Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista EIA
versão impressa ISSN 1794-1237
Rev.EIA.Esc.Ing.Antioq no.18 Envigado jul./dez. 2012
DIELECTRIC PROPERTIES OF POLY(VINYL ALCOHOL) HYDROGELS PREPARED BY FREEZING/THAWING TECHNIQUE
PROPIEDADES DIELÉCTRICAS DE HIDROGELES DE ALCOHOL POLIVINÍLICO OBTENIDOS POR LA TÉCNICA DE CONGELAMIENTO/DESCONGELAMIENTO
PROPRIEDADES DIELÉCTRICAS DE HIDROGEIS DE ÁLCOOL POLIVINÍLICO OBTIDOS PELA TÉCNICA DE CONGELAMENTO/ DESCONGELAMENTO
Martha Elena Londoño1, Juan Manuel Jaramillo2, Roser Sabater3 y Juan Manuel Vélez4
1 Física, Universidad de Antioquia; Magíster en Ingeniería, Área Ingeniería Biomédica, Universidad Pontificia Bolivariana; Doctora en Ingeniería, Ciencia y Tecnología de Materiales, Universidad Nacional de Colombia. Docente e Investigadora, Grupo de Investigación GIBEC, Ingeniería Biomédica, Escuela de Ingeniería de Antioquia. Envigado, Colombia. pfmalon@eia.edu.co.
2 Físico, Universidad de Antioquia; Magíster, Universidade Estadual de Campinas; Doctor en Ingeniería Eléctrica, Universidade de São Paulo, Brasil e integrante del Grupo de Investigación Electromagnetismo Aplicado. Profesor Asociado, Universidad EAFIT. Medellín, Colombia. jjaram44@eafit.edu.co.
3 Ingeniera en Electrónica y Doctora en Ingenieria Industrial, Universitat Politècnica de València. Profesora Titular e investigadora, Centre de Biomaterials i Enginyeria Tissular, Universitat Politècnica de València. Valencia, España. rsabater@die.upv.es.
4 Magíster y Doctor en Ingeniería, Universidade de São Paulo. Profesor e integrante del Grupo de investigación Ciencias y Tecnología de Materiales, Universidad Nacional de Colombia, Medellín, Colombia. jmvelez@unal.edu.co.
Artículo recibido 12-IX-2011. Aprobado 20-VIII-2012
Discusión abierta hasta junio de 2013
ABSTRACT
The dielectric behavior of poly(vinyl alcohol) -PVA- crosslinked hydrogels obtained by the repeated freezing/ thawing (F/T) technique have been investigated by dielectric relaxation spectroscopy (DRS) and differential scanning calorimetry (DSC). The crosslinked polymer is produced by the clustering of chains caused by the association of a polar group of the dissolved polymer followed by polymer crystallization. The dielectric spectra obtained from -50 °C to -10 °C show a broad secondary relaxation process associated to local mobility, ß relaxation, which is related to the terminal polar groups (OH). This process is strongly affected by the freezing/thawing cycles applied. The values of tan δ are below 1 indicating the dielectric phenomenon is predominant in all samples. The effect of crosslinking PVA on the dynamics of the secondary relaxation process was further analyzed.
KEY WORDS: dielectric properties; hydrogels; freezing/thawing; dielectric spectroscopy.
RESUMEN
Por medio de espectroscopia dieléctrica (DRS) y calorimetría diferencial de barrido (DSC) se investigaron las propiedades dieléctricas de los hidrogeles de alcohol polivinílico -PVA- entrecruzados por ciclos repetidos de la técnica congelamiento/descongelamiento. La asociación de los grupos polares del polímero disuelto seguida de su cristalización produce la reticulación del polímero. La espectroscopia dieléctrica obtenida entre -50 °C y -10 °C demostró la existencia de un proceso de relajación secundario asociado con la movilidad de grupos laterales polares OH de la cadena principal, la relajación β. Los ciclos de congelamiento/descongelamiento afectan fuertemente este proceso. El fenómeno dieléctrico predomina en todas las muestras como lo evidencia el valor menor de 1 de tan δ. Además, se analizó el efecto del entrecruzamiento en la dinámica de la relajación secundaria.
PALABRAS CLAVE: propiedades dieléctricas; hidrogeles; congelamiento/descongelamiento; espectroscopia dieléctrica.
