de esos materiales en la escala micrométrica y nanométrica, ya sea topográfica o químicamente. Este artículo revisa las técnicas de microfabricación mas usadas para introducir modificaciones en las superficies de los materiales y sus implicaciones en el comportamiento celular. Esta revisión termina mostrando las posibles aplicaciones médicas derivadas como una fuente de futuros desarrollos en este campo.
PALABRAS CLAVE: Adhesión celular; proliferación celular; superficie de los materiales; microfabricación; topografía de la superficie; química de la superficie.
1. INTRODUCCIÓN
Lately, a great variety of microfabrication techniques have been used to micropattern materials surfaces in order to regulate cell functions. This technology is a powerful tool to study different factors such as cell attachment and growth, and to create organized cell cultures for applications in tissue engineering. Patterned substrates are used principally in cell cultures, but the medical implants science has also shown interest for these approaches since the interaction of cells with the materials surfaces is clearly important in the rejection or effectiveness of any implant. Variations on the surface chemistry and topology have been done with different techniques like photolithography, micro contact printing, and lift-off. This paper reviews the most used and recent techniques to micropattern surfaces, and describes potential medical applications trying to open a door for future work in the field.
2. SURFACE TOPOGRAPHY
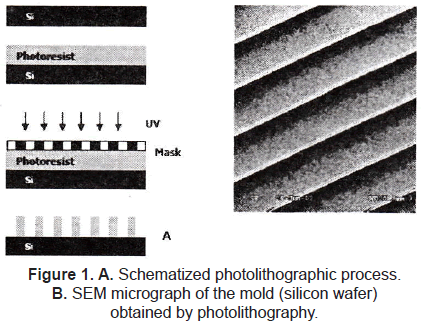
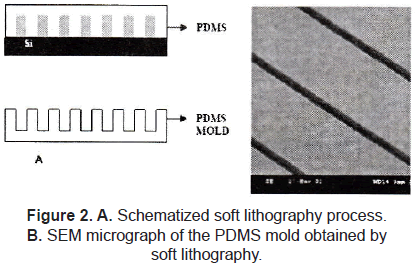
Topographical cues play a crucial role in mediating not only cell orientation and biocompatibility, but also factors such as protein (and gene) expression and cell differentiation. The semiconductor industry allows us to fabricate well-defined surface features in the 100's of micrometers down to a few nanometers; the most common technique used to introduce topographical changes to the surfaces is photolithography (figure 1), which consists of selectively exposing a photoresistance to UV light through a defined mask. Soft lithography represents a non-photolithographic strategy based on self-assembly and replica molding for carrying out micro- and nanofabrication; it is an efficient and low-cost method for the formation and manufacturing of micro and nanostructures ranging from 30 nm to 100 µm (figure 2) [1-3].
The integration of micro and nanotechnology techniques with the biological field has led researchers to make cell growth experiments on topographically modified surfaces. For long time experiments have reported that comparing with smooth surfaces; slightly roughened surfaces could improve the osteointegration, reduce fibrous encapsulation and increase the integration of percutaneous implants. These evidences could lead to the development of new prosthetic and medical implant devices.
Van Kooten et al. [4] showed that using micro textured PDMS (rubber) substrates to culture fibroblast could reduce significantly capsule formation. In this study the interaction of human fibroblasts with rubber surfaces was observed using a cell cycle analysis. PDMS substrates were textured with 2 µm, 5 µm, and 10 µm wide grooves or kept smooth. Cells on 10 µm showed less proliferation than cells with 2 µm and 5 µm grooves.
Cheroundi et al. [5] went into in-vivo experiments, studying the performance of percutaneous micro machined materials. They found that their implants were able to induce epithelial downgrowth around the implant. Finally, the response of fibroblast was greatly affected by the dimensions of the structures fibroblasts oriented along 3 µm and 10 µm deep grooves whereas they inserted obliquely into 22 µm deep grooves).
