Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Lasallista de Investigación
Print version ISSN 1794-4449
Rev. Lasallista Investig. vol.10 no.1 Caldas Jan./June 2013
Artículo original / Original article / Artigo original
Epidemiological study of bovine leukemia virus in dairy cows in six herds in the municipality of Pasto, Nariño*
Estudio epidemiológico del virus de leucemia bovina en vacas de seis hatos del municipio de Pasto, Nariño
Estudo epidemiológico do vírus de leucemia bovina em vacas de seis curais do município de Pasto, Nariño
Bibiana Benavides Benavides**, Darío Alejandro Cedeño Quevedo***, María Fernanda Serrano De La Cruz****
*This article was derivative of research Project: "Prevalence of bovine leukemia virus (BLV), and different forms of disease presentations, in dairy herds in the municipality of Pasto, Nariño". Realized in the University of Nariño between January and December 2011; financed for the Research and International Relations Vicerrectoria (VIPRI) at the Universidad de Nariño.
**MV, MSc. Docente Grupo de Investigación Buiatria Programa Medicina Veterinaria, Facultad de Ciencias Pecuarias, Universidad de Nariño, Colombia
***DMV, MSc. Docente Grupo de Investigación Buiatria Programa Medicina Veterinaria, Facultad de Ciencias Pecuarias, Universidad de Nariño, Colombia.
****Estudiante Grupo de Investigación Buiatria, Programa Medicina Veterinaria, Universidad de Nariño.
Mail: Bibiana Benavides Benavides, e-mail: bibibenavides@gmail.com
Article received: 09/12/2011; Article approved: 10/05/2013
Abstract
Introduction. Enzootic bovine leukosis is a highly infectious disease caused by a deltaretrovirus of the retroviridae family, which affects bovines of all ages and that generates a high economic impact on the dairy herds. This is caused by the high costs of symptomatic treatments, premature deaths and replacement of ill animals, the reduction of the milk production and the restrictions of importation and exportation imposed by some countries. Objective. To determine the prevalence of bovine leukemia virus (BLV) in its two different forms of disease presentations (persistent lymphocytosis and lymphosarcoma) and the factors associated with the seropositivity of the virus in dairy herds from Pasto, (Nariño, Colombia). Materials and methods. The study included six specialized dairy herds from Pasto, Colombia. A total of 242 blood samples were taken from 24 months of age or older cows and were analyzed using the indirect ELISA test to determine the seropositivity. The management practices were evaluated in each herd and an analysis binary logistic regression was used to find associations with seropositivity. Results. A seroprevalence of 19.8% was determined. Out of 48 positive animals, 13 had a total count over 10000 leukocytes/mm3, and 6 of these (12.5%) developed persistent lymphocytosis (PL) according to their age. No cases of lymphosarcoma or malignant lymphoma were found in the study. Concerning the management practices, the replacement of animals in different herds or in livestock fairs is associated to farms with a higher prevalence. Conclusions. Surveillance programs for dairy herds should include diagnostic tests for BLV. Only a small number of animals show consistent changes with lymphocytic or clinical disease. In addition, early diagnosis allows efficient control programs in the replacement of animals and it also prevents the spread of the virus in dairy herds.
Key words: bovine leukemia, dairy herds, persistent lymphocytosis, lymphosarcoma.