RESUMO
Por médio de espectroscopia dielétrica (DRS) e calorimetria diferencial de varredura (DSC) pesquisaramse as propriedades dielétricas dos hidrogeis de álcool polivinílico  PVA
PVA entrecruzados por ciclos repetidos da técnica congelamento/descongelamento. A associação dos grupos polares do polímero dissolvido seguida de sua cristalização produz a reticulação do polímero. A espectroscopia dielétrica obtida entre -50 °C e -10 °C demonstrou a existência de um processo de relaxação secundário associado com a mobilidade de grupos laterais polares OH da corrente principal, a relaxação β. Os ciclos de congelamento/descongelamento afetam fortemente este processo. O fenômeno dielétrico predomina em todas as mostras como o evidência o valor menor de 1 de tan δ. Ademais, analisou-se o efeito do entrecruzamento na dinâmica da relaxação secundária.
entrecruzados por ciclos repetidos da técnica congelamento/descongelamento. A associação dos grupos polares do polímero dissolvido seguida de sua cristalização produz a reticulação do polímero. A espectroscopia dielétrica obtida entre -50 °C e -10 °C demonstrou a existência de um processo de relaxação secundário associado com a mobilidade de grupos laterais polares OH da corrente principal, a relaxação β. Os ciclos de congelamento/descongelamento afetam fortemente este processo. O fenômeno dielétrico predomina em todas as mostras como o evidência o valor menor de 1 de tan δ. Ademais, analisou-se o efeito do entrecruzamento na dinâmica da relaxação secundária.
PALAVRAS-CÓDIGO: propriedades dielétricas; hidrogeis; congelamento/descongelamento; espectroscopia dielétrica.
1. INTRODUCTION
Hydrogels are defined as a physically or chemically crosslinked polymer network able to absorb large amounts of water without being dissolved. Physically cross-linked PVA hydrogels prepared by repeated freezing and controlled thawing show excellent properties, when a PVA solution is subjected to freezing, the pure solvent crystallizes initially, while the solute stays in the liquid part of the specimen. Such state leads to stronger polymer-polymer interaction, which results in a stable three-dimensional cryogel network (Lozinsky, 1998). Such a technique produces stable hydrogels that are physically crosslinked because of the presence of crystalline regions arising upon the interaction between neighbor polymer chains due to the hydrogen bonding of pendant hydroxyls of PVA groups (Hassan and Peppas, 2000; Hatakeyema et al., 2005; Lozinsky et al., 2008). PVA hydrogels offer several beneficial properties: biocompatibility, biodegradability, high water content, bioinertness, and sterilizability, also they can be molded into desired shapes (Fergg y Keil, 2001; Ricciardi et al., 2003; Cascone et al., 2004). On the other hand, they have been examined in separation processes (Pissis and Kyritsis, 1997; Konsta et al., 1999) and for their application as polymer electrolyte membranes in fuel cells due to the fact they enhance ionic conductivity (Awadhia, Patel and Agrawal, 2006).
Different characterization techniques, such as cryo-transmission electron microscopy (cryo-TEM), chromatographic or scattering techniques, mechanical measurements, DSC, X ray diffraction (Wilcox et al., 1999, McGann et al., 2009), carbon nuclear magnetic resonance (CNMR), proton and high resolution solid-state (Ricciardi, Auriemma and De Rosa, 2005) have been used to study the structure of PVA hydrogels. It is a complicated network structure, based on different phenomena: crystallization, hydrogen bonding, liquid-liquid phase separation, and covalent bonds. However, most of the characterizations require the manipulation of the hydrogel sample, driving changes in the original structure. For example, by NMR Valentín et al. (2009) showed that PVA hydrogels obtained by F/T cycles exhibit a complex heterogeneous network structure. The crystallites are quantitatively detected as a rigid-like fraction of quickly relaxing magnetization and the mesh size constraints on the motion of the mobile chains (i.e., cross-links) that render the segmental motion locally anisotropic (Valentín et al., 2009). Dielectric measurements were carried out in PVA films of different crystallinities (not hydrogels) and a single broad relaxation region was observed at a low temperature. These investigations show that the dielectric relaxation process is associated with the motions of dipole in the amorphous regions of the polymer (McCrum, Read and Williams, 1991; Schartel, Wendling and Wendorff, 1996; De La Rosa, Heux and Cavaillé, 2001). Although numerous papers have been devoted to PVA hydrogels obtained by freezing/thawing, their dielectric behavior has not yet been studied.
