Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Avances en Psicología Latinoamericana
versão impressa ISSN 1794-4724versão On-line ISSN 2145-4515
Av. Psicol. Latinoam. v.26 n.1 Bogotá jan./jun. 2008
Neural networks of human nature and nurture
Daniel S. Levine*
* University of Texas at Arlington, U. S. A. Please correspond with: Daniel S. Levine Department of Psychology University of Texas at Arlington. 501 S. Nedderman Drive, Arlington, TX 76019-0528, USA. E-mail: levine@uta.edu. http://www.uta.edu/psychology/faculty/levine.
Fecha de recepción: septiembre de 2007
Fecha de aceptación: marzo de 2008
Abstract
Neural network methods have facilitated the unification of several unfortunate splits in psychology, including nature versus nurture. We review the contributions of this methodology and then discuss tentative network theories of caring behavior, of uncaring behavior, and of how the frontal lobes are involved in the choices between them. The implications of our theory are optimistic about the prospects of society to encourage the human potential for caring.
Key words: neural networks, nature, nurture, caring behavior, frontal lobe.
Resumen
Los métodos de redes neuronales han facilitado la unificación de varias desafortunadas divisiones en psicología, incluyendo la de naturaleza versus crianza. Revisamos las contribuciones de esta metodología para luego examinar las propuestas teóricas basadas en redes acerca de la conducta de cuidado, la conducta de descuido y cómo los lóbulos frontales están involucrados en las elecciones entre éstas. Las implicaciones de nuestra teoría son optimistas acerca de los prospectos de la sociedad para fortalecer el potencial humano para el cuidado.
Palabras clave: redes neuronales, naturaleza, crianza, conducta de cuidado, lóbulo frontal.
The interdependent web
Nature versus nurture? This is one of the many false splits and dichotomies that have retarded research in psychology at different times in its history. Tryon (1993, 1995) summed up some of these dichotomies as follows:
(1) Mind vs. Body; (2) Biological (Nature) vs. Environmental (Nurture); (3) Language Learning (Innate vs. Learned); (4) Subjective vs. Objective; (5) Learning vs. Development; (6) Holistic vs. Atomistic; (7) Individual (Ideographic) vs. General (Nomothetic); (8) Experimental vs. Naturalistic; (9) Awareness vs. Conditioning; (10) Freedom vs. Determinism; (11) Human (Cognitive) vs. Animal (Associative) Learning (Tryon, 1995, p. 303).
Why should we care about these academic distinctions? They are of more than academic interest: they have influenced the popular culture by promoting a fragmented view of human beings. Ultimately, such fragmenting of our consciousness tends to encourage the development of toxic us versus them relationships between people (see also Papini, 2008).
But since about the mid-1980s, there has been a surge of interdisciplinary research findings that promise to heal some of these psychological splits. The development of modern brain imaging techniques has spurred the growth of cognitive neuroscience and greater productive collaboration between experimental psychologists and neuroscientists. The development of modern computational neural network (or connectionist) techniques has fostered a greater understanding of dynamical interconnections among elements and subsystems of the mind. The new disciplines that have emerged—cognitive neuroscience and neural network theory—have the potential to influence popular culture in ways that promote better mutual understanding.
In particular, biologically relevant and plausible neural networks have helped to heal Tryons dichotomy (2), namely, nature versus nurture. The naive conventional belief is that a theoretical, quantitative approach to understanding human personality would favor genetic over environmental causes. But the naive belief is wrong because the brains function is to mediate between the internal world of the body and the external world of the environment. And in order to mediate successfully, the brain is able to change as a result of experience. As the neural network pioneer Stephen Grossberg put it, brains self-organize on a relatively fast time scale through development and life-long learning, and do so in response to non-stationary, or rapidly changing, statistical properties of their environments (Grossberg, 2000, p. 244).
Neural networks, because they are built on the mathematics of dynamical systems, treat genetic and environmental influences as interacting parts of a seamless whole, or as some religions call it, an interdependent web. Genetics provides behavioral tendencies, whereas environments influence the expression of these tendencies. The enhancement or inhibition of any genetic capacity is a function of the interaction of inherited temperament and personality with experiences. These experiences affect temperament and personality, and thus behavior, by altering brain chemistry and structure (see Eisler & Levine, 2002).
What neural networks are and are not
The Chunnel across the English Channel between Britain and France was built from both ends, with laser technology used to make them meet in the middle. By analogy, if one wants to understand the biological basis of human behavior, one needs to go back and forth between the biology of the brain and behavioral functions, and try to get them to meet in the middle.
If the biology of the brain is analogous to Britain, and the psychology of behavior, emotion, and cognition to France, then the growing interdisciplinary field of neural networks, along with cognitive neuroscience, is one of the tunnels between them (for a history and overview, see Levine, 2000). Neural networks are mathematical and computer models that are composed of simulated brain regions, or in some cases psychological constructs, and connections between those regions or constructs. At the same time, the networks are designed with the goal of achieving with computer simulations some results that can be interpreted as analogous to some set of behavioral data.
So how is a neural network defined? The best definition so far, though a very imperfect one, is probably the one developed in 1988 by a team of neural network experts from a study commissioned by the United States Department of Defense (DARPA):
a neural network is a system composed of many … processing elements operating in parallel whose function is determined by network structure, connection strengths, and the processing performed at computing elements or nodes. ... Neural network architectures are inspired by the architecture of biological nervous systems, which use many simple processing elements operating in parallel...
This definition is not completely satisfactory because not all mathematically studied neural networks can be parceled cleanly into nodes and connections: some are studied more as continuous, even chaotic, processes (see, e.g., Freeman, 2003).
What do the nodes or elements in the DARPA definition mean? Typically, though not always, scientists conceptualize the nodes as large groups of neurons (brain cells). Experiments from neurophysiology laboratories have suggested that the electrical patterns of single neurons and the biochemistry of the synapses between neurons are irregular in their organization. But if some irregularities at the levels of single neurons and synapses are averaged out across large groups or brain regions, regular connection patterns emerge that are important for behavior.
Sometimes nodes sometimes correspond to brain areas or specific cell types in those brain areas. At other times, when not enough is known about brain processes or when modeling at a functional level is desired, nodes correspond to cognitive entities such as the memory of a specific word, the tendency to approach a specific object, or the intensity of a specific emotion.
Biologists and clinicians frequently talk informally of the neural networks involved in this or that function, meaning networks in the actual brain: that usage is accurate enough. But what is inaccurate and misleading is the tendency of some researchers to restrict the term neural network to a particular type of network structure that has become popular. These structures are multilayer (usually three-layer) feedforward networks that learn prescribed responses from a set of training data (Rumelhart & McClelland, 1986). Such networks are often called back propagation networks because learning takes place by propagation of changes in connection weights back from the output nodes of the network to other nodes representing stored input pattern categories. Back propagation networks are widely used in both industrial applications (e.g., White & Sofge, 1992) and neuropsychological models (e.g., OReilly, Noelle, Braver, & Cohen, 2002). Yet this class of networks are not the type that best captures either the structure of the brain (which has extensive feedback connections almost everywhere) or the important subfunctions of mental processes (see Levine, 2000, for review).