Resumen
Introducción. La leucosis enzoótica bovina es una enfermedad altamente infecciosa causada por un deltaretrovirus de la familia retroviridae, que afecta a bovinos de todas las edades y especialmente genera un gran impacto económico en los hatos lecheros. Esto se debe a los grandes costos de los tratamientos de los síntomas, muertes prematuras y reemplazo de los animales enfermos, disminución de la producción de leche y restricciones a la importación y la exportación por parte de algunos países. Objetivo. Determinar la prevalencia del virus de leucemia bovina (VLB) en dos formas de presentación de la enfermedad (linfocitosis persistente y linfosarcoma) y los factores asociados con seropositividad del virus en hatos de Pasto, Nariño, Colombia. Materiales y métodos. El estudio incluyó a seis hatos lecheros especializados de Pasto, Nariño, Colombia. Un total de 242 muestras de sangre se tomaron de vacas con 24 meses de edad o mayores y se analizaron con la prueba ELISA indirecta, para determinar la seropositividad. Las prácticas de manejo fueron evaluadas en cada hato y un análisis de regresión logística binaria se practicó para encontrar asociaciones con la seropositividad. Resultados. Se determinó una seroprevalencia de 19.8%. De 48 animales positivos, 14 tuvieron un conteo total mayor a 10000 leucocitos /mm3 y 6 de ellos (12.5%) desarrollaron linfocitosis persistente (LP) según la edad. No se encontraron casos de linfosarcoma ni de linfoma maligno en el estudio. Entre las prácticas de manejo, el reemplazo de animales en distintos hatos o ferias ganaderas se asocia con las fincas de más prevalencia. Conclusiones. Los programas de vigilancia para hatos lecheros deberían incluir exámenes de diagnóstico para VLB. Solamente un pequeño número de animales muestra cambios consistentes con enfermedad linfocítica o clínica. Además, el diagnóstico precoz permite programas de control eficiente en el reemplazo de animales, además de prevenir la propagación del virus en los hatos lecheros.
Palabras clave: leucemia bovina, hatos lecheros, linfocitosis persistente, linfosarcoma.
Resumo
Introdução. A leucosis enzoótica bovina é uma doença altamente infecciosa causada por um delta-retrovirus da família retroviridae, que afeta a bovinos de todas as idades e especialmente gera um grande impacto econômico nos curais leiteiros. Isto se deve aos grandes custos dos tratamentos dos sintomas, mortes prematuras e substituição dos animais enfermos, diminuição da produção de leite e restrições à importação e a exportação por parte de alguns países. Objetivo. Determinar a prevalência do vírus de leucemia bovina (VLB) em duas formas de apresentação da doença (linfocitosis persistente e linfosarcoma) e os fatores associados com soro positivo do vírus em curais de Pasto, Nariño, Colômbia. Materiais e métodos. O estudo incluiu a seis curais leiteiros especializados de Pasto, Nariño, Colômbia. Um total de 242 mostras de sangue se tomaram de vacas com 24 meses de idade ou maiores e se analisaram com a prova ELISA indirecta, para determinar a seropositividade. As práticas de manejo foram avaliadas em cada cural e uma análise de regressão logística binária se praticou para encontrar associações com a seropositividade. Resultados. Determinou-se uma seroprevalencia de 19.8%. De 48 animais positives, 14 tiveram uma contagem total maior a 10000 leucocitos /mm3 e 6 deles (12.5%) desenvolveram linfocitosis persistente (LP) segundo a idade. Não se encontraram casos de linfo sarcoma nem de linfoma maligno no estudo. Entre as práticas de manejo, a substituição de animais em diferentes curais ou feiras de gado se associa com as herdades a mais prevalência. Conclusões. Os programas de vigilância para curais leiteiros deveriam incluir exames de diagnóstico para VLB. Somente um pequeno número de animais mostra mudanças consistentes com doença linfocítica ou clínica. Ademais, o diagnóstico precoce permite programas de controle eficiente na substituição de animais, além de prevenir a propagação do vírus nos curais leiteiros.
Palavras importantes: leucemia bovina, curais leiteiros, linfocitosis persistente, linfo sarcoma.
Introduction
Enzootic Bovine Leukosis (EBL) is a neoplastic disease of the reticuloendothelial system caused by bovine leukemia virus (BLV)1. All breeds are susceptible, although the incidence is higher in dairy cows. It rarely occurs in animals which are younger than 2 years old and the incidence increases with age2, 3.
In Colombia, the EBL was identified for the first time in 1957 from clinical and necropsy cases4. The prevalence of the disease is variable- in dairy cattle there are reports of prevalence of 24.9% in the Andean region, 14.4% in the Caribbean region and 15.3% in the Orinoquia region5. Other studies show a higher prevalence in central and northern Colombia. The prevalence was 32% and 40% respectively6 and in Antioquia the prevalence was 37.5% in heifers and 79.1% in adult cows7.