Dielectric spectroscopy is sensitive to the heterogeneous system and can provide insights into the structures and electrical properties of the materials at molecular and macroscopic levels (Ni and Zhao, 2007). The dielectric constant and dissipation factor are crucial quantities required in the design of devices and furthermore, as a function of temperature or frequency, they reveal much information on the chemical or physical state of polymer (Abd El-kader et al., 2008). The dielectric behavior of polymers is determined by the charge distribution and also the statistical thermal motion of its polar groups. Electrical properties of pure and doped PVA films (Kulanthaisami, Mangalaraj and Narayandass, 1995; Singh and Gupta, 1998; Abd El-kader et al., 2008), complex electrolytes of PVA and PVA blends (Mishra and Rao, 1998), solution grown PVA films (Chandar et al., 1999), polyaniline-polyvinyl alcohol composites (Dutta, Biswas and Kumar, 2002), PVA gel electrolytes (Awadhia, Patel and Agrawal, 2006), solution poly(vinyl alcohol) + poly(vinyl pyrrolidone) (Sengwa and Sankhla, 2007) have been studied using different models. To our best knowledge there is no work about the dielectric behavior studies of PVA hydrogels obtained by a F/T technique and how this process and the number of cycles affect the relaxation processes.
The main aim of this work is to investigate the dielectric behavior of polyvinyl alcohol hydrogels with different crosslinking degree obtained by an F/T technique, between -50 °C and -10 °C, and 1-1 Hz to 106 Hz using dielectric spectroscopy. The regimes of cryogenic treatment processes have a pronounced effect on the properties of PVA hydrogels, and temperatures lower than -10 °C are usually employed in the F/T technique, so our interest is in this temperature range.
2. EXPERIMENTAL
2.1 Materials and preparation of PVA hydrogels
PVA with molecular weight of approximately 89,000 to 98,000 and a degree of hydrolysis 99+ % (Sigma-Aldrich) was used to prepare an aqueous solution of 15 % wt PVA by heating the solution at 80 °C and stirring the solution until it was completely homogeneous. The solution was then cast onto a glass mold and the samples (dimensions 7,0 x 7,0 x 0,1 cm) were exposed to four, six and twelve cycles of freezing for 12 hours at -20 °C and thawing 20 °C at a rate of 1.8 °C/min to obtain physical crosslinked hydrogels; the degree of crosslinking varies with the number of F/T cycles. After that, the samples were dried in a vacuum oven (at 25 °C) until constant weight.
2.2 Characterization
2.2.1 Differential scanning calorimetry
Differential scanning calorimetry (DSC Jade, Perkin Elmer) was used to analyze the crystalline nature, melting point and glass transition temperature of PVA hydrogels prepared by F/T processes. In a typical experiment, 6-8 mg of a dried sample were placed in an aluminum pan and cooled until -40 °C to freeze the sample and then heated at a scanning rate of 5 °C/min from -50 °C to 250 °C.
2.2.2 Dielectric relaxation spectroscopy
The dielectric measurements were performed using an impedance analyzer Alpha-S from Novocontrol Technologies, covering a frequency range from 1×10-1 Hz to 1×106 Hz. The samples of the PVA system were placed between two electrodes (10 mm of diameter) of a parallel plate capacitor. The sample cell with active head dielectric converter was mounted on a controlled temperature cryostat (BDS 1100) and exposed to a heated gas stream being evaporated from a liquid nitrogen dewar. The temperature control was assured by the Novocontrol Quatro cryosystem and performed within ±0.5 °C. The isothermal experiments were performed from -50 °C to -10 °C every 5 °C and the dielectric response (complex permittivity ε* and conductivity σ) were determined as a function of frequency.
3. RESULTS AND DISCUSSION
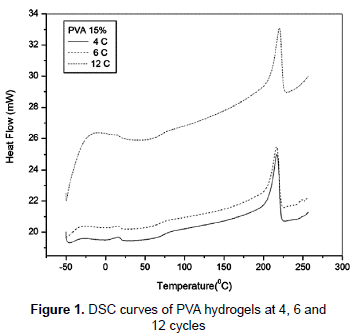
The crosslinked nature of the hydrogels obtained in this study can be proved putting the samples in polar and apolar solvents and noting the insoluble character of samples (Hickey and Peppas, 1995). Figure 1 shows the DSC curves of PVA hydrogel samples. The peaks around 217, 216 and 220 °C correspond to the apparent melting point of the PVA hydrogel crystallites, where the temperature became lower with 4 and 6 cycles F/T. The degree of crystallinity rises with increasing the number of cycles of cryogenic treatment (Hickey and Peppas, 1995; Lozinsky et al., 2008), the increases in the crystallinity for the samples with 6 and 12 cycles F/T are probably due to the oriented crystallization (Nakano et al., 2007). This is evident with the corresponding heat of fusion value. The samples that were exposed to four, six and twelve cycles of F/T had a similar glass transition temperature from 66 to 70 °C. The small changes in the glass transition temperature can be due to a decrease in the number of hydroxyl groups available for hydrogen bonding caused by an increase of the entanglement which hinders other hydrogen bonding formation from weakening of physical network and decreasing Tg (Shafee and Naguib, 2003).