In fact, some more biologically realistic, and functionally rich, neural network architectures have been inspired by organizational principles that tailor anatomical structure to the required psychological functions. Grossberg (2000) and Levine (2000) extensively discuss how such principles have been used to build models of complex cognitive functions from models of simpler functions. Some networks based on such principled models are able to self-organize their responses instead of requiring explicit training.
As neural networks have evolved, the resulting models have covered an expanded range of psychological data (see Levine, 2002b). In the 1970s, network modeling was most advanced in the area of visual perception, and next most advanced in serial learning and short-term memory. The early and middle 1980s saw the growth of models of animal learning and conditioning data. The late 1980s and early 1990s, buoyed by the interdisciplinary cognitive science revolution, saw early models of high level cognition, including language acquisition, and its breakdown in various mental disorders. All these areas are still active, and now a few models have appeared in social psychology. By now, while there is little agreement on the right model for any of these phenomena, the available network tools available and empirical knowledge are sophisticated enough that practically any area of psychology, whether cognitive, behavioral, physiological, social, developmental, or clinical, is amenable to neural network modeling.
Variability of human behavior
By now there is considerable neurophysiological evidence that strengths of many synapses between pairs of neurons change when the neurons on both sides of the synapse are repeatedly electrically active at the same time (Bliss & Lømo, 1973; Byrne, 1987; Kandel & Tauc, 1965). Psychologists interested in learning suggested the idea of synaptic change long before neurophysiologists observed it (Freud, 1895; Hebb, 1949).
The brains plasticity is the key to the marvelous adaptability of human beings. As we have built more layers onto the neural structures inherited from other mammals, the amount of plasticity has grown radically. The parts of the brain most responsible for our linguistic and intellectual and cultural development have the most plastic connections of all.
During the critical period of childhood, the plasticity of neural circuits is greater than at other times and is supplemented by the ability to lay down brand new synapses. But the circuits involving the frontal lobes, the part of our brains most implicated in planning and moral development, remain fairly plastic through adolescence (Webster, Weickert, Herman, and Kleinman, 2002). And even the adult brain is subject to strengthening or weakening of existing synapses, which may underlie significant character development through experience.
Our capacity at birth to be raised to learn English, Italian, Chinese, or Hindi, for example, is a function of the plasticity of connections representing specific sound patterns playing on the hard-wired structures involved in what has been called a language instinct (Pinker, 1994). The brains role in the development of cultural mores and customs is less well understood. Yet cultural mores are likely to be a similar blend of plasticity of specific connections with hard-wired general functional capabilities. That is, we have the universal instinct to create cultures and to make rules governing interpersonal relations within these cultures, but the content of these rules is extremely flexible. Our brains can, for example, create rules that either encourage hierarchies of dominance held together by force, or caring partnership relations held together by the rewards of pleasure (Eisler, 1995).
What do neural networks tell us about all this? Neural networks are a metaphor for the fact that all of mental life is dynamically interrelated. Perceptions, categorizations, beliefs, emotions, plans, and actions cannot be fully separated from each other, but instead form an interdependent web. Results from experimental psychology show, for example, that cognitive ambiguity can lead to emotional discomfort (Elkin & Leippe, 1986; Harmon-Jones et al., 1996), and that emotional biases can influence how categories are chosen (DeHouwer et al., 2002; Pashler & Medin, 2002). Both learning and individual differences strongly influence the results of these interrelationships in model neural networks, as they do in actual people.
In addition, some specific neural network architectures can function as useful metaphors for specific human attitude tendencies. One neural network that my colleagues and I developed serves as a metaphor for the human tendency to get stuck in habitual yet unrewarding behaviors (Levine & Prueitt, 1989). Another network serves as a metaphor for jumping between polar emotional opposites (such as love and hate) (Grossberg, 1972; Grossberg & Schmajuk, 1987). But there is also a neural network metaphor for the creative process that encourages self-actualization (Levine, 1994)!
What can a neural network, or computational, approach add to our understanding of human psychology over and above what can be gained by just thinking intelligently about mental processes? Neural networks do not change our view of the brain and behavior dramatically. They merely help us tackle problems of human behavior using a systems approach. This means that each of our personalities, like any other complex system, is seen as a web of different subsystems, all influencing each other dynamically but each somewhat autonomous. These systems are simulated and studied through the mathematical theory of dynamical systems (also sometimes known as chaos theory). This is the study of how interacting variables in a complex system influence each other over time, and applies to a wide range of other types of physical and social systems (Abraham, Abraham, & Shaw, 1992).
Moreover, the process of building theories and computer models helps focus our understanding on the detailed requirements for an organisms intelligent functioning in a complex, changing, and not fully predictable world. In particular, neural network modeling inspired Grossbergs (2000) notion of the c omplementary brain. He coined this term to mean that intelligent functioning often depends on jointly satisfying two sets of requirements that are complementary, and somewhat paradoxical. For example, in learning and memory, we need to be open to learning new information without forgetting some important information that we learned previously. In conditioning, we need to process cognitive information and also to be sensitive to motivational feedback. And in visual perception, we need to define boundaries and also to define surfaces between the boundaries.
Neural networks and the nature-nurture inquiry
The complementary brain theme can be extended still further to personality and to social interactions. For example, the interdisciplinary social scientist Gerald Cory described the function of individuals within society as a constant effort to mediate between the complementary claims of self-interest and of concern for others (Cory, 1999, 2002). He rooted both self-interest and altruism in different evolutionary programs based in different brain pathways carried over respectively from reptiles and from nonhuman mammals, pathways the pioneering behavioral neuroscientist Paul MacLean had mapped earlier (MacLean, 1990). Cory repeatedly used system-theoretic terms like algorithms to describe these processes. One of the current goals of my research group is to develop computational neural network models of the large number of interacting brain processes involved in the tug-of-war Cory described between altruism and selfishness (see Levine, 2006).
How close are we to applying neural networks to a real understanding of human nature and nurture? Naturally, neural network models of behavioral functions have evolved from the outside in. Perceptual and motor processes have been the easiest to understand, and more complex cognitive functions and cognitive-emotional interactions have taken longer to understand. And the interaction between nature and nurture takes us into the most complex of all this territory, the interrelationship between our brains and society.
Yet neural network modeling has come much closer to this goal since the mid-1990s. A few neural network models of social psychological phenomena are reviewed in the collection edited by Read and Miller (1998). These models cover person perception and impression formation; stereotyping and social categorization; causal attribution; personality and behavior; attitudes and beliefs, including cognitive dissonance; and social influence and group interaction. Other models in this same vein include Brown, Tumeo, Larey, and Paulus (1998); Brown and Paulus (2002); Leven and Levine (1996); Read and Miller (2002); and Van Rooy et al. (2003).