The main form of iatrogenic transmission is attributed to management practices such as injections, vaccinations, dehorning8, castration, rectal palpation9 and tattoos, carried out in minimum hygiene, which have been demonstrated experimentally10 Vertical transmission has been reported transplacentally or through the ingestion of colostrum and milk11-13. It has also been described the role played by blood-sucking insects such as Tabanus sp14.
The BLV is integrated into the DNA of lymphocytes and can produce a polyclonal B cell expansion which is usually manifested as an increase of lymphocytes in blood persistent lymphocytosis (PL)15 and monoclonal expansion accumulating lymphoid tissue of cattle (lymphosarcoma or lymphoma)16.
Most animals remain asymptomatic throughout their life. However, about 30% of cattle infected with BLV develop PL between their 3rd and 6th year of age17. Lymphosarcoma or lymphoma occur in less than 5% of infected animals and it is present in animals 5 years old or older; which may be preceded by PL but it is not necessary for its appearance18, 19. Clinical signs may include enlarged lymph nodes, weight loss, decreased milk production, and other clinical signs related to the location of the tumor20, 21.
BLV proviral load is correlated with the titer of antibodies against envelope antigens, specifically against the viral proteins gp51 and p2422. BLV infection stimulates a strong humoral immune response against these proteins which form the basis for the detection of serological tests23. The most common diagnostic tests are the Agar Gel Immunodiffusion (AGID) and Absorbent Immunosorbent Assay (ELISA) which have been developed in order to detect antibodies against gp5124. In a study in Chile, the ELISA test had a similar ability to detect positive animals to the PCR with a sensitivity of 97%25. That is why this method was used in the present study.
There is evidence that the virus can infect in vitro cells from different animal species, as sheeps. Hence it is important to monitor the BLV diagnostic, which must include constant surveillance of the genome in order to detect genetic variations and emergence of new strains acquiring the ability to affect humans26.
In most countries nowadays, the EBL is a notifiable disease27. In Colombia there are not established health policies to prevent, control and eradicate BL. Additionally to date it is not considered a reportable disease to any entity.
The objective of this study was to determine the prevalence of BLV; its forms of manifestation in dairy herds in the municipality of Pasto-Colombia and to establish the association of population management variables in regards to the seropositivity to BLV.
Materials and methods
A cross sectional study was performed in six specialized dairy farms in the municipality of Pasto, Nariño, ecosystems above 2500 m to 3200 m where dairy herds, randomly selected, with the following inclusion criteria: farms with information system or veterinary assistance who keeps records of population practices.
The sample size was determined by a simple random sampling strategy, based on cow population over 24 months of age (Census of 2010 FMD vaccination) with the purpose of correlating their age with the appearance forms of the disease.
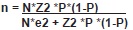
Where:
- N: number of dairy cows in the region (14145 cows)
P: prevalence expected of EBL (30%)
e: accepted error (in this study 10%)
Z: confdence level (α= 0.05)
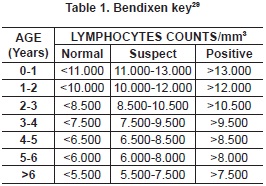
A number of 242 blood samples were obtained by venipuncture of the coccygeal vein whit vacutainer and red tubes without anticoagulant for obtaining serum. Samples were processed at the Clinical Laboratory of the Universidad de Nariño, where white blood counts (WBC) was held. Those samples with counts greater than 10000 leukocytes/mm3 were performed blood smears to obtain the differential count. Animals which had lymphocytosis were resampled 6 months after the first sample to determine whether they developed PL or not (table 1). This count was corrected according to the animal age by Bendixen key which confirms that the increase is due to the BLV cells and not due to their age. Under normal conditions the number of lymphocytes decreases with age of the animal28. In the same laboratory, serum samples were processed to detect the presence of antibodies against BLV by indirect Enzyme Linked Immunosorbent Assay -ELISA (Commercial Kit SV ANOVA BLV GP51). BLV apparent prevalence was calculated as the proportion of positive reactors animals (48 cows) to ELISA over the total number of animals sampled (242) for the study. Positive animals were subject to a clinical examination to establish the development of neoplasias and corroborate histopathologically with lymphosarcoma.