The cellular migration (speed and orientation of the movement) is also affected by the surface topography. Jian and Saltzman [6] studied the effect of micropatterned surfaces on neutrophil cells. In this study, parallel ridges/grooves were micropatterned on glass surfaces using a photosensitive polyimide. The width (2 µm) and length (400 µm) of the ridges were kept constant, the height (5 µm or 3 µm) and the repeat spacing (6 µm -14 µm) of the ridges were changed to investigate the effect of micro topography on neutrophil migration. They found that more than 95% of neutrophils moved in the direction of the long axis of ridges/grooves according with a phenomenon termed "contact guidance"; the rate of cell movement was strongly dependent on the micro topography of the ridges, the highest speed of movement was obtained at a ridge height of 5 µm and spacing of 10 µm, indicating that the movement was about 10 times faster than that on smooth glass surfaces.
The configuration of grooves/ridges has been widely explored by the researchers. Von Recum and Van Kooten as well as Walboomer et al. have deeply studied the effects of this configuration on different cell lines such as fibroblasts, epithelial cells, and endothelial cells [7, 8]. These studies showed that the cells aligned along with the groove's axis; and that the groove depth and width play crucial roles in the degree of alignment.
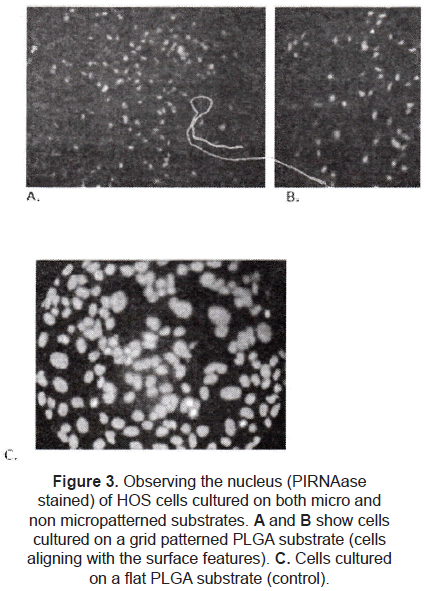
A few studies are focused on different configurations. Hansford et al. [9] studied the effect of a grid pattern (45 µm side squares and 10 µm deep) on the cell alignment. Human osteosarcoma cells (HOS) were cultured on PLGA micropatterned substrates; comparing with flat films (controls), the cells on the micropatterned surfaces were truly affected by the micro topography (figure 3), influencing factors as cell alignment, orientation, proliferation, and morphology.
Craighead et al. [10] studied the effects of micro-pillars geometry on nervous system cells. Silicon pillars were 0.5 µm in width, 1.0 µm or 2.7 µm in height, and separated by 1.0 µm. Results showed that both, the LRM55 cells and the primary astrocytes demonstrated a preference for the pillars rather than the flat silicon surface, and no discernable preference for either pillar height. On the other hand, Wilkinson et al. have looked at the attachment of rat epitenon cells to nanometer-sized polystyrene pillars; results showed a significant decrease in the number of cells attached to the pillars configuration, in comparison with the smooth control surfaces; they proposed that such nanopatterned surfaces could be useful as non-adhesive layers for implant devices.
Not only the cellular migration, but also the cellular activation is affected by the microphotography. Changes in the cytoskeleton condensation produced by the contact of a cell with the micro-topography determine the cellular activation. Wojciak-Stothard et al. [11] studied the activation of a macrophages line and rat peritoneum by topographic features on a nanometric scale; they found that cells cultured on nanogrooves showed a higher phagocytotic activity than cells cultured on plain substrata. In other study, Chou et al. [12] showed that the expression of mRNA for fibronectin was improved by culturing the fibroblast on microgrooved substrates.
Both micro and nano-scale topographical patterning have been shown to influence cell interactions with the material surface. Regulation of different cell activities such as proliferation, adhesion, and apoptosis, etc. could be very useful for the design of medical devices, implants, and biomaterials.
3. SURFACE CHEMISTRY
Some microfabrication techniques allow producing chemically modified surfaces. Chemical patterning has been widely used to study the interactions of the cells with their substrates. The most used techniques are: microcontact printing (µCP), dry lift-off and chemical photo-immobilizing. These techniques have been exploited for producing micro patterns of biomolecules in order to study and manipulate cellular interactions with different substrates.
