Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Lasallista de Investigación
Print version ISSN 1794-4449
Rev. Lasallista Investig. vol.11 no.2 Caldas July/Dec. 2014
Artículo original / Original article / Artigo original
Validation of an analytical method for the determination of mercury in shrimp and fish*
Validación de un método por espectroscopia de absorción atómica para la determinación de mercurio total en camarón y pescado
Validação de um método por espectroscopia de absorção atômica para a determinação de mercúrio total em camarão e peixe
Daniel Esteban León Perez**, Marta Lucia Hern ández Ángel***, María Alejandra Salazar Gomez****, Claudio Jiménez Cartagena*****
* This article derives as a result of the project "Validation and standardization of quick analytical tests, sensitive and reliable for determining the presence of mercury and methylmercury in shrimp and fish" approved by Colciencias, code No 127554431945.
** Food Engineer, Researcher of the Environmental Applied Research Group-GAMA, Faculty of Engineering, Corporación Universitaria Lasallista, Antioquia. Colombia.*** Chemical engineer. Specialist in environmental engineering. Master in environmental engineering. Instructor at National Learning Service-SENA, Renewable Natural Resources Center La Salada, Caldas, Antioquia. Colombia.
**** National Learning Service Contractor-SENA, Renewable Natural Resources Center La Salada, Caldas, Antioquia. Colombia.
***** Pharmaceutical Chemist, Master in Basic Biomedical Sciences, Doctor of Environmental Engineering, Head of the Environmental Applied Research Group-GAMA, Faculty of Engineering, Corporación Universitaria Lasallista. Caldas, Antioquia, Colombia.
Correspondencia: Claudio Jiménez Cartagena, email: clajimenez@lasallistadocentes.edu.co.
Artículo recibido: 30/11/2013; Artículo aprobado: 31/10/2014
Abstract
Introduction. In this article, the validation of the methodology of analysis of mercury is shown with appropriate characteristics of performance for the analysis of samples of shrimp and fish. Objective. To develop an analytical methodology for the validation of complete matrix “in -house” for the analysis of Hg in food samples. Materials and methods. The procedure was developed through the digestion with microwaves and the quantification via spectrography of atomic absorption with cold vapor and a system of flow injection (AAS-FIAS). Results. The methodology was optimal for the extraction, for the treatment of samples and the determination. The recoveries were evaluated in enriched samples at two levels (7,0 y 15,00 µg/L Hg) and with two certified reference materials (SRM 2976 y BCR-463) higher than 90% with a relative standard deviation (RSD) lower than 1.5 %. The method showed linearity between 1.00 y 20.00 µg/L, con r2 > 0,995 and residuals lower than 20%. The limit of detection and quantification was 0.331 y 0.992 µg/L respectively, which are inferior to those reported for the analysis of metals in food. Conclusion. The method is reliable for routine essays and is applicable for the evaluation of the food exposition in accordance with the quality parameters of the Resolution 122 of 2012 from the Ministry of Health and Social Protection of Colombia.
Key words: mercury, analysis, standards, Validation Studies, instrumentation.
Resumen
Introducción. En este artículo, la validación de la metodología de análisis de mercurio se muestra con características apropiadas de rendimiento para el análisis de muestras de camarones y peces. Objetivo. Desarrollar una metodología analítica para la validación de matriz completa “in -house” para el análisis de Hg en muestras de alimentos. Materiales y métodos. El procedimiento fue desarrollado a través de la digestión con microondas y la cuantificación mediante espectrografía de absorción atómica con vapor frío y un sistema de inyección en flujo (AAS-FIAS). Resultados. La metodología fue óptima para la extracción, para el tratamiento de muestras y la determinación. Las recuperaciones fueron evaluadas de muestras enriquecidas en dos niveles (7,0 y 15,00 µg/L Hg) y con dos materiales de referencia certificados (SRM 2976 y BCR-463); dichas recuperaciones siempre fueron mayores del 90 % con una desviación estándar relativa (RSD) menor que 8.15%. El método tubo linealidad entre 1.00 y 20.00 µg/L, con r2 > 0,9911 y residuales menores al 20%. El límite de detección y de cuantificación fue 0.331 y 0.992 µg/L respectivamente, los cuales son inferiores a los reportados para el análisis de metales en alimentos. Conclusión. El método es aplicable para la evaluación de la exposición alimentaria y cumple con los parámetros de calidad establecidos por la Resolución 122 de 2012 del Ministerio de Salud y la Protección Social de Colombia.