So our neural network theories are still far from capturing the essence of human nature and nurture. But progress has been rapid in the 1990s and early 2000s, in empirical understanding of brain-behavior relationships as well as in network modeling itself. Neuroscience has made increasing contact with social psychology, as epitomized by the emergence of a distinct field called social neuroscience (see, e.g., Cacioppo et al., 2002; Wood, 2003). To bridge the gap from neural networks to nature and nurture, we must first revisit some neuropsychological data about caring and uncaring behavior.
Caring and uncaring behavior
No set of questions is more basic or more prone to speculation than questions about our ethical and moral makeup. How much of a mixture are humans of selfishness and altruism? Is our concern for others welfare simply disguised selfishness — or is it a genuine part of a biologically derived need for community with others? If the latter, why are we so uncaring and violent much of the time, both as individuals and societies? These questions have long posed a challenge for behavioral biologists and psychologists steeped in the theory of natural selection.
Some degree of caring is essential for mammal, and particularly human, babies to survive—so caring for offspring clearly has an evolutionary function. But this does not explain the great variance of parental behaviors. Nor does it explain the emergence of altruism toward those who are not blood relatives. Darwin himself doubted that survival of the fittest could account for or altruism, because those who are more self-sacrificing would not have a survival or reproductive advantage over those who are more selfish (Darwin, 1871/1981, p. 163).
This insight has led evolutionary scholars to develop a variety of extensions of the natural selection paradigm. Some come from broader interpretations of Darwins own work; for example, David Loye highlighted Darwin's emphasis in The Descent of Man and other writings on love and cooperation rather than pure self-interest as a factor in mammalian, especially primate, evolution (Loye, 1999, 2002). He cited Darwins tracing of love and cooperation as offshoots of the sexual and parental instincts.
Probably the most widely known theory to explain cooperative behaviors (including empathy and caring) has been group selection. As Bateson (2000, p. 19) put it: ... some assemblages of individuals may, through their concerted efforts, generate an outcome that puts their group at an advantage over other groups.
Empathy and caring within a group facilitate cooperative activities and so provide a clear survival advantage for that group. Yet group selection still does not explain why caring, respectful parenting, and altruism are sometimes present and sometimes absent.
Saying that evolution selects for a trait does not tell us what the biological or neuropsychological mechanisms are for expressing that trait. Nor does it shed light on what environmental contexts enhance or suppress that trait. Such contextual knowledge is required to draw conclusions about how the trait affects, and is affected by, social and cultural interactions.
In other words, we need to move from questions about genes to questions about gene expression: to the physical and social environment that will lead to the expression or inhibition of the human capacity for caring and altruistic behaviors. Neither nature alone nor nurture alone provides adequate explanations for important features of our personalities or our characteristic action patterns (see the articles in Levine, 2002a).
We now sketch three competing characteristic classes of neural activity patterns that may underlie caring or uncaring behaviors in humans, following the development in Eisler and Levine (2002). Based on the work of several generations of behavioral biologists, we identify these pattern classes as tend-and-befriend, fight-or-flight, and dissociative. Each one of these pattern types is part of every healthy persons genetic makeup. Each serves an adaptive purpose and therefore has been selected for in evolution. Yet the circumstances of each of their expression, and the contexts in which each one is expressed, are heavily dependent on social conditioning and not necessarily adaptive.
We outline some tentative neural pathways that, as they become refined by better understanding, will be subject to neural network investigation. A key brain region for deciding between the competing expressions of different behavior patterns is the underside of our frontal lobes ― as neuroscientists call it, the orbitofrontal cortex (or ventromedial prefrontal cortex). This is the part of the brain that the neuroscientist Walle Nauta identified as our censor which filters out acceptable plans (Nauta, 1971). In his widely known somatic marker theory, Antonio Damasio described that same brain region as essential for effective decision making based on our bodily needs (Damasio, 1994). It is the major communication link between Paul MacLeans three brains: the crocodile, horse, and person from our evolutionary history that we bring to expression of our deepest selves (MacLean, 1962).
First we look at some possible neural pathways for caring behavior. Then we look at some neural pathways for fight-or-flight behavior, noting that noncaring behavior can also take on the form of withdrawal or dissociation rather than fighting. Then we sketch a theory for how the orbitofrontal cortex might mediate the choices between such overall behavior patterns.
Neural pathways for caring: Role of oxytocin and vasopressin
There appears to be in all mammals (it has been studied most extensively in rodents) a system of neurotransmitters and peptide hormones, in which the peptide oxytocin is particularly pivotal, for affect regulation and mediation of social bonding. While this neural system has been most studied in mother-child interactions and in female-male sexual pair bonding, there is some evidence that the same system also operates in other bonding relationships such as non-kin friendships, as well as in relaxation responses and general stress reduction.
The social psychologist Shelley Taylor and her colleagues (Taylor et al., 2000) described what they called the tend-and-befriend response that women and female animals often employ as a response to stress, in preference to the traditionally studied fight-or-flight response. The tend-and-befriend response, mediated by the brains oxytocin system, includes both the tending of offspring and social bonding between females (mutual grooming for nonhuman animals, friendship for humans) around mutual protection of selves and offspring. Other results we will cite hint that these mechanisms exist in male animals as well, despite gender differences in amounts of some biochemical substances involved. For this reason, even though Taylor applied the term tend-and-befriend specifically to grooming between female animals and friendship between women, I use the term for bonding responses in general.
Rewards and positive emotions have long been associated with the neurotransmitter dopamine. Yet whereas dopamine is involved in a wide range of positive emotions, oxytocin is specifically important for positive emotions relating to social and family connections. This hormone, found only in mammals, was first discovered to be essential for maternal behaviors such as uterine contraction and milk ejection. But Thomas Insel, James Winslow, and their colleagues discovered that oxytocin has broader importance for bonding, in male as well as female animals (Insel, 1992; Insel & Winslow, 1998; Winslow, Shapiro, Carter, & Insel, 1993).
Insel and Winslow studied two species of North American rodents that are closely related but have radically different social organization: the prairie vole, which is monogamous with strong male-female pair bonding and both parents involved in care of young, and the montane vole, which is promiscuous with fathers uninvolved with young. They found that oxytocin attaches to receptor molecules in reward-related areas of the brain in the pair-bonding prairie vole but not in the non-bonding montane vole. (Young and Wang, 2004, note that the two species do not differ significantly in total brain oxytocin level, only in where oxytocin binds in the brain.). Also, in female prairie voles, pair bonding—with the first male they smell after reaching puberty—can be induced by direct injections of oxytocin, and abolished by drugs that reduce the amount of oxytocin (Insel, Winslow, Wang, & Young, 1998).