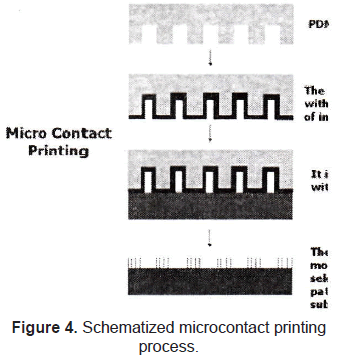
The method of microcontact printing was initially developed by Kumar et al. [13] and Hidber et al. [14] to pattern surfaces with self-assembled monolayers (SAMs) of alkanethiolates and colloids, to use them as masks in dry etching and chemical process. It involves two of the microfabrication techniques used for topographical patterning (photolithography and soft lithography). Briefly, the desired pattern is printed onto a silicon wafer using a standard photolithography technique. An inverse replica of this mold is made of polydimethylsiloxane (PDMS, thermally cured silicone elastomer) using soft lithography. After that, the PDMS mold is covered with the desired molecule and it is put in contact with the surface intended to pattern (figure 4).
Shinghvi et al., Chen et al. and Mrksich et al. [15-17] have deeply applied the Microcontact printing technique for cell patterning. The technique was successfully used to imprint gold surfaces with specific patterns of alkanethiols SAMs, creating "islands" of defined shape and size that supported extracellular matrix protein adsorption, and therefore cell attachment. Results showed that it was possible to place cells in defined locations and arrays, and to dictate their shape.
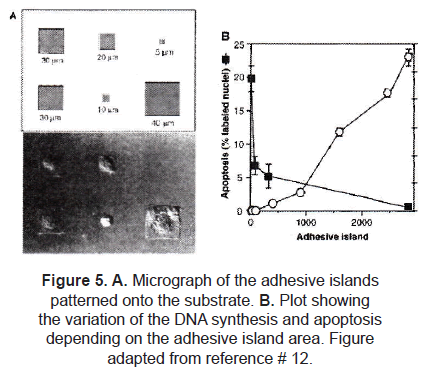
Defining the degree of cell extension provided control over cell growth and protein secretion. In a different experiment, Chen et al. [16] controlled the growth and apoptosis of human and bovine capillary endothelial cells by using micropatterned substrates that contained extracellular matrix-coated adhesive islands of decreasing size, to progressively restrict cell extension. Using µCP, fibronectin adhesive square islands were patterned onto a single substrate. After the cell culture, they found out that square-shaped cells were produced almost matching the size and shape of the adhesive island (figure 5A). Apoptosis as well as DNA synthesis were affected by the size of the island (figure 5B).
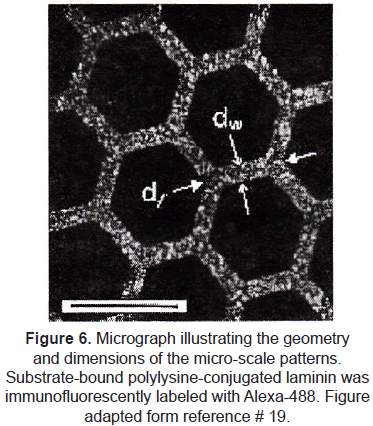
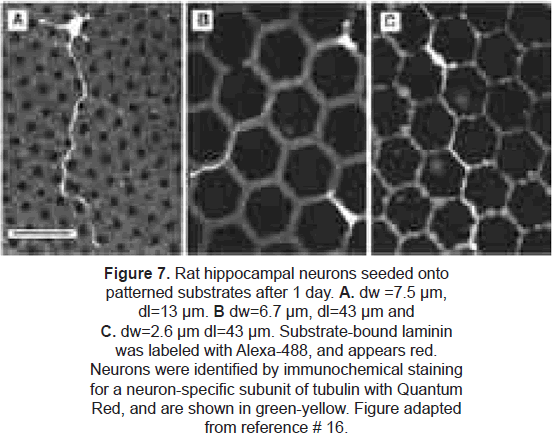
µCP has been exploited specifically to control neuron cell attachment and neurite morphology [18-20]. Random growth of axons and dendrites in cultures makes difficult to control synapse formation and development of neural networks. The group of Kam et al. [20] used microcontact printing to define an interconnected lattice network of polylysineconjugated laminin (a protein-polypeptide ligand that is an effective promoter of neuron outgrowth on material surfaces). Rat hippocampal neurons selectively adhered to the patterned regions. Long axonal processes were extended by adhered neurons; they did not deviate from the prescribed patterns, demonstrating that neurons respond to this protein with high selectivity and that these techniques could provide long-range guidance of axonal outgrowth. (figures 6 and 7).