Palabras clave: mercurio, análisis, normativa, estudios de validación, instrumentación.
Resumo
Introdução. Neste artigo, a validação da metodologia de análise de mercúrio se mostra com características apropriadas de rendimento para a análise de mostras de camarões e peixes. Objetivo. Desenvolver uma metodologia analítica para a validação de matriz completa "in -house" para a análise de Hg em mostras de alimentos. Materiais e métodos. O procedimento foi desenvolvido através da digestão com microondas e a quantificação mediante espectrografia de absorção atômica com vapor frio e um sistema de injeção em fluxo (AAS-FIAS). Resultados. A metodologia foi ótima para a extração, para o tratamento de mostras e a determinação. As recuperações foram avaliadas de mostras enriquecidas em dois níveis (7,0 e 15,00 µg/L Hg) e com dois materiais de referência certificados (SRM 2976 e BCR-463); ditas recuperações sempre foram maiores de 90 % com um desvio regular relativa (RSD) menor que 8.15%. O método tubo lineal entre 1.00 e 20.00 µg/L, com r2 > 0,9911 e residuais menores a 20%. O limite de detecção e de quantificação foi 0.331 e 0.992 µg/L respectivamente, os quais são inferiores aos reportados para a análise de metais em alimentos. Conclusão. O método é aplicável para a avaliação da exposição alimentaria e cumpre com os parâmetros de qualidade estabelecidos pela Resolução 122 de 2012 do Ministério de Saúde e a Proteção Social da Colômbia.
Palavras importantes: mercúrio, análise, regulamento, estudos de validação, instrumentação.
Introduction
The anthropogenic emissions of mercury (Hg) become the main way of pollution in the environmental and food matrices (Leermakers, Baeyens, Quevauviller, & Horvat, 2005). The World Health Organization (WHO) has established a daily tolerable intake for methylmercury (MeHg) of 0.48 μg/kg of body weight and in several countries have set limits of total mercury (HgT) in species of fish of 1.0 mg/kg and in other fishing products 0.5 mg/kg. In Colombia, the Ministry of Health and Social Protection and the Ministry of Environment, Housing and Territorial Development established the maximum acceptable value for the Hg of 0.001 mg/L in the water intended for human consumption (Social, 2012).
The Hg at room temperature is found as vapor and its atomization is even more simple (Baklanov & Chmilenko, 2001). Its determination is founded on the reduction of the inorganic forms to elemental Hg by using tin chloride (SnCl2) or sodium tetrahydroborate (NaBH4) as shown in the reaction (Rı, amp, x, o-Segade, & Bendicho, 1999), for its further determination. This method is known as cold vapor (CV) is the most used since there are no loss for the introduction of the sample, which can occur when using other methods (Hight & Cheng, 2005). After the generation of the CV, the detection takes place for the atomic absorption by using a hollow cathode lamp of Hg and analyzing a wavelength of 253.7 nm. The limit of detection that can be reached is <1 ng/mL (Shah, Kazi, Baig, Afridi, & Arain, 2012).
The treatment of the sample has incorporated digestion for microwaves (MW), which use electromagnetic radiation to desorb the analyte and digest the organic matter. The difference between this kind of warming and the conventional systems is that the digestion is produced in the bosom of the dissolution and not for convection from an external source, producing an homogeneous and effective warming (Reyes, Mizanur Rahman, Fahrenholz & Skip Kingston, 2008). The advantage of the digestion with MW oven versus the digestion in a conventional open glass is the possibility of controlling the parameters of pressure and temperature, or the possibility of digest the sample in reduced times; hence the speed of warming is very high. The analysis of Hg allows to obtain information of concentration, bioavailability and toxicity in samples of products originated from fishing boats and environmental samples, among others.
This work presents a simple methodology for the determination of Hg in fish and shrimp by using atomic absorption spectrometry with hydride generation and flow injection system, combined with digestion with MW. There was a significant reduction in the volume of the oxidant agent used in relation to the traditional method. An easy, quick and effective validation was guaranteed, which assured the parameters of quality and the elimination of interferences; besides, the trueness as part of the accuracy of the method was established through two certified reference materials, the SRM 2976 from: National Institute of Standards and Technology-NIST and the BCR-463 from the Institute for Reference Materials and Measurements-IRMM.