Another peptide hormone, vasopressin (which is closely related chemically to oxytocin), is particularly important for pair bonding in male prairie voles (Young & Wang, 2004). Vasopressin is particularly related to male aggression in defense of the mate and young, and to paternal care (Insel et al., 1998). Yet other results argue against a neat gender dichotomy between these two hormones. Cho, DeVries, Williams, and Carter (1999) showed that while oxytocin is more associated with maternal behavior and vasopressin with paternal behavior, pair bonding could be abolished in either male or female prairie voles by drugs that blocked brain receptors for either of the two peptides. This suggests that both peptides are required for pair bonding in both sexes.
Insels group has bred male mice (which are closely related to voles) that lack a gene for producing oxytocin and found that these animals are selectively deficient in social memory. Unlike normal mice, they cannot remember the smell of another mouse with whom they have engaged in affiliative behavior (e.g., sex, play, or grooming), despite intact memory for other kinds of smells (Ferguson et al., 2000). Vasopressin has also been found to be critical for social memory in mice (for a review, see Bielsky & Young, 2004).
Social recognition and bonding pathways in humans and other primates are less well worked out than in rodents, but the evidence so far points to roles for oxytocin and vasopressin similar to those in voles. For example, Rosenblum et al. (2002) studied two closely related species of macaque monkeys with analogous differences to those between prairie and montane voles: bonnet macaques that tend to be gregarious, affiliative, and affectively stable, and pigtail macaques that tend to be emotionally volatile and socially distant. These researchers found that the more affiliative bonnet macaques had higher levels of oxytocin in their cerebrospinal fluid than did the less affiliative pigtail macaques. The bonnets also had lower levels of a substance called CRF that is important in the production of the stress-related hormone cortisol.
In humans, Kosfeld et al. (2005) found that intranasal administration of oxytocin to men playing a simulated investment game increased their ability to trust their partners. The same hormone has been found to modulate neural circuits for social cognition and for fear in humans (Kirsch et al., 2005). The evidence for a social bonding role of vasopressin in humans is less clear, but Fries, Ziegler, Kurian, Jacoris, and Pollak (2005) found urinary levels of both peptides to be below normal in children reared in orphanages with too few social contacts.
Other results suggest that oxytocin inhibits fight-or-flight responses to stress. By contrast, oxytocin promotes responding to stress by seeking positive social interactions and nonnoxious sensory stimulation, as Taylor et al. (2000) studied in female animals. Uvnäs-Moberg (1997, 1998) reviewed evidence from her laboratory and others that oxytocin administration in both male and female rats counteracts many typical physiological and behavioral effects of stress. For example, oxytocin causes decrease in blood pressure and in the amount of cortisol.
The physiological antistress effects of oxytocin are known to occur in association with both lactation and sexual intercourse. Oxytocin is also likely to be released by other forms of pleasurable social contact, such as mutual grooming in animals and supportive friendship in humans. Indeed Turner, Altemus, Enos, Cooper, and McGuinness (1999) found that oxytocin levels in the blood of women who had never been pregnant increased in response to relaxation massage.
As an approach to a later, more complete, theory, one can assume that both oxytocin and dopamine are involved in brain mechanisms common to a range of caring or bonding responses across different species of mammals. This generates a first approximation to a neural network theory of human bonding responses based on the simpler brains of voles and their involvement in male-female pair bonding (Figure 1). Our starting point is the results of Insel et al. (1998) showing that oxytocin and vasopressin both have different binding patterns in the brain of the pair-bonding prairie vole than in the brain of the non-pair-bonding montane vole. We also build on the results of Cho et al. (1999) on gender differences in prairie voles.
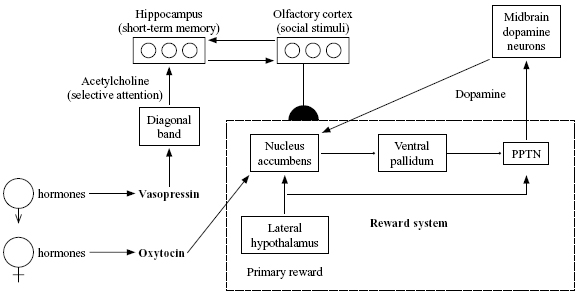
Figure 1. Proposed network for bonding effects of oxytocin and vasopressin. PPTN is the pedunculopontine tegmental nucleus, a part of the midbrain. Ventral pallidum is a part of the basal ganglia. Both of these areas along with the lateral hypothalamus and nucleus accumbens are part of the neural circuit for processing rewards. Arrows represent excitatory connections; fi lled circles represent inhibitory connections; semicircles represent modifi able connections. (Reprinted from Eisler and Levine, 2002, with the permission of Kluwer Academic Publishers.)
Our theory of bonding (Figure 1) is based on the assumption that brain regions to which oxytocin and vasopressin bind more in the prairie vole than in the montane vole are regions that play roles in bonding (tend-and-befriend) behavior. Insel et al. (1998) reviewed data suggesting that the key area for oxytocin binding is the nucleus accumbens, well known to be a key part of the dopamine-modulated stimulus-response system. The key area for vasopressin bonding seems to be an area called the diagonal band that produces the neurotransmitter acetylcholine, which is believed to be involved in selective attention and to modulate memory consolidation (Everitt & Robbins, 1997). These data suggest complementary roles for the two peptides in bonding, with oxytocin more related to the part of the process that drives behavior via reward, and vasopressin more related to the part of the process that focuses attention on relevant stimuli—in this case, on the opposite-sex vole with which the animal is forming a pair bond.
The other parts of the network of Figure 1 (particular regions of the hypothalamus, midbrain, and basal ganglia) are inspired by the previous neural network model by Brown, Bullock, and Grossberg (1999) of how behaviors can become conditioned due to the effects of unexpected rewards. This network illustrates some of the major brain pathways likely to be involved in any type of conditioned response. This includes partner preference, a conditioned association whereby the smell (for prairie voles) or visual appearance (for primates) of a particular fellow member of one's species becomes linked to social and/or sexual rewards.
As our modeling proceeds from voles to humans, the kind of conditioning that occurs will of course be far more complex. It will also be more susceptible to change through new experiences and learning. However, the social factors peculiar to humans probably build on the same underlying mechanisms that operate in nonhuman mammals (see MacLean, 1990).
In addition, humans undergo conditioning not just about whom to bond with, but about how strong is the tendency to bond with anybody, as opposed to engaging in fight-or-flight or dissociative behavior. In humans, much of this conditioning is socially and culturally based (e.g., pressures to bond with some groups of people and not bond with others). To see how this might occur, we first need to review how fight-or-flight and dissociative behaviors are organized.
Neural pathways for fight-or-flight and dissociation
The fight-or-flight response involves various parts of the brain as well as the endocrine glands, the immune system, and the cardiovascular system, which coordinate to produce characteristic biochemical changes in response to unpleasant or potentially threatening environmental events. This interconnected system serves useful functions in evolution: its hyperarousal prepares the body for either fighting the stressful event or withdrawing from it.