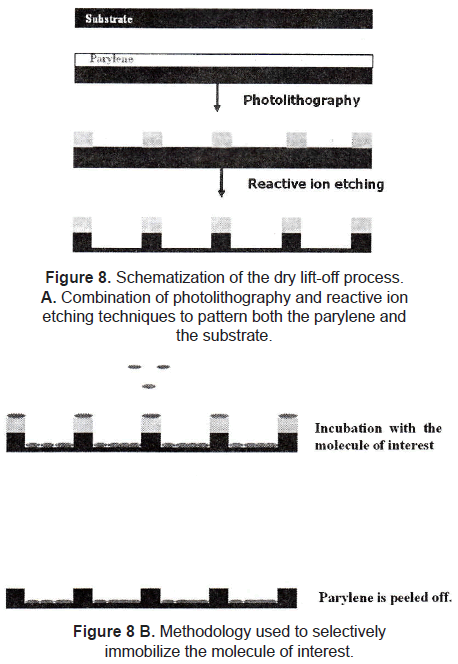
Dry lift-off is a more recent technique applied to pattern biological active molecules. Ilic and Craighead [21] described the lift-off process using parylene as photosensitive polymer. The parylene is patterned onto the substrate by using a combination of photolithography and reactive ion etching. The substrate is incubated with a solution of the desired molecule to allow its adsorption. After that, the parylene is peeled off of the substrate mechanically, leaving the molecule selectively adsorbed on the surface (figure 8). Using this method, they patterned aminopropyltriethoxysilane (APTS), antibodies, and poly-L-lysine. Results showed that there were binding of E. Coli and rat basophilic cells to antibodies and poly-L-lysine, respectively, indicating that biological activity is maintained with this pattern technique.
Bhatia et al. [22] proposed a variation of the lift-off technique. A photoresist is patterned on a glass substrate by photolithography. After the development, the desirable molecule is deposited onto the patterned glass surface, making chemical bonds with the exposed glass region. Finally, the photoresist is selectively removed with acetone, leaving the molecule pattern on the glass surface. The main goal of the research was to make organized cell co-cultures (hepatocytes and 3T3 fibroblast). The results were quite satisfactory, demonstrating that this technique is also useful to make successful chemical patterning, therefore controlling cell adhesion.
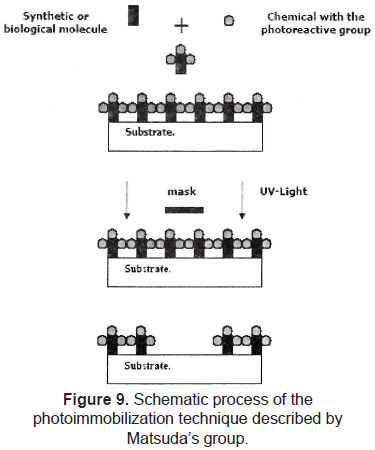
Photo-immobilization of molecules is also a widely used technique to perform chemical patterns. Matsuda et al. [23-25] reported the development of a micropatterning technology for cultured cells, by precise surface regional modification via photochemical fixation of phenyl azido-derivatized polymers on polymer surfaces. The process consists in coupling the molecule of interest with a photoreactive group, finally, this complex (molecule and photoreactive group) is patterned on a substrate by using a conventional photolithographic process (figure 9). This technique was used to pattern hydrophilic regions onto a hydrophobic polymer surface; then, endothelial cell behavior was evaluated, showing that these cells adhered, spread, and proliferated only on the hydrophobic regions.
Chen et al. [26] used Matsuda's method to pattern immobilized sulphated hyaluronic acid on a polystyrene plate and examine the interaction with blood cells. Platelet adhesion was reduced on the regions immobilized with sulfated hyaluronic acid. In the same way, Park and Ito [27] micropatterned heparin onto polystyrene substrates; and in the presence of fibroblast growth factor (FGF), the growth of mouse fibroblast STO cells was enhanced only on the heparin-immobilized regions. These results indicated that micropatterned-immobilized heparin activated FGF for cell growth activity.