Materials and methods
Instrumentation
An Atomic Absorption Spectrophotometer AAnalyst TM 200 was used with a Flow Injection System FIAS 100 by Perkin Elmer, in which the time of permanence of the Hg vapor in the cell, the length of baseline, the temperature of operation, the pulsations, the speed of pumping and the flow of carrier gas were optimized (PerkinElmer, 2008). In addition, a reactant purifier was used for the elimination of Hg traces, and for the treatment of the samples, a Microwave ETHOS ONE digester was used, under conditions pre-established by the manufacturer (Milestone, 2009). Both the distillation system and the digester were provided by MILESTONE, Italy. A Black and Decker food processor.
Reactants
Nitric acid (HNO3) Suprapur® was used for the preparation of the calibration curve and quality Emsure® for the digestion of the samples. hydrochloric acid (HCl) at 37% EMSURE® and borohydride (NaBH4), sodium hydroxide (NaOH) EMSURE®, hydrogen peroxide (H202) at 30% Suprapur®, standard solution of Hg traceable at SRM of NIST in HNO3, quality Certipur®; all the reactants used were provided by Merck Colombia. The certified reference materials SRM 2976 -Mussel Tissue (trace elements & methylmercury) freeze-dried National Institute of Standards and Technology (NIST, Gaithersburg, EE.UU) and BCR-463 tuna fish Institute for Reference Materials and Measurements European Commission (IRMM, Geel, Belgium).
Samples
Samples of rainbow trout (Oncorhynchus mykiss) and tiger shrimp (Penaeus monodon ) obtained in supermarkets in the city of Medellin -Colombia; they were packed in ziploc® plastic bags and stored at -30 °C until they were processed. In the initial treatment of the samples, the non -edible parts were removed preserving only the tissue and skin, these parts were reduced in particle-size in a food processor until an homogeneous paste, which was homogenized and stored in conic tubes of polypropylene of 50 ml at -4° C until its analysis.
Treatment of samples
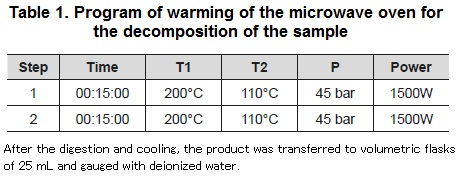
The digestion of the samples was carried in the microwave ETHOS ONE digester, provided of a rotor with hermetic closing Teflon vials; the power of operation was 1500 W and it was applied to all the procedures. The procedure of digestion was made in accordance with the application notes SK 16 HPR-FO-17 for freezedried fish (used for the reference material) and HPR-FO-07 for fresh fish suggested by the manufacturer (Milestone, 2009). An amount of 0.1 and 0.4 g (fresh fish and shrimp, samples of fortified fish and shrimp at 30 and 70% of the values of the calibration curve and for the reference materials) was taken; also, 2 ml of H202 at 30% with 8 ml of HNO3 at 65% was added. The program of microwaves is shown in table 1.
Solutions
The standard solutions for the calibration curve were prepared from a standard solution of 1000 mg/L of Hg, following the recommendations of the 3111 method of the Standard Methods Committee (American Public Health Association, 2005), at concentrations of 1.0, 5.0, 10.0, 15.0y 20.0 µg/L. All the solutions were stabilized in free HNO3 of Hg 5M.
The reducing solutions were prepared for each analysis with a fresh solution of NaBH4 at 1% of then consecutive dilutions were made at known concentrations until it was possible to obtain a response of the instrument. Additionally, 10 withe samples were analyzed and the signals were measured. The limit of detection calculated was the minimum concentration at which the analyte was detected with a signal-to-noise ratio of 3:1. The limit of quantification (LOQ) was calculated in the point where the signal-to-noise ratio was lower than 10 and the relative standard deviation calculated was lower than 10%, it was also possible by using a factor between 2 < f < 10, this concentration obtained is a theoretical result, hence it was evaluated by performing 5 trials at the theoretical concentration obtained, in order to demonstrate that this result was the minimum quantifiable amount, precise and accurate that the method sets up.
Results and discussion
The procedure of the treatment of the sample was successfully applied, as much for the certified reference material as for the samples of fresh shrimp and fish. Any interference due to the residual organic matrix was verified during the process demonstrating the not affectation for the matrix effect.
