Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Avances en Psicología Latinoamericana
Print version ISSN 1794-4724
Av. Psicol. Latinoam. vol.31 no.1 Bogotá Jan./Apr. 2013
3, 4-methylenedioximethamphetamin reverses anxiety induced by chronic mild stress
3, 4-metilen dioximetanfetamina revierte la ansiedad inducida por estrés crónico moderado
3, 4-metilenedioximetafetamina reverte a ansiedade induzida por estresse crônico moderado
LAURA ANDREA LEÓN A*
FERNANDO P. CARDENAS**
* Psychologist and Master of Science in Psychology by the Universidad de los Andes, Bogotá, Colombia. Ph.D., by the Universidade de São Paulo, Ribeirão Preto, Brasil. Laboratory of Neuroscience and Behavior, Department of Psychology, Universidad de los Andes, Bogotá, Colombia.
** Psychologist, Universidad Nacional de Colombia, Master of Sciences and Ph.D, by the Universidade de São Paulo, Ribeirão Preto, Brazil. Chair of the Laboratory of Neuroscience and Behavior, Department of Psychology, Universidad de los Andes, Bogotá, Colombia. Laboratory of Neuroscience and Behavior, Department of Psychology, Universidad de los Andes, Cra. 1 #18A-12, Bogotá, Colombia. Tel: +57133949993854; Fax +5713394999-2597.
e-mail address: lucarden@uniandes.edu.co.
Para citar este artículo: León, L. A. & Cardenas, F. P. (2013). Subchronic 3, 4-methylenedioximethamphetamine reverses anxiety induced by chronic mild stress. Avances en Psicología Latinoamericana, 31 (1), pp. 266-278.
Fecha de recepción: 1° de agosto de 2012
Fecha de aceptación: 29 de enero de 2013
Abstract
Here we report the effects of subchronic 3, 4-methylenedioximethamphetamine (MDMA) on the elevated plusmaze, a widely used animal model of anxiety. Rats exposed to a mild chronic stress (MCS) protocol received intracerebroventricular microinjections of the selective serotonin reuptake inhibitor (SSRI) - fluoxetine (2.0 ug/ ul) or MDMA, (2.0 ug/ul) for seven days. On the eighth day rats were tested in the elevated plus-maze. Our results showed that sub-chronic MDMA interacted with MCS leading to a decrease in anxiety-related behaviors including: percentage of open arms entries (F[2, 26] = 4.00; p = 0.031), time spent in the open arms (F[2, 26] = 3.656; p = 0.040) and time spent in the open arms extremities (F[2, 26] = 5.842; p = 0.008). These results suggest a potential effect of MDMA in the reversion of the emotional significance of aversive stimuli.
Keywords: MDMA, fluoxetine, elevated plus-maze, anxiety, serotonin, mild chronic stress.
Resumen
Reportamos aquí los efectos de la administración subcrónica de 3, 4-metilendioximetanfetamina (MDMA) sobre el laberinto en cruz elevado, un modelo animal de ansiedad ampliamente utilizado. Las ratas fueron expuestas a un protocolo de estrés crónico moderado (MCS) y recibieron microinyecciones intra-cerebroventriculares del inhibidor selectivo de la recaptación de serotonina (SSRI)-fluoxetina (2,0 ug/ul) o de MDMA (0,2 ug/ul) durante siete días. En el octavo día las ratas fueron probadas en el laberinto en cruz elevado. Los resultados mostraron que la administración subcrónica de MDMA interactuó con el MCS, llevando a un decremento de los comportamientos relacionados con la ansiedad, incluyendo: porcentaje de entradas a los brazos abiertos (F 26] = 4.00; p = 0.031), el tiempo empleado explorando los brazos abiertos (F[2, 26] = 3.656; p = 0.040) y el tiempo empleado explorando las extremidades de los brazos abiertos (F[2, 26] 26] = 5.842; p = 0.008). Estos resultados sugieren un potencial efecto de MDMA en la reversión del significado emocional de los estímulos aversivos.
Palabras clave: MDMA, fluoxetina, laberinto en cruz elevado, ansiedad, serotonina, estrés crónico moderado.