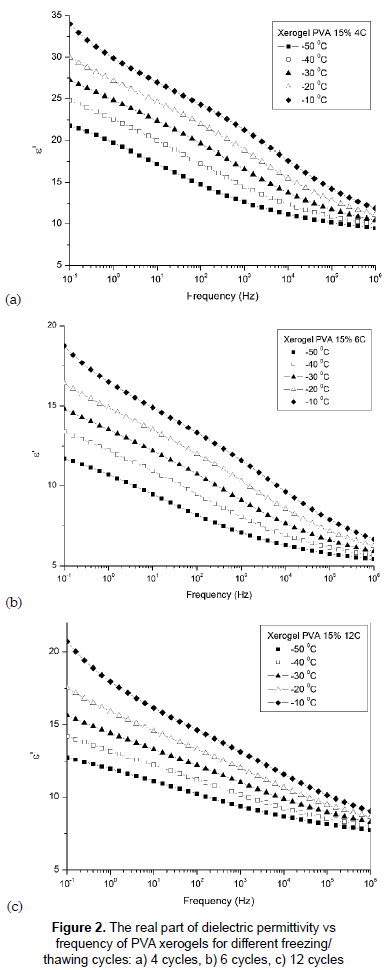
The dielectric behaviors of PVA hydrogels obtained by different cycles of F/T have been studied and results are analyzed below in terms of different parameters. Figures 2a, 2b and 2c show the variation of the dielectric constant ε' with frequency of temperatures between -50 and -10 °C for xerogels of PVA with different crosslinking degrees (4, 6 and 12 F/T cycles respectively). It is observed from the figures that the dielectric constant ε' continuously decreases with increasing frequency. The rapid decreases in the dielectric constant noticed over the frequency range 10-1 to 104 Hz may be attributed to the tendency of dipoles in macromolecules to orient themselves in the direction of the applied field in the low frequency range. However, in the high frequency range the dipoles will hardly be able to orient themselves in the direction of the applied field, and hence the value of the dielectric constant decreases (Blythe and Bloor, 2005). The sample with fewer cycles, 4, shows high ε' values. This can be understood in terms of the degree of crosslinking and changes in crystallinity. Crystallization prevents the movement in the chains; the crystalline phase is rigid and shows no dielectric relaxation processes and, as a consequence, the dielectric response is lower (Blythe and Bloor, 2005). The increase in the number of dipoles is due to less crosslinking in the sample. It was also observed that temperature affects the dielectric properties of PVA hydrogels. In fact, the rise in the temperature and the resulting drop in relaxation time of the sample increase the degree of the dipole orientation and consequently enhance the value of the dielectric constant (Singh and Gupta, 1998).
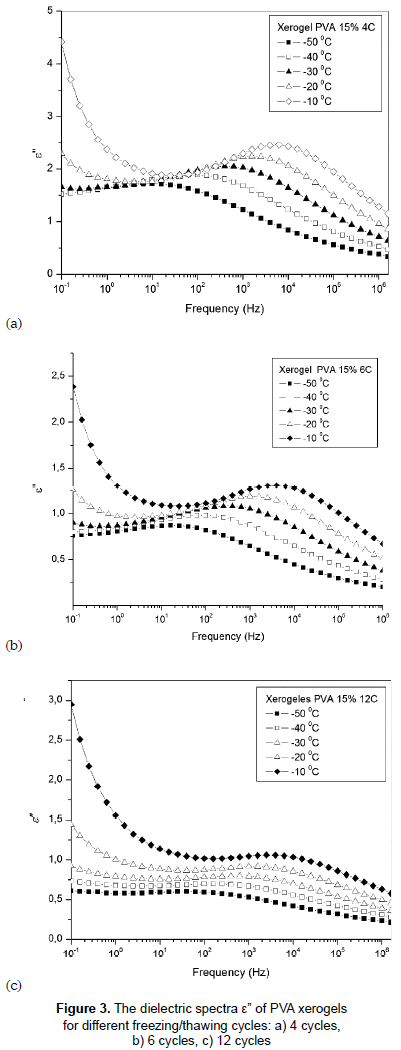
Figures 3a, 3b and 3c show the variation of dielectric loss, ε'', with frequency of temperatures between -50 and -10 °C for PVA hydrogels with 4, 6 and 12 F/T cycles. All samples exhibit relaxation processes, b relaxation; this relaxation is caused by some local movement of side dipole groups, in this case by motions of hydroxyl groups (Hickey and Peppas, 1995). High dielectric loss values are observed for the samples with fewer F/T cycles, which may be due to the lower degree of crosslinking present in the sample with 4 cycles which in turn makes the neighboring network chain more flexible; hence the conductivity increases. From figure 3 it can also be observed that the temperature largely influences the dielectric loss of the samples and the intensity of this relaxation rises with the increases of temperature (De La Rosa, Heux and Cavaillé, 2001).