Micropatterning with cell growth factors has been of great interest for the researchers, since it was demonstrated that immobilized growth factor proteins could regulate cell functions without the cellular internalization [28-30]. Chen et al. [31] found that immobilized insulin had an extremely high mitogenic effect, which was much higher than that of native insulin. Similar results were obtained using immobilized epidermal growth factor (EGF) [32]. Most of the researchers in the field agree that the immobilization inhibits the down-regulation induced by the cellular internalization, thus continuing the stimulation for a long time.
Ito et al. [33] and Chen et al. [34] photoimmobilized insulin and EGF on a poly (ethylene terephthalate) or polystyrene. Chinese hamster ovary (CHO) cells overexpressing the cognate receptors were cultured on the patterned surfaces. After 8 hours, phosphorylation (activation) was investigated by staining with an anti-phosphotyrosine antibody. The signaling proteins were activated only in cells cultured on the areas with the immobilized biomolecules. The important result was that the cell proliferation was accelerated only in the photo-immobilized areas.
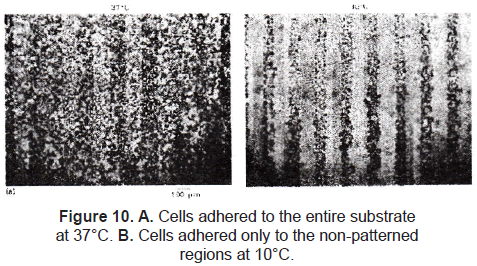
A relatively new approach is worked with stimuli-responsive polymers. Ito et al. [35] and Chen et al. [36] immobilized a thermo-responsive polymer onto a polystyrene substrate using the method described by Matsuda. The irradiated regions cross-linked the polymers to the polystyrene film. The non-exposed regions were washed away with water. Mouse fibroblast STO cells were cultured on this patterned film at 37 °C. There was no significant difference of cell attachment at this temperature, but when the film was maintained at 10 °C, cells on the photo-immobilized regions detached (figure 10).
4. POSSIBLE MEDICAL APPLICATIONS
As we could see, surface micropatterning to regulate cell functions has been deeply investigated; this field may have a strong impact on the medical science, specifically in areas such as implants, drug delivery systems (using cell therapy), tissue engineering, and biomaterials.
There are several disorders related to alterations on the retinal pigment epithelium (RPE), these are: Stargardt's disease, age-related macular degeneration and some forms of retinitis pigmentosa. Recent studies propose cell transplantation of RPE as a successful therapy for diseases like retinal degeneration; the problem is that RPE cells are polar, they have an apical and a basal domain, so random placement of these cells into the subretinal space does not always result in RPE regeneration.
Lu et al. [37] studied a way to culture RPE cells, proposing a transplantation of an organized sheet of cells with appropriate orientation as therapy. The experiments were focused on how could be affected the shape and function of RPE cells by changing the surfaces properties of the substrate with micro-scale patterns of octadecyltrichlorosaline (OTS, hydrophobic organosilane). The studied configuration was circles of 10 µm or 50 µm in diameter (borosilicate glass-hydrophilic region), surrounded by OTS (hydrophobic region). Cells were seeded, and after 4 h they found that most of the cells on the 10 µm pattern had round morphology, the spread cells were few and the average size was approximately 10 µm; cells on the 50 µm pattern were organized in clusters confined within the hydrophilic region, the average size was 20 µm; cells on the control substrates were spread and more elongated. When the cells reached confluence, they were closely packed and formed a continuous monolayer; the cytoskeleton organization was similar for the two micropatterned surfaces (actin organized into circumferential rings at the apical side and short filaments at the basal side); this array of the actin filaments has been found in differentiated epithelial cells, and it is associated with tight junctions that are crucial to maintain cell polarity. These results demonstrated that is possible to modulate RPE cell shape and phenotypic expression using micropatterned surfaces.
Several sportsmen and people with degenerative (or autoimmune) diseases have problems with the articular cartilage. Petersen et al. [38] worked on the design of micro-engineered scaffolds for cartilage tissue engineering applications. Chondrocites behavior was studied when cultured on micropatterned agarose substrates. Immunofluorescent assay for type II collagen was used to check the chondrocite's activity. Results showed that the cell adhesion was higher on the micropatterned surfaces than that on the smooth controls; the chondrocites grew up within the squares, and factors such as cell adhesion and proliferation were directly related to the square dimensions. Immunofluorescence assays demonstrated the production of type II collagen in the matrix.