An epidemiological survey was conducted on each campus of the study, aimed to find variables associated with seroprevalence of BLV. These variables were: 1. Source of replacement animals; 2. Biosecurity practices in the population management (changing palpation sleeves, disinfection of surgical and obstetric instruments, needles and syringes exchange among animals), 3. Management during calving and reuse of medical equipment of individual treatments (needles, syringes).
Data was recorded on an Excel spreadsheet. Each variable was identified as "0" when the response was negative and as "1" when it was positive. Polytomous variables were transformed into dummy variables to be examined dichotomously. These variables were analyzed descriptively and the association with the presence or absence of antibodies to BLV was determined by logistic regression in the statistical program STATA 9®
This project was approved in the record 001 of October 13, 2010, by the Ethics Committee On Animal Research of the Universidad de Nariño, and it was classified as minimal risk.
Results
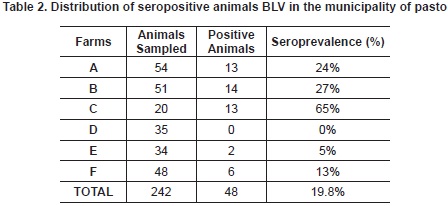
Out of 242 animals sampled, 48 animals were found seropositive, giving a seroprevalence of 19.8% for the VLB in the municipality of Pasto. The distribution of positive animals in sampled herds was as follows in table 2:
Out of 48 seropositive animals, 13 (27.08%) had counts over 10000 leukocytes/mm3 and 6 (12.5%) developed persistent lymphocytosis according to their age. Only one animal infected to present an ocular neoplasia, which -at the histopathology study - was diagnosed as squamous cell carcinoma.
A descriptive analysis was performed of the most important variables associated with seropositivity transmission and BLV (table 3).
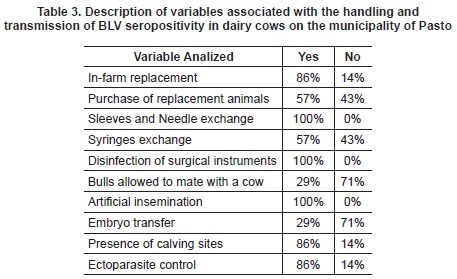
When performing logistic regression, co linearity was found in most variables. Therefore, the variables could not be associated with seropositivity to BLV. Nonetheless, the replacement variable was found to have an increased risk of infection when the replacement is from other farms. Infarm replacement has an inverse association in accordance with the information of table 4.
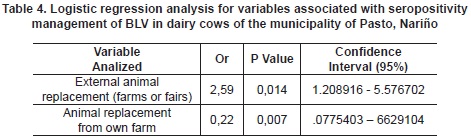
Discussion
The seroprevalence found in this study (19.8%) is lower than expected in dairy farms because it assumes a greater risk due to management practices and the time animals live in farms30. Nonetheless, the result is similar to the ones found in the following specific studies: animals with reproductive disorders where the prevalence was 21% (Monteria, Cordoba)31, and dairy herd in high altitude tropical areas with seroprevalence of 15.4%32. In this region, geographical conditions and management are similar to the one in the study area. It is evident that the variety of results among farms found in the study (table 1) ranges from 0% -farm D- to 65% -farm C-, confirming that there is a variety of farm prevalence with a low number of positive reactors33.
It is important to clarify that only some animals develop all stages of the disease and that the order of their appearance does not suggest a sequence of them34. For this reason, the infection is not always visible; therefore, it must be implemented a diagnostic routine test in order to identify the virus at the farm.
The PL condition is an important factor because transmission is more effective when the infecting cattle have it35. These cattle have approximately 25-35% of infected cells with pro-virus, whereas animals which do not develop PL have less than 5% of infected lymphocytes22. The number of animals that developed PL is low6. This coincides with those reported by Van et al who found that only one third of infected animals develop this condition36.