Hyperarousal involves an increase in activity of the brain's system for distribution of the neurotransmitter norepinephrine. Norepinephrine is the transmitter most involved with pumping up the brain's connections to the cardiovascular and endocrine systems involved in active responses to stressful situations. Normally, when the stressful events have ceased, the stress-based profile disappears and the body recovers its pre-stress biochemical configuration. When the stresses are too severe or persistent, however, as with children who are physically or sexually abused repeatedly, the recovery cannot take place fast enough to keep up with the new stresses that occur (Perry et al., 1995). The child may survive into adulthood, but damaging changes in the brain remain. Specifically, there is sensitization of the pathways in the nervous system and other bodily organs (including the heart and endocrine glands) responsible for fight-or-flight responses to danger. This means the person becomes more likely to have an arousal response to stimuli milder than the initial traumatic event.
The dissociative response to stress is opposite to hyperarousal in that it involves freezing rather than fighting or fleeing. Dissociation is often accompanied by depression or a tendency toward fantasy or daydreaming. It helps account for the withdrawal of some traumatized individuals into addiction to alcohol or drugs.
The long-term physiological changes in children exhibiting a dissociative pattern have not yet been extensively studied. However, the key neural transmitter for the expression of that pattern is known to be dopamine (Perry et al., 1995), the transmitter mainly involved with reward (both from natural positive reinforcers and addictive drugs). Rather than mobilizing the organism toward a fighting or other coping response, the dissociative response mobilizes the organism to withdraw emotionally from the current aversive situation and try to feel good. In contrast to the tendency of hyperaroused children to show a resting rapid heart rate, dissociated children tend toward hyperactivity of the vagus nerve which slows down the heart.
In the Perry studies, neither hyperarousal nor dissociative responses were uniformly found in all abused children. Each was more likely to occur in children who had a family history of particular types of disorders. This could signal inherited genetic predispositions, or it could signal patterns of emotional and physical response passed on from generation to generation through both conscious and unconscious learning.
Perry and his colleagues point out that the brain is malleable all through life, but much more so in the early years. Neural transmitter changes that influence learning in adult life actually impinge on neuron and nerve pathway growth in the young child. And what happens is that states become traits (Perry et al., 1995).
Fight-or-flight and dissociative responses both involve activity of pathways connecting the hypothalamus with two important endocrine glands, the pituitary and adrenal glands. These pathways, known as the hypothalamic-pituitary-adrenocortical (HPA) axis, are involved in production of the hormone cortisol, typically produced in response to trauma.
Another substance that is typically released during fight-or-flight responses is the neurotransmitter associated with arousal, norepinephrine. An extensive system has been mapped out of interactions in the brain between these two major fight-or-flight substances, cortisol and norepinephrine (Koob, 1999; Nestler, Alreja, & Aghajanian, 1999). In addition to the HPA axis, this stress system, common to all mammals, includes parts of the amygdala, which process the degree of fearfulness associated with stimuli in the environment, and a part of the hypothalamus, the paraventricular nucleus (PVN). This stress system also includes loci in the brainstem that connect to the autonomic nervous system, especially a cell nucleus called the locus coeruleus, which is the source of most of the norepinephrine synapses going to other parts of the brain.
These chronic states of hyperarousal would typically get in the way of positive social interactions, making the individual less receptive, more suspicious, and more prone to uncaring and even violent behaviors. This in turn would mean that caring from others would be discouraged, making the biochemical and neural responses associated with receiving caring less likely in a self-perpetuating cycle of chronic hyperexcitablility.
In dissociative responses, based on work summarized by Perry et al. (1995) and Henry and Wang (1998), some of the same brain areas are likely to be involved as in fight-or-flight responses (Figure 2) but with differences in biochemical activation patterns. For example, dissociated individuals should show low norepinephrine activity, combined with high cortisol (Koopman et al., 2003) and low oxytocin. The decrease in oxytocin means that chronically active dissociation, like fight-or-flight, severely reduces the likelihood of caring behavior.
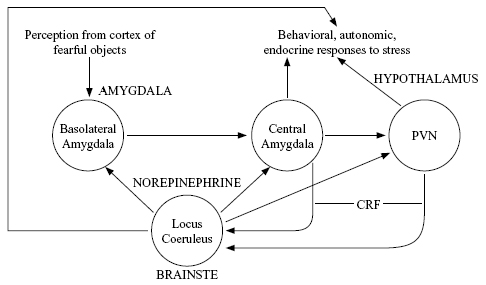
Figure 2 shows a very simplified picture of these interactions. The precursor to cortisol, corticotrophin releasing factor (CRF), is utilized as a neural transmitter in some regions (Koob, 1999). There is pharmacological evidence that cortisol signals reach the norepinephrine-producing locus coeruleus, and that this nucleus in turn sends norepinephrine signals to the amygdala and hypothalamus. All those areas in turn generate behavioral responses to stress (fighting or fleeing) as well as responses of both the HPA axis (endocrine) and the sympathetic autonomic nervous system (which affects the viscera). Thus a positive feedback loop tends to enhance and perpetuate the stress response once it gets going, unless the external environment becomes substantially less stressful. In the case of chronic stress, such as childhood abuse, the system shown in Figure 2 becomes more excitable so that even mildly unpleasant events can generate activity in this positive feedback loop.
Dissociative responses involve dysfunctions of the reward system in which dopamine is the most important neurotransmitter. Dissociation then also typically means that the enduring rewards of positive social interactions are less available. Brain pathways by which this occurs are likely to be analogous to pathways, not shown here, by which the reward system gets taken over by drugs of abuse (see Koob & LeMoal, 2001).
Role of the orbitofrontal cortex
The orbitofrontal cortex has long been recognized as the part of the human brain that uniquely mediates complex emotional responses including social responses. The famous 19th century patient Phineas Gage lost the ability to make plans and respond appropriately to social situations after a railroad accident in which an iron rod went through that part of his brain (see Damasio, 1994, for reconstruction of the case). This region is unique in the extent of its connections both to high-order sensory and association areas elsewhere in the cortex and to emotion-related, viscerally-projecting areas below the cortex (hypothalamus, amygdala, and basal ganglia).
Varied clinical and lesion studies suggest that the orbitomedial prefrontal cortex forms and sustains mental linkages between specific sensory events in the environment and positive or negative emotional states (see Öngür & Price, 2000, and Price, 1999). It is widely believed that, through a process that is still little understood, the prefrontal cortex links neural activity patterns in the sensory cortex that reflect the influence of past sensory events with other neural activity patterns in subcortical regions that reflect innate or learned expressions of emotional states.
It seems likely that the area of the brain mediating the emotional significance a person attaches to objects and classes of objects also mediates the prevalence of large classes of emotionally based responses such as tend-and-befriend, fight-or-flight, and dissociation. This might occur through reciprocal connections of the orbitomedial prefrontal cortex with several subcortical brain areas. One of them is the paraventricular nucleus (PVN) of the hypothalamus, which is important in control of endocrine secretion. Different parts of the PVN contain, among other hormones, oxytocin, vasopressin, and CRF, the precursor to cortisol. The prefrontal cortex does not synapse directly on PVN, but synapses on other parts of the hypothalamus that in turn connect to PVN. In particular there are prefrontal connections to an area called the dorsomedial hypothalamus that sendsinhibitory neurons to the PVN, as shown in Figure 3. These dorsomedial-PVN connections use GABA, the brain's commonest inhibitory transmitter.