It is worth notice that the sample with 12 F/T cycles has a different behavior both in ε' and ε'' due a greater crowding caused by the increase of the mass density due to the entanglement and crosslinking in the sample.
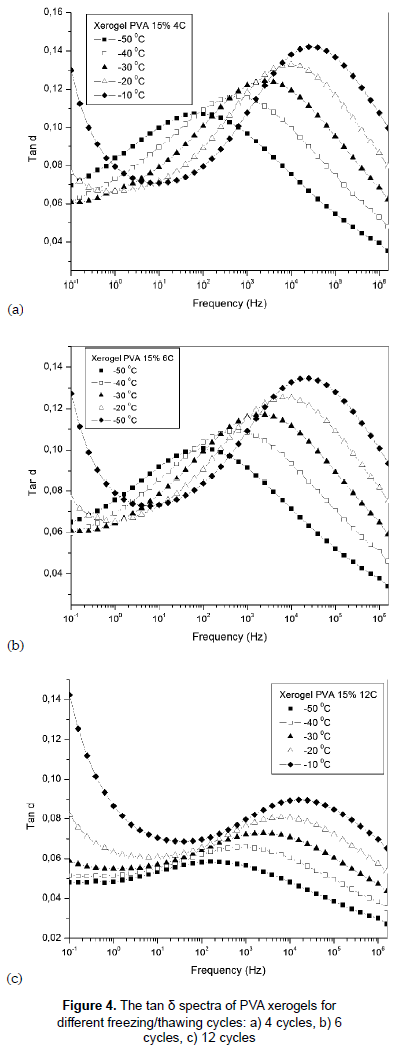
3.1 Dielectric loss tan δ (f)
Figures 4a, 4b and 4c present the frequency dependence of tan δ, equal ε'' / ε', for PVA hydrogels with different F/T cycles at temperatures between -50 and -10 °C. The tan δ spectrum shows relaxation process for all samples, and as the degree of crosslinking increases, the magnitude of the relaxation peak decreases (Kao, 2004) and the breadth increases (Casalini and Roland, 2010). The values of tan δ are below 1 indicating the dielectric phenomenon is predominant in all samples.
3.2 Conductivity (AC)
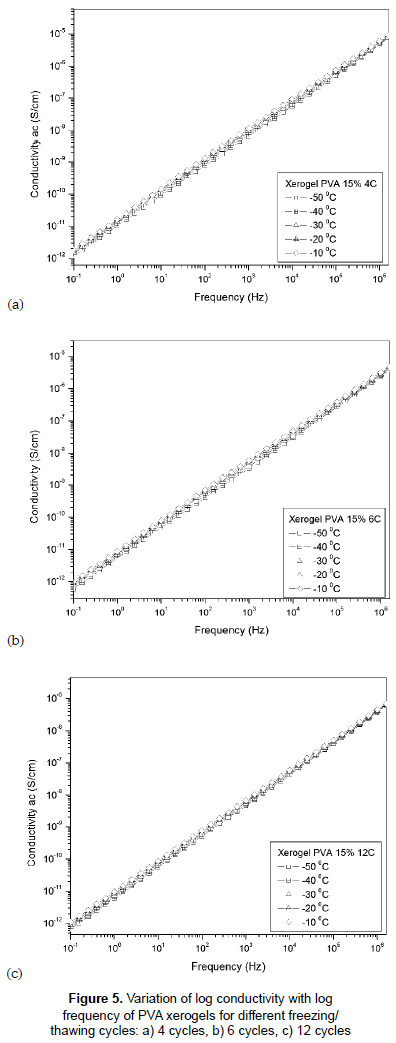
The plots of A.C. conductivity vs frequency for hydrogels with different cycles of F/T are shown in figures 5a, 5b and 5c. The conductivity increases linearly with the frequency at temperatures below zero (-50 to -10 °C). This linear variation is described by σαc = αωn; where α is a constant, ω is the angular frequency and n is a value close to 1. This conduction takes place via localized hopping of carriers between randomly distributed trapping centers (Kulanthaisami, Mangalaraj and Narayandass, 1995). The low values of conductivity may suggest the electrode polarization is not significant in these samples (Pietrucha and Marzec, 2005).