Nerve regeneration is one of the biggest issues related to patient's rehabilitation. Leng et al. [39] proposed the use of microcontact printing as a prototype visual prosthesis interface. Micropatterned regions of growth factors were created with a final resolution of 5 µm (the substrates used were glass and a hydrophobic polymer). Stimulation electrodes were used to improve the growth of the rat retinal ganglion cells (RGCs). Results showed that using microcontact printing they were able to direct the growth of isolated rat RGCs on both glass and hydrophobic plastic surfaces. Finally, the authors concluded that the ability to direct the growth of RGCs opens the possibility of a visual prosthesis interface based on the regeneration of retinal cells.
In the same way, there are a lot of researches showing that micro and nanotechnology are an option to solve different problems on the medical science. A few are briefly mentioned below.
- Bhadriraju and Chen [40] say that the facilities provided by different microfabrication techniques to spatially organize cells and their adhesion has enhanced our ability to engineer cell functions ex vivo. They propose the use of these tools to create more in vivo-like cultures. This could be useful for cell-based drug discovery and target validation.
- The group of Lee [41] proposes microcontact printing as an effective tool to pattern inhibitory molecules in order to regulate the growth of retinal pigment epithelial cells or iris pigment epithelial cells on human lens capsule for transplantation.
- Hongchun and Yoshihiro [42] studied how could be controlled the cell attachment and detachment on micropattern-immobilized poly(N-isopropylacrylamide) with gelatin. Research on this field has great impacts on any implantable device.
5. CONCLUSIONS
Now it is clear that the materials surface properties affect the cell behavior. Both, chemical and topographical surface features have effects on different cell biology factors such as attachment, proliferation, differentiation, activation, gene expression and apoptosis. Semiconductors industry became an important tool to modify those features at the nano or micro scale allowing the scientific community to engineer materials surfaces with determined micro features (chemically or topologically) in order to find a way to control the cell behavior. Regulation of cell functions by micropatterning surfaces has several applications in Medicine. Controlling the amount of proteins secreted by the cells (gene expression) could be useful for cell therapy (drug delivery system); regulating the migration of the cells (direction and speed) could be useful for nerve regeneration; controlling the attachment and detachment of the cells could be useful for any medical implant (maybe avoiding some immune response or fibroblast encapsulation); organized cell co-cultures could open the door for in-vitro reconstruction of organs. In general, all these are new fields that should be investigated to get a better understanding on this area and to finally find the appropriate way to be applied.
REFERENCES
[1] Whitesides G. M., Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001; 3: 335-73. Review. [ Links ]
[2] Yu T., Ober C. K. Methods for the topographical patterning and patterned surface modification of hydrogels based on hydroxyethyl methacrylate. Biomacromolecules. 2003 Sep-Oct; 4(5): 1126-31. [ Links ]
[3] Wang C., Madou M. From MEMS to NEMS with carbon. Biosens Bioelectron. 2005 Apr 15; 20(10): 2181-7. [ Links ]
[4] Van Kooten T. G., Whitesides J. F., von Recum A. Influence of silicone (PDMS) surface texture on human skin fibroblast proliferation as determined by cell cycle analysis. J Biomed Mater Res 1998; 43: 1-14. [ Links ]