In technified production systems such as those included in this study, it has been demonstrated a relationship among the genetic potential for milk production (genotype BoLA-A), the resistance, the susceptibility to BLV, the development of PL, and proviral load. The latter suggests that the resistance to PL is associated with longevity in the herd when the infection is prevalent37. This was observed in farm A, which has high production cows with high longevity --14 years of age-- in some animals in production. In this herd, there were no cases of PL, alterations in milk production, or its quality. This is consistent with studies in Canada where it was found that BLV infection did not affect the quantity and quality of milk during the 305 days of lactation38, 39.
In this study only one animal presented an ocular neoplasia compatible with one of the anatomical locations of lymphosarcoma in Holstein cows40. However, this was diagnosed as squamous cell carcinoma. There were no other cases with neoplasias because less than 5% of infected cattle with BLV develop lymphosarcoma or malignant lymphoma41. Additionally, the difficulty and the limitation of their diagnosis it is the result of animals dying without evident clinical signs. These dead animals are sent for human consumption and they are never subject to necropsy in order to establish tumor development e.g. lymphosarcoma. These tumors are often found in the rumen, heart, spleen, intestine, liver, kidney, lung and uterus42.
In the variables analyzed in the survey, horizontal transmission was considered because of infected lymphocytes present in biological fluids such as blood, semen and rarely saliva43. This transmission is higher in situations of confinement where the risk increases by the presence of tissues and fluids especially during calving44.This risk decreases when using birth sites or paddocks, which were found in 86% of the farms under study.
Other variables in the iatrogenic transmission of BLV are management practices with minimum hygiene conditions which have been experimentally demonstrated45. In the analyzed farms, the criteria of biosecurity were met- change of needles/ gloves and disinfection of surgical instruments (table 2). In terms of the role of blood-sucking insects, it was taken into account the implementation of control programs for ectoparasites used in 86% of the properties. The entry of replacement animals from other farms and fairs was the only variable associated with seropositivity. This can be explained because the health status of these animals is not evaluated and required by their owners. The only diagnostic tests are the ones from Official Control Diseases (Foot and mouth disease, brucellosis and bovine tuberculosis). The official control measures for bovine leucosis adopted by countries such as Lithuania in the process of its eradication include the following: detection or virus monitoring, border surveillance, movement of animals control inside the country, and sanitary slaughter46.
The risk that represents this type of management was evident in farm C where, on one hand, the entrance of animals is free and permanent without any sanitary control. This coincides with the highest BLV prevalence found in this study (65%). On the other hand, the lowest infection prevalence (0%) was found in farm D, where replacement animals come from the same farm and bought animals are subject to various diagnostic tests in order to ensure their health. The previous biosecurity measures were recommended by a study conducted in Minneapolis about the considerations that must be taken at the time to establish replacement programs47.
The economic importance of this disease is that BLV infection generates premature replacements, reduced slaughter value, mortality loss, abortion, reproductive alterations, and treatment costs. Additionally, there is an immunodeficiency and increased susceptibility to other diseases48, resulting not only in the decreased exports competitiveness, but also in the increased concerns about consumer safety49.
Conclusions
Control of BLV in infected dairy farms in the municipality of Pasto must be improved by focusing particularly on the risk and protective factors. A small number of animals showed consistent changes with lymphocytic or clinical disease, however among the surveillance programs for dairy herds should include diagnostic tests for BLV. In addition, early diagnosis allows efficient control programs in replacement animals preventing the spread of the virus in the region. Future research is required to compare the influences of each factor responsible for within-herd transmission and to facilitate more rational prioritization of control measures. Health policies for prevention, control, and eradication of the BLV should be set
Acknowledgements
To the students of the Buiatria Research Group; Alejandro Moncayo, Claudia Rodríguez, Carlos Herrera, Guillermo Cárdenas, Andrés Narváez. To the Bacteriologist Nancy Galindes, the Pathologist Laura Laverde T., and the farmers who facilitated the study in their farms.