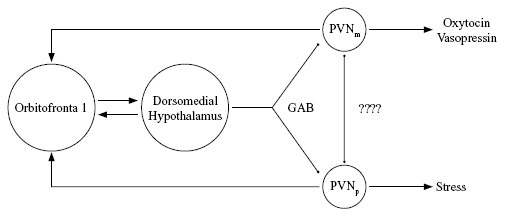
Figure 3. Part of the stress-regulating interactions between the prefrontal cortex and hypothalamus, as discussed by Buijs and Van Eden (2000). Arrows denote excitatory synapses, circles inhibitory ones. PVNp = parvocellular part of paraventricular nucleus, PVNm = magnocellular part. Direct inhibitory connections between the PVN oxytocin and stress hormone systems are suggested but not verifi ed. (Adapted from Eisler and Levine, 2002, with the permission of Kluwer Academic Publishers.)
Based on the simplified schema in Figure 3, we suggest that at any given time the orbitofrontal cortex sends different strengths of inhibitory signals to the different parts of PVN that contain oxytocin or the cortisol precursor, and that this can be a means of influencing the relative likelihood of oxytocin-mediated (tend-and-befriend) versus cortisol-mediated (fight-or-flight or dissociative) responses. Because the orbitofrontal cortex stores the emotional or visceral significance of social memories, the relative strengths of these pathways could be influenced by the amount of stress in the organism's early experiences. The types of behavior that prefrontal regulation would tend to disinhibit are likely to be those encouraged by the society, family, and other people that a person interacts with.
A second set of prefrontal pathways for response selection is the loops between cortex, thalamus, and basal ganglia. These loops are a basis for several neural network models of reward-based behavioral regulation (e.g., Bullock et al., 1999; Monchi & Taylor, 1999). In particular, the strong connections between the prefrontal cortex and the nucleus accumbens (a primary site for both natural and drug-related dopamine rewards) are likely to play a role in both tend-and-befriend and dissociative responses.
A third set of prefrontal pathways is the reciprocal connections with areas of the amygdala involved in emotional evaluation of stimuli (Schoenbaum, Setlow, Saddoris, & Gallagher, 2003). These areas (central and basolateral amygdala) are also part of the stress-related positive feedback loop of Figure 2.
The orbitofrontal cortex is influenced by neural signals from the same brain areas to which it sends signals. Connections to this prefrontal region from other parts of the cortex representing social stimuli should be strengthened or weakened with experience, including the previous stresses and previous responses. This in turn influences the tendency for a given social context to generate fight-or-flight, dissociative, or tend-and-befriend behavior.
Neural network theory-building
Understanding the dynamics involved in regulation of fight-or-flight, dissociative, and tend-and-befriend responses requires integrating disparate kinds of data. Animal lesion studies, human brain imaging studies, and clinical observations of abused children, for example, all involve different measurement techniques. As the wealth of scientific tools grows, so do the complexity of relating one result to another and the need for a common theoretical language. Increasingly, researchers in all areas of behavioral neuroscience have turned to neural network modeling, and drawn on the expertise of established schools of computational modelers, to provide just such a common language.
This type of modeling involves building theories of the behaviorally significant dynamic interactions among interconnected brain regions, then, when these interactions have been specified to some level of precision, simulating them on a computer. As recent neural network models have become more sophisticated, the mathematical dynamics of network variables have come closer to reproducing functional roles of specific brain areas (Levine, 2000, Chapter 7).
The first step in developing a plausible neural network theory of any complex behavioral process is to break it down into subprocesses that can be modeled by smaller networks. The next step is to synthesize these smaller network models and their interconnections into a larger model. Our eventual aim is to develop as accurate and predictive as possible a theory of how, and in what contexts, prefrontal-subcortical pathways influence selective expression among genetically derived neural patterns representing tend-and-befriend, fight-or-flight, and dissociation. This will build on the network theories instantiated in Figures 1-3 combined with networks for dissociative responses (Koob & LeMoal, 2001).
Conclusions: Family, society, and genes all matter
The results reviewed here indicate that selfishness and altruism, ethical and unethical behavior, caring and noncaring interactions all result from complex interactions between nature and nurture. Quantitative simulation via biologically realistic neural networks should be useful in helping to unravel the dynamics of the interacting cortical and subcortical regions that instantiate these characteristic behavior patterns. Such networks can incorporate individual differences, whether those differences are based on genetic predispositions or cultural learning.
The premise of this article is that normal humans carry all three of the major classes of behavior patterns that have arisen as evolutionary adaptations for different purposes — tend-and-befriend (caring or bonding), fight-or-flight (hyperarousal), and dissociative (withdrawal) patterns. Hence, the choices we make between activation of these patterns are a matter of selective disinhibition or gene expression, not of natural selection. This premise does not hinge on the particular set of pathways outlined in Figures 1-3. New results on the behavioral functions of specific brain areas in many mammalian species could alter some of these diagrams, but will not change the importance of selective gene expression.
Differences in capacity for caring behavior between different people probably exist. Just as there are genetic differences in the strengths of brain pathways involved in mathematical or motor skills, there are differences in the pathways involved in tend-and-befriend behavior. Yet cultural influences also play a strong role in causing any of these skills to be expressed or suppressed. Hence, ultimately our neural network studies should provide explanations for cross-cultural, training-based differences in the amount of caring behavior, such as Eisenbergs (1992) finding that caring children are produced by societies such as the Papago Indians of Arizona in which parents tend to be lovingly attached to their children and not to use physical punishment.
The adults brain is less malleable than the childs because no new connections are being made. Yet plasticity of existing connections still allows for social influences to make radical changes in adult patterns of caring and uncaring behavior. An example was Larry Trapp, a Grand Dragon of the Ku Klux Klan in Nebraska who converted to a speaker for racial tolerance (and a Jew!) as a result of a life-changing friendship with Michael and Julie Weisser, a Jewish cantor and his wife who moved into Trapps neighborhood (Dallas Morning News, September 9, 1992). Trapp was stunned when the Weissers returned his harassing hate phone calls with kindness and offers of assistance (he was going blind and needed help getting groceries).
The neural network approach therefore supports the trend among both natural and social scientists to discard the question Is it nature or nurture? in favor of the question How does nurture selectively influence the expression of nature?