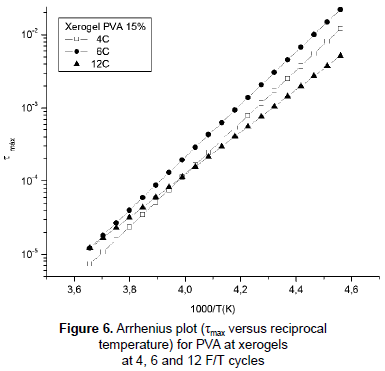
Figure 6 represents the plot τmax(1/2πf) as a function of reciprocal temperature (as calculated experimentally from the maximum of ε'' ) and fitted to a straight line. The relaxation process can be modeled by Arrhenius temperature dependence equation:
τ(T) = τ0eEa/kT (1)
where τmax is the relaxation frequency at which tan δ is maximum, Ea is the energy barrier for dipole relaxation (activation energy) and τ0 is the constant parameter characteristic for a particular relaxation process. The activation energies, taken from slopes on the straight line, for the PVA xerogels at 4, 6, 12 F/T cycles were 68.027, 68.917 and 55.537 kJ/mol respectively. These results suggest that the samples submitted to 4 and 6 cycles had the lowest binding forces which oppose dipolar reorientation (Abd Elkader et al., 2008). The difference in the apparent activation energy between the samples with less F/T cycles and the sample with 12 F/T cycles is around 13 kJ/mol indicating the influence of the crosslinking existence in the hydrogels (Ghilarducci, Salva and Marzocca, 2009) and how the number of F/T cycles affects the activation energy. The lack of dependence of the activation energy on the degree of crosslinking is caused by the counterbalance exerted between the concentration of hydrogen bonds and the density of chemical branch points in local mobility (Shteinberg et al., 1980), but chemical branch points do not exist in PVA hydrogels and therefore no balance exists and this could cause the change in the energy of activation with crosslinking. The increases in the value of activation energy may be related with the increases of crosslinking degree which depends on the number of F/T cycles; high degree of crosslinking severely reduces molecular mobility (Kaiser, 1989) due to increases of the mass density (Casalini and Roland, 2010). Likewise, relaxation times become larger for the sample with 12 cycles, 3,0x10-16 s, shorter for 6 and 4 cycles, 8,0x10-19 s and 7,0x10-19 s respectively. The increase in the relaxation time for the sample with 12 cycles is evidence of the effect of greater crowding caused by the increase of the mass density due to the entanglement and crosslinking in the sample (De La Rosa, Heux and Cavaillé, 2001). However, this effect is less visible between samples with 4 and 6 F/T cycles.
4. CONCLUSIONS
Study of dielectric behavior of PVA hydrogels obtained by F/T technique with different crosslinking degrees showed that dielectric constant and dielectric loss diminish due to the increase of crosslinking degree. Only one relaxation was observed in all samples, the β dielectric relaxation which increases with the increase of temperature and corresponds to some local movement of OH groups. In the samples studied, the values of tan δ below 1 suggest that the dielectric phenomena are predominant, whereas the conduction takes place via localized hopping of carriers. Energy of activation and relaxation time have a complex dependence on number of F/T cycles due to different structures obtained by this process. The greater crowding caused by the increase of the mass density due to the entanglement and crosslinking in the polymer disturb the dielectric properties of the hydrogels. The PVA hydrogels show a complex dielectric behavior, unlike other polymers, and more effort will be necessary to understand the influence of the structure in the dielectric behavior.