References
1. VAN REGEN MORTEL, M. H.; et al. Virus Taxonomy. Classification and Nomenclature of Viruses. Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, 2000. p. 378-380. [ Links ]
2. JOHNSON, R. & KANEENE, J. Bovine leukemia virus. Part I. Descriptive epidemiology, clinical manifestations, and diagnostic tests. En: Comp Cont Educ Proc Vet. 1991. Vol. 13, No. 2, p. 315-328. [ Links ]
3. ASSANDRI, M. Leucosis bovina, enfermedad de gran importancia y limitante para la exportación de ganado en pie. En: Rev. Sci. Tech. 2007. Vol. 17, p. 727-732. [ Links ]
4. MARIÑO, O. C. Situación de la investigación en leucosis bovina en Colombia. En: Acovez. 1984. Vol. 8, N° 27, p. 22-26. [ Links ]
5. ORJUELA, J.; NAVARRETE, M. y BETANCOURT, L. Salud y productividad en bovinos de la costa norte de Colombia. [En línea]. Roma: FAO. Recuperado el 09 de junio de 2010]. Url disponible en: http://www.fao.org/ag/aga/agap/fg/feedback/war/u9900bOg/htm. [ Links ]
6. ALFONSO, R.; ALMANSA, J. E. y BARRERA, J. Prevalencia serológica y evaluación de los factores de riesgo de leucosis enzoótica en la Sabana de Bogotá y los Valles de Ubaté y Chiquinquira, Colombia. En: Rev. Sci. Tech. 1998. Vol. 17, p. 727-732. [ Links ]
7. RAMÍREZ, N.; et al. Diagnóstico epidemiológico referente a varias patologías de bovinos en tres haciendas de la Universidad de Antioquia. (Trabajo de investigación). Medellín: Universidad de Antioquia, 2001. p. 22-23. [ Links ]
8. DARLINGTON, R. L.; DIGIACOMO, R. F. & EVERMANN, J. F. Bovine leukemia virus Transmission by dehorning in dairy heifers. En: Bovine Pract. 1985. Vol. 19, p. 144-146. [ Links ]
9. DIVERS, T.; BARTHOLOMEW, R. C. & GALLIGAN, D. Evidence for transmission of bovine leukemia virus by rectal palpation in a commercial dairy herd. En: Prev Vet Med. 1995. Vol. 23, p. 133-141. [ Links ]
10. LUCAS, M. H.; et al. Immunosuppression and the spread of viral infections in districts contaminated in different ways. En: Arch. Com. Environ. Studies (ACES). 1999. Vol. 11, Vol. 3-4, p. 17-28. [ Links ]
11. HOPKINS, S. G. & DIGIACOMO, R. F. Natural transmission of bovine leukemia virus in dairy and beef cattle. En: Vet Clin North Am Food Anim Pract. 1997. Vol. 13, p. 107-128. [ Links ]
12. NAGY, D.; TYLER, W. & KLEIBOEKER, S. Decreased Periparturient Transmission of Bovine Leukosis Virus in Colostrum-Fed Calves. En: J. Vet Intern Med. 2007. Vol. 21, p. 1104-1107. [ Links ]
13. MEAS, S, T. Vertical transmission of bovine leukemia virus and bovine immunodeficiency virus in dairy cattle herds. En: Vet Microbiol. 2002. Vol. 84, p. 275-282. [ Links ]
14. MANET, G. Natural Mode of Horizontal Transmission of Bovine Leukemia Virus (BLV): the Potential Role of Tabanids (Tabanus spp.). En: Vet Immunol Immunopat. 1989. Vol. 22, p. 255-263. [ Links ]
15. FLORINS, A, M. Emphasis on cell turnover in two hosts infected by bovine leukemia virus: A rationale for host susceptibility to disease. En: Vet Immunol Immunopat. 2008. Vol. 125, p. 1-7. [ Links ]
16. BRUNNER, M.; LEIN, D. & DUBOVI, E. Experiences in the New York State bovine leukosis virus eradication and certification program. En: Vet. Clin North Am Food Anim Pract. 1997. Vol. 13, p. 143-150. [ Links ]