Any interference due to the residual organic matrix was verified during the process demonstrating the not affectation for the matrix effect. The digestion of this sample was made by using the same process of the fish and shrimp samples. For the certified samples SRM 2976, the average value in the concentration of the samples (n =7 for each one), a content of 58.2 µg/ kg of Hg was obtained according to the nominal value of 61.0 ± 3.6 µg/kg and for the BCR-463, a content of 2.71 μg/g of Hg was obtained with the nominal value of 2.85 ± 0.16 μg/g; the calculated veracity and repeatability were 95.40 % y 95,09%, besides the RSD = 13.8 and 11 respectively. The results obtained with the reference material verify the quality of the method and the correction of errors, in comparison to Mao Tseng et al 1997(Mao Tseng, De Diego, Martin, Amouroux, & F. X. Donard, 1997) developed a method for digestion in microwaves, but they used a methanolic potassium hydroxi de solution, obtaining recoveries of 102%, for our method, this solution is not considered since the analysis of Hg requires this compound to be in a solution to form the nitric salts for its stabilization and realize a basic digestion requires extra procedures which can introduce errors in the measurement.
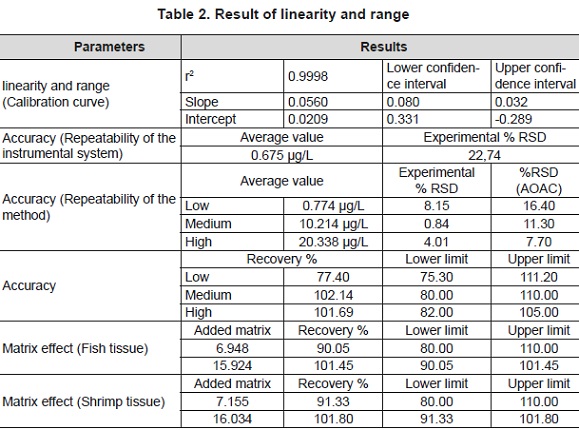
It gathers the results of the validation of the method, it is established that the calibration curves showed good linearity (> 0,99), high sensibility and selectivity, being these the main advantages of the presented technique in this study. A good repeatability was reached, being confirmed by the low standard deviations (<11%). This analysis also certified that there was no loss of Hg during the digestion of the sample by the system of microwaves in the adopted conditions. The proof of the hypothesis for the regression, the method showed that there is a relation between the variables, in the intervals of confidence for the slope it was concluded that the method suitably responds to changes of concentration; besides, for the intervals of confidence for the intercept, the method exhibits acceptable proportionality in the interval tried. The criteria of acceptance for the repeatability of the method, accomplished in the % RSD of the determinations for the levels 1, 3 and 5, are lower than the theoretical percentage, accomplishing the repeatability of the method for each level. The method accomplishes with the criteria of acceptance for the accuracy, according to the average of percentages of recovering en the three levels. The proof of the hypothesis of the method shows that is exact in the range of concentration tried; besides, recoveries within the specified limits are achieved in each level, which presented an alpha of 0.05, a t calculated of -0.11 and a t tabulated of 2.16. LOD and LOQ estimated were 0.331 and 0.992 µg/L respectively, accomplishing a %RSD=8.15. Leal et al 2006 (Leal, Elsholz, Forteza, & Cerdà, 2006) reported a limit of detection of 5 ng/L, a lower concentration than the one reported by our method, although this is analytically considered at the level ultra-trace as a good methodology for the elemental analysis, the level of sensibility of our method is considered more adequate because it accomplishes with the instrumental requirements for the analysis of Hg in fish and shrimp samples, being able to determine concentrations under the limits suggested by the legislation (table 2).
Conclusions
The methodology suggested for the determination of Hg in fish and shrimp by microwave digestion has lots of advantages. The use of nitric acid in the mixture with hydrogen peroxide is more favorable than the use of traditional digestion with the mixture with nitric acid, sulfuric acid and potassium permanganate solution. Regarding the quantity of analysis sample, even though the process of digestion implies a reduced quantity of sample, the reduction of losses of samples, the low consumption of acids (which can add analyte to the sample), a volume of capacity small and the optimization of variables for the analysis allow us to reach the conditions of toughness suggested in the objective of the study. The method proposed for the determination of Hg showed to be simple, efficient and easy to be made in the analyzed matrices and its validation process was developed in an optimal way, the use of analysis carried out allowed us to evaluate diverse parameters at the time, securing the quality of the protocol of analysis and the procedure of validation established within the quality system suggested in the laboratory.