REFERENCES
Abd El-kader, F. H.; Osman, W. H; Mahmoud, K. H. and Basha, M. A. F. (2008). "Dielectric investigations and ac conductivity of polyvinyl alcohol films doped with europium and terbium chloride". Physica B: Condensed Matter, vol. 403, No. 19-20 (October), pp. 3473-3484. [ Links ]
Awadhia, A.; Patel, S. K. and Agrawal, S. L. (2006). "Dielectric investigations in PVA based gel electrolytes". Progress in Crystal Growth and Characterization of Materials, vol. 52, No. 1-2 (March- June), pp. 61-68. [ Links ]
Blyte, T. and Bloor, D. Electrical properties of polymers. Cambridge, UK: Cambridge University Press, 2005. 480 p. [ Links ]
Casalini, R. and Roland, C. M. (2010). "Effect of crosslinking on the secondary relaxation in polyvinylethylene". Journal of Polymer Science. Part B: Polymer Physics, vol. 48, No. 5, pp. 582-587. [ Links ]
Cascone, M. G.; Lazzeri, L.; Sparvoli, E.; Scatena, M.; Serino, L. P. and Danti, S. (2004) "Morphological evaluation of bioartificial hydrogels as potential tissue engineering scaffolds". Journal of Materials Science: Materials in Medicine, vol. 15, No 12 (December), pp. 1309-1313. [ Links ]
Chandar B. V. S.; Veeravazhuthi, V.; Sakthivel, S.; Mangalaraj, D. and Narayandass, S. K. (1999). "Growth, structure, dielectric and AC conduction properties of solution grown PVA films". Thin Solid Film, vol. 348, No. 1-2 (July), pp. 122-129. [ Links ]
De La Rosa, A.; Heux, L. and Cavaillé, J. Y. (2001). "Secondary relaxations in poly(allyl alcohol), PAA, and poly(vinyl alcohol), PVA. II. Dielectric relaxations compared with dielectric behaviour of amorphous dried and hydrated cellulose and dextran". Polymer, vol. 42, No. 12 (June), pp. 5371-5379. [ Links ]
Dutta, P.; Biswas, S. and Kumar, S. (2002). "Dielectric relaxation in polyaniline-polyvinyl alcohol composites". Materials Research Bulletin, vol. 37, No. 1 (January), pp. 193-200. [ Links ]
Fergg, F. and J. Keil, F. (2001). "Diffusion and reactions of multicomponent electrolytes in poly(vinyl alcohol) hydrogels: Modeling and experiment". Chemical Engineering Science, vol. 56, No. 4 (February), pp. 1305-1315. [ Links ]
Ghilarducci, A.; Salva, H. and Marzocca, A. J. (2009). "About the activation energies of the main and secondary relaxations in cured styrene butadiene rubber". Journal of Applied Polymer Science, vol. 113, No. 4 (August), pp. 2361-2367. [ Links ]
Hassan, C. M. and Peppas, N. A. (2000). "Structure and applications of poly(\(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods". Advances in Polymer Science, vol. 153, pp. 37-65. [ Links ]
Hatakeyema, T.; Uno, J.; Yamada, C.; Kishi, A. and Hatakeyama, H. (2005). "Gel-sol transition of poly(vinyl alcohol) hydrogels formed by freezing and thawing". Thermochimica Acta, vol. 431, No. 1-2 (June), pp. 144-148. [ Links ]
Hickey, A. S. and Peppas, N. A. (1995). "Mesh size and diffusive characteristics of semicrystalline poly(vinyl alcohol) membranes prepared by freezing/thawing techniques". Journal of Membrane Science, vol. 107, No. 3 (November), 229-237. [ Links ]
Kaiser, T. (1989). "Highly crosslinked polymers". Progress in Polymer Science, vol. 14, No. 3, pp. 373-450. [ Links ]
Kao, K. C. Dielectric phenomena in solids. San Diego, CA: Elsevier Academic Press, 2004. 112 p. [ Links ]
Konsta, A. A.; Daoukaki, D.; Pissis, P. and Vartzeli, K. (1999). "Hydration and conductivity studies of polymer-water interactions in polyacrylamide hydrogels". Solid State Ionics, vol. 125, No. 1-4 (October), pp. 235-241. [ Links ]
Kulanthaisami, S.; Mangalaraj, D. and Narayandass, S. K. (1995). "Conduction studies on polyvinyl alcohol films". European Polymer Journal, vol. 31, No. 10 (October), pp. 969-975. [ Links ]
Lozinsky, V. I. (1998). "Cryotropic gelation of poly(vinyl alcohol) solutions". Russian Chemical Reviews, vol. 67, No. 7, pp. 573-586. [ Links ]