Resumo
Os efeitos da administração sub-crônica de 3, 4-metilenedioximetanfetamina (MDMA) no labirinto em cruz elevado, um modelo amplamente utilizado no estudo da ansiedade, foram testados. Ratos submetidos a um protocolo de estresse crônico moderado (MCS) receberam micro injeções intra cérebro ventriculares do inibidor seletivo da recaptação de serotonina (SSRI)-fluoxetina (2.0ug/ul) ou MDMA (2.0 ug/ul) durante sete dias. No oitavo dia, os ratos foram testados no labirinto em cruz elevado. Os resultados sugerem que a administração sub-crônica de MDMA interagiu com o MCS levando à diminuição dos comportamentos relacionados com ansiedade, incluindo: porcentagem de entradas aos braços abertos (F[2, 26] = 4.00; p = 0.031), o tempo gasto explorando os braços abertos (F[2, 26] = 3.656; p = 0.040) e o tempo gasto explorando as extremidades dos braços abertos (F[2, 26] = 5.842; p = 0.008).
Palavras chave: MDMA, fluoxetina, labirinto em cruz elevado, ansiedade, serotonina, estresse crônico moderado.
Generalized anxiety disorder (GAD) is a common mental illness. Given its co-morbidity with depression, it has received much attention in recent years. Some of the symptoms present in GAD depend on serotonergic mechanisms. It has been reported that increased serotonin (5-HT) release induces anxiety enhancement in both animal models and human clinics (Kakui et al., 2009; Khan et al., 2006). Several animal models of anxiety have been used in the screening of anxiolytic or anxiogenic properties of compounds, including the elevated plusmaze (EPM), social interaction tests, open fields, etc. (Dunn et al., 1989). Some 5-HT1a agonists (i.e. buspirone) show an inverted-U-shaped doseresponse curve in some animal models of anxiety such as the Vogel conflict test (Vaidya et al., 2005).
Evidences supporting the role of 5-HT in the physiopathology of GAD include the anxiogenic-like effect of selective 5-HT reuptake inhibitors (SSRIs) —as fluoxetin— in early treatment for depression in human clinics. SSRIs induce an initial period of enhanced anxiety and their anxiolytic or antidepressant effects only arise after prolonged treatment. This delayed effect has been explained by the ability of SSRIs (similar to that found in 5-HT1a agonists) to inhibit the firing rate of serotonergic neurons through the activation of somatodendritic receptors (Zhang et al., 2000).
3, 4-Methylenedioximethamphetamine (MDMA) is a strong monoaminergic reuptake inhibitor with an extremely high affinity for 5-HT transporters (SERTs). MDMA reverses SERTs action, leading to a rapid enhancement in 5-HT release, even in the absence of incremented firing rate. The initial enhanced release is followed by the depletion of serotonergic terminals. In addition MDMA blocks 5-HT reuptake and it also maybe inhibits monoaminooxidase (MAO). All these actions enhance the availability of 5-HT (Colado et al., 2001; Green et al., 1995; Iravani et al., 2000; Mlinar & Corradetti, 2003).
Different effects for MDMA on anxiety have been reported. For example acute administration of "moderate" doses of systemic MDMA (8-15mg/ Kg) in rats increase anxiogenic-like behaviors in the EPM and in the light-dark box (Lin et al., 1999; Maldonado & Navarro, 2000; Navarro & Maldonado, 2002). Assuming that the commonly used dose by adult humans range between 100 and 120 mg (2mg/Kg approximately) the 8-15 mg/Kg dose used by those authors range between fourfold and sevenfold and is "high" rather than "moderate". Acute administration of (0, 1.25, 2.5, 5 mg /kg) of MDMA seems to have paradoxal effects, enhancing anxiety in EPMs and cat odor avoidance tests and reducing it in social interaction and footshock-induced ultrasonic vocalization tests (Morley & McGregor, 2000). It was also found that lower doses of MDMA (1.25 mg/Kg) significantly reduce aggressive behavior in the social interaction test. These paradoxal results could be explained assuming that at lower doses there exists an interaction between MDMA and basal anxiety conditions leading to different effects.