References
1. Abraham, F. D. (with R. H. Abraham and C. D. Shaw). A visual introduction to dynamical systems theory for psychology. Santa Cruz, CA: Aerial Press, (1992). [ Links ]
2. Bateson, P. The biological evolution of cooperation and trust. In D. Gambetta (Ed.), Trust: Making and breaking cooperative relations, electronic edition. Department of Sociology, University of Oxford, Chapter 2, 14-30, <papers/bateson14-30.pdf>. [ Links ]
3. Bielsky, I. F., & Young, L. J. Oxytocin, vasopressin, and social recognition in mammals. Peptides, 25, (2004), 1565-1574. [ Links ]
4. Bliss, T. V. P., & Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. Journal of Physiology (London), 232, (1973), 331-356. [ Links ]
5. Brown, J., Bullock, D., & Grossberg, S. How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. Journal of Neuroscience, 19, (1999), 10502-10511. [ Links ]
6. Brown, V. R., & Paulus, P. B. Making group brainstorming more effective: Recommendations from an associative memory perspective. Current Directions in Psychological Science, 11, (2002), 208-212. [ Links ]
7. Brown, V. R., Tumeo, M., Larey, T., & Paulus P. B. Modeling cognitive interactions during group brainstorming. Small Group Research, 29, (1998), 495-526. [ Links ]
8. Buijs, R. M., & Van Eden, C. G. The integration of stress by the hypothalamus, amygdala, and prefrontal cortex: Balance between the autonomic nervous system and the neuroendocrine system. Progress in Brain Research, 127, (2000), 117-132 [ Links ]
9. Byrne, J. H. Cellular analysis of associative learning. Physiological Reviews, 67, (1987), 329-439. [ Links ]
10. Cacioppo, J. T., Berntson, G. G., Adolphs, R., Carter, C. S., Davidson, R. J., McClintock, M. K., McEwen, B. S., Meaney, M. J., Schacter, D. L., Sternberg, E. M., Suomi, S. S., & Taylor, S. E. (Eds.). Foundations in social neuroscience. Cambridge, MA: MIT Press, (2002). [ Links ]
11. Carpenter, G. A., & Grossberg, S. A massively parallel architecture for a self-organizing neural pattern recognition machine. Computer Vision, Graphics, and Image Processing, 37, (1987a), 54-115. [ Links ]
12. Cho, M. M., DeVries, C., Williams, J. R., & Carter, C. S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 113, (1999), 1071-1079. [ Links ]
13. Cory, G. A., Jr. The reciprocal modular brain in economics and politics: Shaping the rational and moral basis of organization, exchange, and choice. New York: Plenum Press, (1999). [ Links ]
14. Cory, G. A., Jr. MacLeans evolutionary neuroscience, the CSN model, and Hamiltons rule. Brain and Mind, 3, (2002), 151-181. [ Links ]
15. Damasio, A. Descartes' error: Emotion, reason, and the human brain. New York: Grosset/Putnam, (1994). [ Links ]
16. Darwin, C. The descent of man. Princeton, NJ: Princeton University Press, (1871/1981). [ Links ]
17. De Houwer, J., Hermans, D., Rothermund, K., & Wentura, D. Affective priming of semantic categorisation responses. Cognition & Emotion, 16, (2002), 643-666. [ Links ]
18. Eisenberg, N. The caring child. Cambridge, MA: Harvard University Press, (1992). [ Links ]
19. Eisler, R. Sacred pleasure: Sex, myth, and the politics of the body. San Francisco: Harper, (1995). [ Links ]
20. Eisler, R., & Levine, D. S. Nurture, nature, and caring: We are not prisoners of our genes. Brain and Mind, 3, (2002), 9-52. [ Links ]
21. Elkin, R. A., and Leippe, Michael R. Physiological arousal, dissonance, and attitude change: Evidence for a dissonance-arousal link and a dont remind me effect. Journal of Personality and Social Psychology, 51, (1986), 55-65. [ Links ]
22. Everitt, B. J., & Robbins, T. W. Central cholinergic systems and cognition. Annual Review of Psychology, 48, (1997), 649-684. [ Links ]
23. Ferguson, J. N., Young, L. J., Hearn, E. F., Matzuk, M. M., Insel, T. R., & Winslow, J. T. Social amnesia in mice lacking the oxytocin gene. Nature Genetics, 25, (2000), 284-288. [ Links ]
24. Freeman, W. J. Neurodynamic models of brain in psychiatry. Neuropsychopharmacology, 28 Suppl 1, (2003), S54-63. [ Links ]
25. Freud, S. Project for a scientific psychology, (Stanford Edition), London: Hogarth, (1895/1966). [ Links ]
26. Fries, A. B. W., Ziegler, T. E., Kurian, J. R., Jacoris, S., & Pollak, S. D. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences, USA, 102, (2005), 17237-17240. [ Links ]
27. Grossberg, S. A neural theory of punishment and avoidance. I. Qualitative theory. Mathematical Biosciences, 15, (1972b), 39-67. [ Links ]
28. Grossberg, S. The complementary brain: unifying brain dynamics and modularity. Trends in Cognitive Sciences, 4, (2000), 233-246. [ Links ]
29. Grossberg, S., & Schmajuk, N. A. Neural dynamics of attentionally-modulated Pavlovian conditioning: Conditioned reinforcement, inhibition, and opponent processing. Psychobiology, 15, (1987), 195-240. [ Links ]
30. Harmon-Jones, E., Brehm, J. W., Greenberg, J. Simon, L., & Nelson, D. E. Evidence that the production of aversive consequences is not necessary to create cognitive dissonance. Journal of Personality and Social Psychology, 70, (1996), 5-16. [ Links ]
31. Hebb, D. O. The organization of behavior. NewYork: Wiley, (1949). [ Links ]
32. Insel, T. R. Oxytocin: A neuropeptide for affiliation - evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology, 17, (1992), 3-33. [ Links ]
33. Insel, T. R., & Winslow, J. T. Serotonin and neuropeptides in affiliative behaviors, Biological Psychiatry, 44, (1998), 207-219. [ Links ]
34. Insel, T. R., Winslow, J. T., Wang, Z., & Young, L. J. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. In H. H. Zingg, C. W. Bourque, & D. G. Bichet (Eds.), Vasopressin and oxytocin: Molecular, cellular, and clinical advances (pp. 215-230). New York: Plenum Press, (1998). [ Links ]
35. Kandel, E. R., & Tauc, L. Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans. Journal of Physiology (London), 181, (1965), 1-27. [ Links ]
36. Kirsch, P., Esslinger, C., Chen, Q., Mier, D., Lis, S., Siddhanti, S., Gruppe, H., Mattay, V. S., Gallhofer, B., & Meyer-Lindenberg, A. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience, 25, (2005), 11489-11493. [ Links ]
37. Koob, G. F. Corticotrophin-releasing factor, norepinephrine, and stress. Biological Psychiatry, 46, (1999), 1167-1180. [ Links ]
38. Koob, G. F., & LeMoal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24, (2001), 97-129. [ Links ]