[5] Chehroudi B., McDonnel D., Brunette D. M. The effects of micromachined surfaces on formation of bone-like tissue on subcutaneous implants as assessed by radiography and computer image processing. J Biomed Mater Res 1997; 34: 279-90. [ Links ]
[6] Tan J., Saltzman W. M. Topographical control of human neutrophil motility on micropatterned materials with various surface chemistry. Biomaterials. 2002 Aug; 23(15): 3215-25. [ Links ]
[7] Von Recum A., van Kooten T. G. The influence of micro-topography on cellular response and the implications for silicone implants. J Biomater Sci Polym Ed. 1995; 7(2): 181-98. Review. [ Links ]
[8] Walboomers X. F., Monaghan W., Curtis A. S., Jansen JA. Attachment of fibroblasts on smooth and microgrooved polystyrene. J. J. Biomed Mater Res. 1999 Aug; 46(2): 212-20. [ Links ]
[9] Hansford D., Gallego D., Patiño D., Calderón D., Ferrell N., Chakrapani A. Microfabrication and in vitro evaluation of Polylactic-co-glycolic Acid (PLGA) Tissue Engineering Scaffolds. First Colombian Congress of Bioengineering and Biomedical Engineering, Medellín - Colombia. October, 2003. [ Links ]
[10] Craighead H. G., Turner S. W., Davis R. C., James C. D., Perez A. M., St. John P. M., Isaacson M. S., Kam L., Shain W., Turner J. N., Banker G. Chemical and topographical surface modification for control of central nervous system cell adhesion. J Biomed Microdev 1998; 1(1): 49-64. [ Links ]
[11] Wojciak-Stothard B., Curtis A., Monaghan W., Macdonald K. and Wilkinson C. Guidance and activation of murine macrophages by nanometric scale topography. Exp Cell Res. 1996 Mar 15; 223(2): 426-35. [ Links ]
[12] Chou L., Firth J. D., Uitto V. J., Brunette D. M. Substratum surface topography alters cell shape and regulates fibronectin mRNA level, mRNA stability, secretion and assemble in human fibroblast. J. Cell Sci. 1995 Apr; 108 (Pt 4): 1563-73. [ Links ]
[13] Kumar A., Biebuyck H. A., Whitesides G. M. Patterning self-assembled monolayers: applications in materials science. Langmuir 1994; 10(5): 1498-511. [ Links ]
[14] Hidber P. O., Helbig W., Kim E., Whitesides G. M. Microcontact printing of palladium colloids: micronscale patterning by electroless deposition of copper. Langmuir 1996; 12(6): 1375-80. [ Links ]
[15] Shinghvi R., Kumar A., Lopez G. P., Stephanopoulos G. N., Wang D. I., Whitesides G. M., Ingber D. E. Engineering cell shape and function. Science 1994; 264: 296-8. [ Links ]
[16] Chen C. S., Mrksich M., Huang S., Whitesides G. M., Ingber D. E. Geometric control of cell life and death. Science. 1997 May 30; 276(5317): 1425-8. [ Links ]
[17] Mrksich M., Chen C. S., Xia Y., Dike L. E., Ingber D. E., Whitesides G. M. Controlling cell attachment on contoured surfaces with self-assembled monolayers of alkanethiolates on gold. Proc Natl Acad Sci U S A. 1996 Oct 1; 93(20): 10775-8. [ Links ]
[18] Leng T., Huie P., Mehenti N. Z., Peterman M. C., Lee C. J., Marmor M. F. Sanislo S. R., Bent S. F., Blumenkranz M. S., Fishman H. A. Directed ganglion cell growth and stimulation with microcontact printing as a prototype visual prosthesis interface. Annual Meeting Abstract Search and Program Planner 2002. Abstract No 4454. [ Links ]
[19] Scholl M., Sproessler C., Denyer M., Krause M., Nakajima K., Maelicke A., Knoll W., Offenhaeusser A. Ordered networks of rat hippocampal neurons attached to silicon oxide surfaces. J Neurosci Methods. 2000 Dec 15; 104(1): 65-75. [ Links ]
[20] Kam L., Shain W., Turner J. N., Bizios R. Axonal outgrowth of hippocampal neurons on micro-scale networks of polylysine-conjugated laminin. Biomaterials. 2001 May; 22(10): 1049-54. [ Links ]
[21] Ilic B., Craighead H. G. Patterning of chemically sensitive biological materials using a polymer-based dry lift off. J Biomed Microdev 2000; 2(4): 317-22. [ Links ]