In order to elucidate the possible interaction between MDMA and basal anxiety here we used the chronic unpredictable stress protocol (CUS) sometimes referred as chronic mild stress (MCS). This protocol involves the single exposure to different mild unpredictable stressors, at least two per day (Papp et al., 1996). There are many available protocols varying between two weeks and four months. Some variations in the amount of the depressive-like effects induced by MCS and CUS have been also reported (Ducottet et al., 2003; Li et al., 2010). MCS induces acute 5-HT release followed by a decrease in serotonergic neurons firing rate and partial desensitizitation of somatodendritic 5-HT1a autoreceptors in the dorsal raphe (Bambico et al., 2009; Ossowska et al., 2001). Previous research has also reported that exposure to unpredictable stressors can increase anxiety in rodents (Matuszewich et al., 2007). However the precise role of MCS in general anxiety levels has not been fully demonstrated. Indeed there are many contradictory reports (Bondi et al., 2008; Cunningham et al., 2009; Fokos & Panagis, 2010; Mitra et al., 2005; Vyas & Chattarji, 2004). For some authors the exposure to CUS has no effect on unconditioned response tasks as the EPM (Matuszewich et al., 2002; Mitra et al., 2005). For some others however, the exposure to stressors induces an enhancement in the level of anxiety (Botelho et al., 2007; Dos Santos et al., 2010; Estanislau & Morato, 2005; Hata et al., 2001). The aim of this study is to compare the interaction between basal anxiety levels and the effects of both MDMA and fluoxetine on the EPM performance of rats previously exposed to MCS.
Experimental procedures
Animals
Thirty-two naive three months old male Wistar rats (320 ± 30 g) were used. All the animals were obtained from the animal facilities of the Universidad de los Andes. The subjects were housed in groups of four, in polypropylene cages (43 x 31 x 18 cm) and kept under a 12 h light/dark cycle (lights on at 07:00h). Rats had free access to food and water throughout the experiment. All experimental protocols employed in this work were performed in compliance with recommendations of the Colombian Guidelines for Laboratory Animal Care (laws 84/1989 and 8430/1993) which are based on the US National Institutes of Health Guide for Care and Use of Laboratory Animals (No. 86-23, revised 1996). After the arrival at the laboratory, rats were allowed three days to acclimate to the vivarium conditions. All rats received two minutes of daily handling (to get familiar with the experimenters) for three days, before the beginning of the experiments.
Surgery
Each animal was anesthetized with a mixture of Ketamine (RotexMédica, 75 mg/Kg) and Xylacine (Bayer, 5 mg/Kg), and fixed in a stereotaxic frame (Narishige). Lidocaine (Ropsohn Therapeutics, 2%) was subcutaneously administrated under the scalp. The incisor bar was set at 3.3 mm below the interaural line such that the skull was horizontal between Bregma and Lambda. A stainless steel guide cannula (9 mm), through which a microinjection needle could be inserted for drug infusion, was implanted aimed at the lateral ventricle (AP=0.8 mm; ML=+1.4 and DV=2.4 mm, using Bregma as reference, Paxinos & Watson, 2007). The cannula was attached to the skull by means of acrylic resin and to two stainless steel screws. The rats were then returned to their cages. At the end of the surgery the cannula was sealed with a wire to protect it from obstruction. Before the rats returned to their cages, they received intramuscular vancomycin (Lilly). All rats were allowed a period of sis days for the recovery of the surgery.
Drugs
MDMA (Radian International) and fluoxetine (Genfar) were dissolved in saline solution (0.9%) to concentrations of 2.5 and 2.0 ug/ul, respectively. Control animals received saline solution (0.9%). Esteban et al. (2001), using microdyalisis showed that a dose of 15 mg/Kg led to a cerebral extracellular concentration of 20 uM. In the same set of experiments, they demonstrated that the intracerebral perfusion of400 mM of MDMA led to an extraellular concentration between 10.4 and 19.5 uM. These extracellular concentrations are neurotoxic. For these reasons and in order to prevent any neurotoxic effect we used a thousand times lower dose. It was also found that the intracerebroventricular infusion of 2.0 ug of MDMA induced self-administration (Braida & Sala, 2002) suggesting that this dose is effective with no potential neurotoxic effect.