17. BEYER, J. Cattle infected with bovine leukaemia virus may not only develop persistent B-cell lymphocytosis but also persistent B-cell lym-phopenia. En: J. Vet Med B Infect Dis Vet Public Health. 2002. Vol. 49, N° 6, p. 270-277. [ Links ]
18. SCHWARTZ, I. & LEVY, D. Pathobiology of bovine leukemia virus. En: Vet Res. 1994. Vol. 25, p. 521-536. [ Links ]
19. FLORINS, A. Emphasis on cell turnover in two hosts infected by bovine leukemia virus: a rationale for host susceptibility to disease. En: Vet Immunol. Immunopathol. 2008. Vol. 125, N° 1-2, p. 1-7. [ Links ]
20. WILLEMS, L. A. Genetic Determinants of Bovine Leukemia Virus Pathogenesis. En: Aids research and human retroviruses. 2000. Vol. 16, p. 1787-1795. [ Links ]
21. CHAMIZO, E. Enzootic Bovine Leukosis. En: Redvet. 2005; Vol. 6. N° 7. [En línea]. [Recuperado el 30 de junio de 2009]. http://www.veterinaria.org/revistas/redvet/n070505.html [ Links ]
22. JULIARENA, M. A.; GUTIERREZ, S. E. & CERIANI, C. Determination of proviral load in bovine leukaemia virus-infected cattle with and without lymphocytosis. En: Am J Vet Res. 2007. Vol. 68, N° 11, p. 1220-1225. [ Links ]
23. MARTÍN, D. et al. Comparación de métodos serólogicos y virológicos para el diagnóstico de la infección por el Virus de Leucosis Bovina Enzoótica (BLV). En: Med Vet. 2000. Vol. 17, p. 133-141. [ Links ]
24. BUZALA, E. & DEREN, W. Comparison of PLA with AGID and ELISA results in serology of bovine leukosis. En: Pol. J. Vet Sci. 2003. p. 9-11. [ Links ]
25. FELMER, R.; et al. Diagnóstico y tipificación del virus de la leucosis bovina mediante una prueba de PCR-RFLP a partir de ADN extraído desde células somáticas de la leche. En: Arch Med Vet. 2006. Vol. 38, N° 3, p. 253-257. [ Links ]
26. OCHOA, A.; URIBE, A. & GUTIÉRREZ, M. Estudio del potencial zoonótico del virus de leucosis bovina y su presencia en casos de cáncer de seno. En: Universitas scientiarum. 2006. Vol. 11, N° 2, p. 31-40. [ Links ]
27. OFFICE INTERNATIONAL DES EPIZOOTIES - OIE. Enzootic bovine leucosis. In: Manual of Standards for Diagnostic Test and Vaccines. Paris, France: OIE, 2006. p. 503. [ Links ]
28. DEBACQ, C.; ASQUITH, B. & REICHERT, M. Reduced cell turnover in bovine leukemia virus-infected, persistently lymphocytotic cattle. En: J Virol. 2003. Vol. 77, p. 13073-13083. [ Links ]
29. BENAVIDES, B. Are All Animals Naturally Infected With Bovine Leukemia Virus Equally Infectious? A Quantitative Field Study; In: XXVI World Buiatrics Congress. Santiago de Chile, noviembre 2010. [ Links ]
30. SORGE, U. K. Short communication: Milk ELISA status for bovine leukosis virus infection is not associated with milk production in dairy cows. En: J. Dairy Sci. 2011. Vol. 94, p. 5062-5064. [ Links ]
31. BETANCUR, C. & RODAS, J. Seroprevalencia del virus de la leucosis viral bovina en animales con trastornos reproductivos de Montería. En: Rev. MVZ Córdoba. 2008. Vol. 13, N° 1, p. 1197-1204. [ Links ]
32. LAVERDE, L.; et al. Seroprevalencia De Leucosis Viral Bovina En El Trópico Alto Colombiano. (Trabajo de Investigación) Medellín: Universidad CES 2010. Memoria del congreso mundial de buiatria 2010. [ Links ]
33. FELMER, R.; et al. Prevalencia y distribución espacial de brucelosis, leucosis bovina, diarrea viral bovina y rinotraqueítis infecciosa bovina a partir del análisis ELISA de estanques prediales en lecherías de la IX Región, Chile. En: Arch Med Vet. 2009. Vol. 41, N° 1, p. 17-26. [ Links ]