[22] Bhatia S. N., Yarmush M. L., Toner M. Controlling cell interactions by micropatterning in co-cultures: Hepatocytes and 3T3 fibroblasts. J Biomed Mater Res. 1997 Feb; 34(2): 189-99. [ Links ]
[23] Matsuda T., Inoue K., Sugawara T. Development of micropatterning technology for cultured cells. Trans Am Soc Artif Intern Organs 1990; 36: M559-63. [ Links ]
[24] Matsuda T., Inoue K. Novel photoreactive surface modification technology for fabricated devices. ASAIO Trans. 1990 Jul-Sep; 36(3): M559-62. [ Links ]
[25] Matsuda T., Sugawara T. Development of surface photochemical modification method for micropatterning of cultured cells. J Biomed Mater Res. 1995 Jun; 29(6): 749-56. [ Links ]
[26] Chen G., Ito Y., Imanishi Y., Magnani A., Lamponi S., Barbucci R. Photoimmobilization of sulphated hyaluronic acid for antithrombogenicity. Bioconjug Chem. 1997 Sep-Oct; 8(5): 730-4. [ Links ]
[27] Park YS., Ito Y. Micropattern-immobilization of heparin to regulate cell growth with fibroblast growth factor (FGF). Cytotechnology, 33(1-3) July 2000. 117-122. [ Links ]
[28] Ito Y. Intelligent materials for tissue engineering. In: Akaike T., Okano T., Akashi M., Terano T., Yui N., editors. Advances in biomaterials science. Tokyo: CMC 1997. p. 421-32. [ Links ]
[29] Ito Y. Tissue engineering with immobilized growth factors. Mater Sci Eng 1998; C6: 267-74. [ Links ]
[30] Ito Y., Chen G., Imanishi Y. Artificial juxtacrine stimulation for tissue engineering. J Biomater Sci Polym Ed. 1998; 9(8): 879-90. Review. [ Links ]
[31] Chen G., Ito Y., Imanishi Y. Mitogenic activity of water-soluble and water-insoluble insulin conjugates. Bioconjug Chem. 1997 Mar-Apr; 8(2): 106-10. [ Links ]
[32] Ito Y., Li J. S., Takahashi T., Imanishi Y., Okabayashi Y., Kido Y., Kasuga M. Enhancement of the mitogenic effect by artificial juxtacrine stimulation using immobilized EGF. J Biochem (Tokyo). 1997 Mar; 121(3): 514-20. [ Links ]
[33] Ito Y., Chen G., Imanishi Y. Photo-immobilization of insulin onto polystyrene dishes for protein-free cell culture. Biotechnol Prog 1996; 12: 700-3. [ Links ]
[34] Chen G., Ito Y., Imanishi Y. Photo-immobilization of epidermal growth factor enhances its mitogenic effect by artificial juxtacrine signaling. Biochim Biophys Acta. 1997 Sep 11; 1358(2): 200-8. [ Links ]
[35] Ito Y., Chen G., Guan Y., Imanishi Y. Patterned immobilization of thermoresponsive polymer. Langmuir 1997; 13: 2756-9. [ Links ]
[36] Chen G., Imanishi Y., Ito Y. Effect of protein and cell behavior on pattern-grafted thermoresponsive polymer. J Biomed Mater Res. 1998 Oct; 42(1): 38-44. [ Links ]
[37] Lu L., Kam L., Hasenbein M., Nyalakonda K., Bizios R., GoKpferich A, Young J. F., Mikos AG. Retinal pigment epithelial cell function on substrates with chemically micropatterned surfaces. Biomaterials. 1999 Dec; 20(23-24): 2351-61. [ Links ]
[38] Petersen E. F., Spencer R. G., McFarland E. W. Microengineering Neocartilage Scaffolds. Biotechnol Bioeng. 2002 Jun 30; 78(7): 801-4. [ Links ]
[39] Leng T., Huie P., Mehenti N. Z., Peterman M. C., Lee C. J., Marmor M. F., Sanislo S. R., Bent S. F., Blumenkranz M. S., Fishman H. A. Directed ganglion cell growth and stimulation with microcontact printing as a prototype visual prosthesis interface. ARVO Annual Meeting Abstract Search and Program Planner. 2002. Abstract No 4454. [ Links ]
[40] Bhadriraju K., Chen C. S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today. 2002 Jun 1; 7(11): 612-20. Review. [ Links ]
[41] Lee C. J., Philip H., Leng T., Peterman M. C., Marmor M. F., Blumenkranz M. S., Bent S. F., Fishman H. A. Microcontact printing on human tissue for retinal cell transplantation. Arch Ophthalmol. 2002 Dec; 120(12): 1714-8. [ Links ]
[42] Hongchun L., Yoshihiro I. Cell attachment and detachment on micropattern-immobilized poly(Nisopropylacrylamide) with gelatin. Lab Chip. 2002 Aug; 2(3): 175-8. Epub 2002 Aug 13. [ Links ]