Procedure
Mild chronic stress
The subjects were randomly assigned to one of two groups: exposed to mild chronic stress; (MCS[+]) or no exposed to mild chronic stress; (MCS[-]). MCS[-] animals were left in the vivarium with no other procedure than five minutes of daily handling. The seven days MCS protocol used was a modified version of that described by D'Aquila et al (1994). Briefly it consisted of one or two different stressors per day i.e. inclination of the cage, change of partners, wet bedding, altered light-dark cycle, etc.
MCS[+] and MCS[-] animals were divided into three subgroups receiving intracerebroventricular fluoxetine, MDMA or saline solution once a day per seven days. All microinjections were done at a rate of 1ul/min, using a dental needle connected to a Hamilton syringe via a Tygo tubing. The total injected volume was 1ul. The displacement of an air bubble inside the tubing was used to monitor the progress of the microinjection. After receiving the microinjection each animal returned to its cage.
Elevated plus-maze
An elevated plus-maze, described in detail elsewhere was used (Cardenas et al., 2001). Briefly it consisted of two open arms (50 x 10 cm) crossed at right angles with two opposed arms of the same size. Two of the opposed arms were enclosed by wooden walls (40 cm high) except for the central part where the arms crossed. The whole apparatus was elevated 50 cm above the floor. To prevent the rats from falling, a rim of Plexiglas (1.0 cm high) surrounded the perimeter of the open arms. The experimental sessions were recorded by a video camera interfaced with a computer in an adjacent room and digitalized for later analysis. In order to record displacements from one place to the other in the plus-maze and to precisely locate where other behaviors occurred, the image of the elevated plusmaze was virtually divided into 10 cm-squares. This also allowed the recording of the number of squares entered by an animal (which made possible to estimate the total distance run). An entry to any kind of arm was scored when all four feet were within that arm.
Along with the number of entries into and the time spent in each kind of arm, other behaviors were also analyzed: (a) number of entries into and time spent in the endings of the open arms; (b) grooming: defined as the species-innate groom behavior beginning with the snout, progressing to the ears and ending with whole-body groom; (c) rearing: partial or total rising onto the hind limbs; (d) head dipping: exploratory movement of head and shoulders over sides of the open arms and down towards the floor; (e) head out (horizontal scanning): exploratory movement of head and shoulders over sides of the open arms without directing towards the floor; (f) freezing: absence of any movements different than breathing for at least three seconds and (g) stretching (flat back approach): defined as the stretched cautious movement from one square to the next within the open arms. Displacements and behaviors were recorded using the software X Plo Rat (version 3.3).
Histology
After the end of experiments, all rats were anesthetized with a lethal dose of penthobarbital (80 mg/ Kg). The animals were then transcardiacally perfused with 120ml of saline solution (0.9%) followed by 200 ml of paraformaldehyde (4%). The brains were then gently removed and maintained in the same paraformaldehyde solution for at least four days. After that period all the brains were coronally sectioned on a sliding vibratome (Vibratome 1500) and 60 um slices were obtained and treated for Nissl staining with Cresyl Violet to confirm the location of the cannula. All animals with cannulae in wrong locations were excluded from the study.
Statistics
All data were analyzed using two-way ANOVA. When necessary, the comparison between the averages of the groups was done using the Newman Keuls test as post hoc test. Alpha was set at 0.05 for all instances.