34. YAKOBSON, B. J. Cellular immune response cytokine expression during the initial stage of bovine leukemia virus (BLV) infection determines the disease progression to persistent lymphocytosis. En: Comp Inmun Microbiol Inf Dis 2000. Vol. 23, p. 197-208. [ Links ]
35. BUXTON, B. A. SCHULTZ, R. D. Factors affecting the infectivity of lymphocytes from cattle with bovine leukosis virus. En: Can J Comp Med. 1984. Vol. 48, p. 365-369. [ Links ]
36. VAN EIJK, M. J. Development of persistent lymphocytosis in cattle is closely associated with DRB2. En: Immunogenetics. 1992. Vol. 37, N° 1, p. 64-68. [ Links ]
37. JULIARENA, M. A. Antibody response against three widespread bovine viruses is not impaired in Holstein cattle carrying bovine leukocyte antigen DRB3.2 alleles associated with bovine leukemia virus resistance. En: J. Dairy Sci. 2008. Vol. 92, p. 375-381. [ Links ]
38. TIWARI, A. Production Effects of Pathogens Causing Bovine Leukosis, Bovine Viral Diarrhea, Paratuberculosis, and Neosporosis. En: J. Dairy Sci 2007. Vol. 90, p. 659-669. [ Links ]
39. SORGE, U. K.; et al. Short communication: Milk ELISA status for bovine leukosis virus infection is not associated with milk production in dairy cows. En: J. Dairy Sci. 2011. Vol. 94, p. 5062-5064. [ Links ]
40. MALATESTINIC, A. Bilateral exphotalmus in a Holstein cow with lymphosarcoma. En: Can Vet J. 2003. Vol. 44, N° 8, p. 664-666. [ Links ]
41. MONTI, G. & GRAU, A. Prevalencia serológica predial e intrapredial para el virus de la leucosis bovina (VLB) en lecherías de las regiones de Los Ríos y de Los Lagos de Chile. En: Arch Med Vet. 2010. Vol. 42, p. 87-91. [ Links ]
42. Willems, L.; et al. Bovine leukemia virus as a model for human T-cell leukemia virus. En: Curr Tropics Virol. 1999. Vol. 1, p. 139-167. [ Links ]
43. MONTI, G. E.; FRANKENA, K. & DE JONG, M. C. Transmission of bovine leukaemia virus within dairy herds by simulation modelling. En: Epidemiol Infect. 2007. Vol. 135, p. 722-732. [ Links ]
44. BEYER, J.; et al. Cattle infected with bovine leukemia virus may not only develop persistent B cell lymphocytosis, but also persistent B-cell lymphopenia. En: J. Vet. Med. 2002. Vol. 49, N° 6, p. 270-277. [ Links ]
45. LUCAS, M. H.; et al. Immunosuppression and the spread of viral infections in districts contaminated in different ways. En: Arch. Com. Environ. Studies (ACES). 1999. Vol. 11, N° 3-4, p. 17-28. [ Links ]
46. ACAITC, J.; TOMOSIONAS, V. & LUKAUSKAS, K. The erradication experience on enzootic bovine leukosis from Lithuania. En: Prev Vet Med 2007. Vol. 82, N° 1-2, p. 83-89. [ Links ]
47. FAUST, M. A.; KINSEL, M. L. & KIRKPATRICK, M. A. Characterizing Biosecurity, Health, and Culling During Dairy Herd Expansions. En: J Dairy Sci. 2001. Vol. 84, p. 955-965. [ Links ]
48. CHI, J.; et al. Management factors related to seroprevalences to bovine viral-diarrhea bovine-leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy herds in the Canadian Maritimes. En: Prev. Vet Med. 2002. Vol. 55, p. 57-68. [ Links ]
49. OTT, S. L.; JOHNSON, R. & WELLS, S. J. Association between bovine-leukosis virus sero-prevalence and herd-level productivity on US dairy farms. En: Prev Vet Med. 2003. Vol. 61, p. 249-262. [ Links ]