39. Koopman, C., Sephton, S., Abercrombie, H. C., Classen, C., Butler, L. D., Gore-Felton, C., Borggrefe, A., & Spiegel, D. Dissociative symptoms and cortisol responses to recounting traumatic experiences among childhood sexual abuse survivors. Journal of Trauma & Dissociation, 4, (2003), 29-46. [ Links ]
40. Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U., & Fehr, E. Oxytocin increases trust in humans. Nature, 435, (2005), 673-676. [ Links ]
41. Leven, S. J., & Levine, D. S. Multiattribute decision making in context: A dynamic neural network methodology. Cognitive Science, 20, (1996), 271-299. [ Links ]
42. Levine, D. S. Steps toward a neural theory of self-actualization. World congress on neural networks, San Diego (Vol. I, pp. 215-220). Hillsdale, NJ: Erlbaum, (1994). [ Links ]
43. Levine, D. S. Introduction to neural and cognitive modeling (2nd edition). Mahwah, NJ: Erlbaum, (2000). [ Links ]
44. Levine, D. S. (Ed.). Special issue of Brain and Mind on Brain Development and Caring Behavior. Vol. 3, No. 1, (2002a). [ Links ]
45. Levine, D. S. Neural network modeling. In J. Wixted et al. (Eds.), Stevens Handbook of Experimental Psychology (Vol. 4 (Methodology), pp. 223-269). NewYork: Wiley,(2002b). [ Links ]
46. Levine, D. S. Neural modeling of the dual motive theory of economics. Journal of Socio-Economics, 35, (2006), 613-625. [ Links ]
47. Levine, D. S., & Prueitt, P. S. Modeling some effects of frontal lobe damage: Novelty and perseveration. Neural Networks, 2, (1989), 103-116. [ Links ]
48. Loye, D. Darwin 's lost theory of love. New York: iUniverse, (1999). [ Links ]
49. Loye, D. The moral brain. Brain and Mind, 3, (2002), 133-150. [ Links ]
50. MacLean, P.D. The triune brain in evolution: Role in paleocerebral functions. New York: Plenum, (1990). [ Links ]
51. Monchi, O., & Taylor, J. G. A hard wired model of coupled frontal working memories for various tasks. Information Sciences Journal, 113, (1998), 221-243. [ Links ]
52. Nauta, W. J. H. The problem of the frontal lobe: A reinterpretation, Journal of Psychiatric Research, 8, (1971), 167-187. [ Links ]
53. Nestler, E. J., Alreja, M., & Aghajanian, G. K. Molecular control of locus coeruleus neurotransmission. Biological Psychiatry, 46, (1999), 1131-1139. [ Links ]
54. O'Reilly, R. C., Noelle, D. C., Braver, T. S., & Cohen, J. D. Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cerebral Cortex, 12, (2002), 246-257. [ Links ]
55. Öngür, D., & Price, J. L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex, 10, (2000), 206-219. [ Links ]
56. Papini, M. R. Colamus humanitatem: Nurturing human nature. Avances en Psicolog ía Latinoamericana, 26, (2008), 15-29 [ Links ]
57. Pashler, H., & Medin, D. Stevens' handbook of experimental psychology (3rd ed.), Vol. 2: Memory and cognitive processes. New York: Wiley, (2002). [ Links ]
58. Perry, B. D., Pollard, R. A., Blakley, T. L., Baker, W. L., & Vigilante, D. Childhood trauma, the neurobiology of adaptation, and use-dependent development of the brain: How states become traits. Infant Mental Health Journal, 16, (1995), 271-291. [ Links ]
59. Pinker, S. The language instinct. NewYork: William Morrow & Co, (1994). [ Links ]
60. Price, J. L. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences, 877, (1999), 383-396. [ Links ]
61. Read, S. J., & Miller, L. C. (Eds.). Connectionist models of social reasoning and social behavior. Mahwah, NJ: Erlbaum, (1998). [ Links ]
62. Read, S. J., & Miller, L. C. Virtual personalities: A neural network model of personality. Personality and Social Psychology Review, 6, (2002), 357-369. [ Links ]
63. Rosenblum, L. A., Smith, E. L. P., Altemus, M., Scharf, B. A., Owens, M. J., Nemeroff, C. B., Gorman, J. M., & Coplan, J. D. Differing concentrations of corticotrophin-releasing factor and oxytocin in the cerebrospinal fluid of bonnet and pigtail macaques. Psychoneuroendocrinology, 27, (2002), 651-660. [ Links ]
64. Rumelhart, D. E., & McClelland, J. L. (Eds.). Parallel distributed processing (Vols. 1 and 2). Cambridge, MA: MIT Press, (1986). [ Links ]
65. Sarnyai, Z., & Kovács, G. L. Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology, 19, (1994), 85-117. [ Links ]
66. Schoenbaum, G., Setlow, B., Saddoris, M., & Gallagher, M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron, 39, (2003), 855-867. [ Links ]
67. Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A. R., & Updegraff, J. A. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107, (2000), 411-429. [ Links ]
68. Tryon, W. W. Neural networks: I. Theoretical unification through connectionism. Clinical Psychology Review, 13, (1993), 353-371. [ Links ]
69. Tryon, W. W. Synthesizing animal and human behavior research via neural network learning theory. Journal of Behavior Therapy and Experimental Psychology, 26, (1995), 303-312. [ Links ]
70. Turner, R. A., Altemus, M., Enos, T., Cooper, B., & McGuinness, T. Preliminary research on plasma oxytocin in healthy, normal cycling women investigating emotion and interpersonal distress. Psychiatry, 62, (1999), 97-113. [ Links ]
71. Uvnäs-Moberg, K. Oxytocin linked antistress effects - the relaxation and growth response. Acta Physiologica Scandinavica, 640 (Suppl.), (1997), 38-42. [ Links ]
72. Uvnäs-Moberg, K. Oxytocin may mediate the benefits of positive social interaction and emotion. Psychoneuroendocrinology, 23, (1998), 819-835. [ Links ]
73. Van Rooy, D., Van Overwalle, F., Vanhoomissen, T., Labiouse, C., & French, R. A recurrent connectionist model of group biases. Psychological Review, 110, (2003), 536-563. [ Links ]
74. Webster, M. J., Weickert, C. S., Herman, M. M., & Kleinman, J. E. BDNF mRNA expression during postnatal development, maturation, and aging of the human prefrontal cortex. Developmental Brain Research, 15, (2002), 139-150. [ Links ]
75. White, D. A., & Sofge, D. A. (Eds.). Handbook of intelligent control: Neural, fuzzy, and adaptive approaches. New York: Van Nostrand Reinhold, (1992). [ Links ]
76. Winslow, J. T., Shapiro, L., Carter, C. S., & Insel, T. R. Oxytocin and complex social behavior: Species comparisons. Psychopharmacology Bulletin, 29, (1993), 403-414. [ Links ]
77. Wood, J. N. Social cognition and the prefrontal cortex. Behavioral and Cognitive Neuroscience Reviews, 2, (2003), 97-115. [ Links ]
78. Young, L. J., & Wang, Z. The neurobiology of pair bonding. Nature Neuroscience, 7, (2004), 1048-1054. [ Links ]