Acknowledgments
The authors thank the Department of Science, Technology and Innovation -COLCIENCIAS, for funding this project and especially to the National Learning Service -SENA-La Salada, for providing the plant of laboratory for the development and obtainment of the results of the project.
Bibliography
American Public Health Association -AWWA. Water Pollution Control Federation. (2005). Metal by Flame Atomic Absorption. Standard Methods for the Examination of Water and Wastewater Method 3111. New York: American Public Health Association, American Water Works Association. [ Links ]
Baklanov, A., & Chmilenko, F. (2001). Use of Ultrasound in Sample Preparation for the Determination of Mercury Species by Cold-Vapor Atomic Absorption Spectrometry. Journal of Analytical Chemistry, 56(7), 641-646. doi: http://dx.doi.org/10.1023/a:1016792205748. [ Links ]
Hight, S., & Cheng, J. (2005). Determination of total mercury in seafood by cold vapor-atomic absorption spectroscopy (CVAAS) after microwave decomposition. Food Chemistry, 91(3), 557-570. doi: http://dx.doi.org/10.1016/j.foodchem.2004.10.004. [ Links ]
Horwitz, W., & Chemists, A. (2000). Official methods of analysis of the AOAC: Association of Official Analytical Chemists. [ Links ]
Instituto de Normas Técnicas y Certificación -ICONTEC. (2003). Norma Tecnica Colombiana NTC-ISO-IEC 17025: requisitos generales de competencia de laboratorios de ensayo y calibracion. Bogotá: ICONTEC. [ Links ]
Leal, L., Elsholz, O., Forteza, R., & Cerdà, V. (2006). Determination of mercury by multisyringe flow injection system with cold-vapor atomic absorption spectrometry. Analytica Chimica Acta, 573-574(0), 399-405. doi: http://dx.doi.org/10.1016/j.aca.2006.04.078. [ Links ]
Leermakers, M., Baeyens, W., Quevauviller, P., & Horvat, M. (2005). Mercury in environmental samples: Speciation, artifacts and validation. TrAC Trends in Analytical Chemistry, 24(5), 383-393. doi: http://dx.doi.org/10.1016/j.trac.2004.01.001. [ Links ]
Mao Tseng, C., De Diego, A., Martin, F., Amouroux, D., & Donard, O. (1997). Rapid Determination of Inorganic Mercury and Methylmercury in Biological Reference Materials by Hydride Generation, Cryofocusing, Atomic Absorption Spectrometry After Open Focused Microwave-assisted Alkaline Digestion. [10.1039/ A700956I]. Journal of Analytical Atomic Spectrometry, 12(7), 743-750. doi: http://dx.doi.org/10.1039/a700956i. [ Links ]
Milestone. (2009). Milestone SK-10 and SK-12 Rotors user manual. Sorisole, Italy: Milestone. [ Links ]
Ortega, L., Garcia, J., & Fontanety, M. (2001). Validacion de métodos analíticos. Barcelona, España: Asociación Española de Farmacéuticos de la Industria. [ Links ]
Reyes, L., Mizanur Rahman, G., Fahrenholz, T., & Skip Kingston, H. (2008). Comparison of methods with respect to efficiencies, recoveries, and quantitation of mercury species interconversions in food demonstrated using tuna fish. Analytical and Bioanalytical Chemistry, 390(8), 2123-2132. doi: http://dx.doi.org/10.1007/s00216-0081966-3. [ Links ]
Rı, amp, x, o-Segade, S., & Bendicho, C. (1999). Selective Reduction Method for Separate Determination of Inorganic and Total Mercury in Mussel Tissue by Flow-Injection Cold Vapor Technique. Ecotoxicology and Environmental Safety, 42(3), 245-252. doi: http://dx.doi.org/10.1006/eesa.1998.1753. [ Links ]
Shah, A.; Kazi, T.; Baig, J.; Afridi, H. & Arain M.B. (2012). Simultaneously determination of methyl and inorganic mercury in fish species by cold vapour generation atomic absorption spectrometry. Food Chemistry, 134(4), 2345-2349. doi: http://dx.doi.org/10.1016/j.foodchem.2012.03.109. [ Links ]