Lozinsky, V. I.; Damshkaln, L. G.; Kurochkin, I. N. and Kurochkin, I. I. (2008) "Study of cryostructuring of polymer systems: 28. Physicochemical properties and morphology of poly(vinyl alcohol) cryogels formed by multiple freezing-thawing". Colloid Journal, vol. 70, No. 2, pp. 189-198. [ Links ]
McCrum, N. G.; Read, B. E. and Williams G. (1991). Anelastic and dielectric effects in polymeric solids. New York, NY: Dover, 332 p. [ Links ]
McGann, M. J.; Higginbotham, C. L.; Geever, L. M. and Nugent, M. J. D. (2009). "The synthesis of novel pHsensitive poly(vinyl alcohol) composite hydrogels using a freeze/thaw process for biomedical applications". International Journal of Pharmaceutics, vol. 372, No. 1-2 (May), pp. 154-161. [ Links ]
Mishra, R. and Rao, K. J. (1998). Electrical conductivity studies of poly(ethyleneoxide)-poly(vinyl alcohol) blends. Solid State Ionics, vol. 106, No. 1-2 (February), pp. 113-127. [ Links ]
Nakano, Y.; Bin, Y.; Bando, M.; Nakashima, T.; Okuno, T.; Kurosu, H. and Matsuo, M. (2007). "Structure and mechanical properties of chitosan/poly(vinyl alcohol) blend films". Macromolecular Symposia, vol. 258, No. 1 (November), pp. 63-81. [ Links ]
Ni, N. and Zhao, K. (2007). "Dielectric analysis of chitosan gel beads suspensions: Influence of low crosslinking agent concentration on the dielectric behavior". Journal of Colloid and Interface Science, vol. 312, No. 2 (August), pp. 256-264. [ Links ]
Pietrucha, K. and Marzec, E. (2005). "Dielectric properties of the collagen-glycosaminoglycans scaffolds in the temperature range of thermal decomposition". Biophysical Chemistry, vol. 118, No. 1 (October), pp. 51-56. [ Links ]
Pissis, P. and Kyritsis, A. (1997). "Electrical conductivity studies in hydrogels". Solid State Ionics, vol. 97, No. 1-4 (May), pp. 105-113. [ Links ]
Ricciardi, R.; Gaillet, C.; Ducouret, G.; Lafuma, F. and Laupretre, F. (2003). "Investigation of the relationship between the chain organization and rheological properties of atactic poly(vinyl alcohol) hydrogels". Polymer, vol. 44, No. 11 (May), pp. 3375-3380. [ Links ]
Ricciardi, R.; Auriemma, F. and De Rosa C. (2005). "Structure and properties of poly(vinyl alcohol) hydrogels obtained by freeze/thaw techniques". Macromolecular Symposia, vol. 222, No. 1 (March), pp. 49-64. [ Links ]
Schartel, B.; Wendling, J. and Wendorff, J. H. (1996). "Cellulose/poly(vinyl alcohol) blends. 1. Influence of miscibility and water content on relaxations". Macromolecules, vol. 29, No. 5 (February), pp. 1521-1527. [ Links ]
Sengwa, R. J. and Sankhla, S. (2007). "Dielectric dispersion study of coexisting phases of aqueous polymeric solution: Poly(vinyl alcohol) + poly(vinyl pyrrolidone) two-phase systems". Polymer, vol. 48, No. 9 (April), pp. 2737-2744. [ Links ]
Shafee, E. E. and Naguib, H. F. (2003). "Water sorption in cross-linked poly(vinyl alcohol) networks". Polymer, vol. 44, No. 5 (March), pp. 1647-1653. [ Links ]
Shteinberg, V. G.; Ol'khov, Yu. A.; Melent'ev, A. G. and Baturin, S. M. (1980). "The effect of crosslinking density of dielectric relaxation in polyurethane elastomers". Polymer Science U.S.S.R., vol. 22, No. 2, pp. 269-274. [ Links ]
Singh, K. P. and Gupta, P. N. (1998) "Study of dielectric relaxation in polymer electrolytes". European Polymer Journal, vol. 34, No. 7 (July), pp. 1023-1029. [ Links ]
Valentín, J. L.; López, D.; Hernández, R.; Mijangos, C. and Saalwächter K. (2009). "Structure of poly(vinyl alcohol) cryo-hydrogels as studied by proton low-field NMR spectroscopy". Macromolecules, vol. 42, No. 1 (January), pp. 263-272. [ Links ]
Willcox, P. J.; Howie Jr., D. W; Schmidt-Rohr, K.; Hoagland, D. A.; Gido, S. P.; Pudjijanto, S.; Kleiner, L.W. and Venkatraman, S. (1999). "Microstructure of poly(vinyl alcohol) hydrogels produced by freeze/thaw cycling". Journal of Polymer Science. Part B: Polymer Physics, vol. 37, No. 24 (December), pp. 3438-3454. [ Links ]