Results
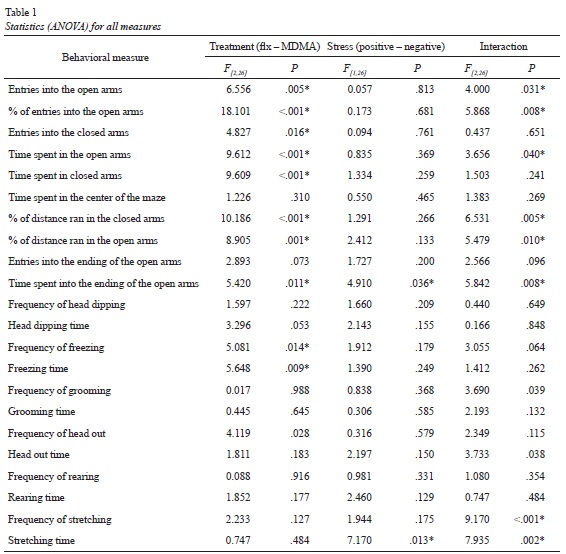
(Please refer to Table 1 for all numeric F values for ANOVAs). Regarding to the number of entries into the open arms ANOVA showed significant differences for the treatment condition but not for the stress condition (F[2, 26] = 6.556; p = .005; F[2, 26] = .057; p = .813). The interaction between the two factors was significant (F[2, 26] = 4.000; p = .031). The post hoc comparison of the averages of the groups (Student Newman-Keuls) showed that animals treated with fluoxetine entered less into the open arms when previously submitted to the stress condition (p < .05). The post hoc test also showed that animals without stress and treated with fluoxetine entered more into the open arms than control subjects (p < .05). The Student Newman-Keuls test even showed that animals submitted to MCS and treated with MDMA entered more into the open arms (p < .05) than did animals treated with saline or fluoxetine.
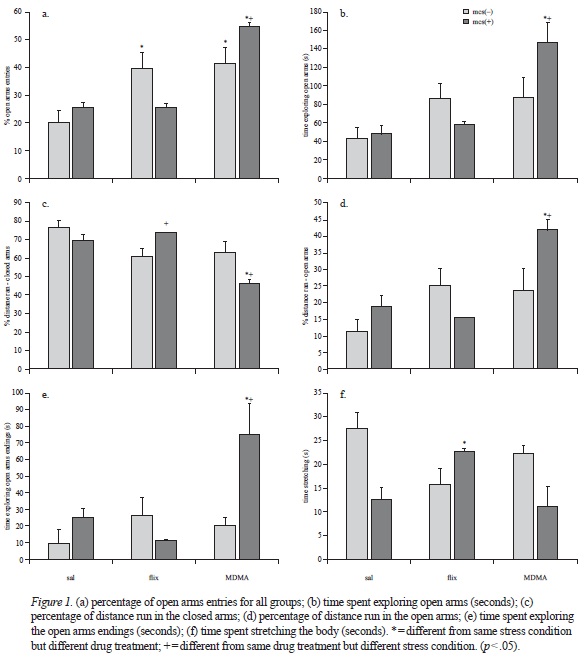
ANOVA showed significant differences in the percentage of entries into the open arms for the treatment condition but not for the stress condition (F[2, 26] = 18.101; p < .001; F[2, 26], = 0.173; p = .681). Figure 1-A shows the percentage of open arms entries for all groups. As in the case of entries into the open arms, the interaction between the two factors was significant (F[2, 26] = 5.868; p = .008). The post hoc comparison of the averages of the groups showed that animals with no exposure to MCS and treated with fluoxetine had a higher percentage of entries into the open arms. Rats treated with MDMA, with or without exposure to MCS showed higher percentages of entries into the open arms (p < .001) than animals treated with saline or fluoxetine. MDMA also increased the percentage of entries into the open arms in rats exposed to MCS in comparison with rats without MCS exposure (p < .01).
In relation with the time spent exploring the open arms, ANOVA showed significant differences for the treatment condition but not for the stress condition (F[2, 26] = 9.612; p < .001; F[1, 26] = 0.835; p = .369). The interaction between the two factors was significant (F[2, 26] = 3.656; p = .040). The Student Newman-Keuls test showed that animals exposed to MCS and treated with MDMA spent more time exploring the open arms than animals treated with saline solution (p = .001; see Figure 1-B).
ANOVA showed significant differences for the treatment but not for the stress condition in the amount of entries into the closed arms (F[2, 26] = 4.827; p = .016; F[1, 26] = 0.094; p = .761). The interaction between the factors was not significant (F[2, 26] = 0.437; p =.651). As a general factor, MDMA treated animals entered less into the closed arms. ANOVA also showed significant differences in the percentage of entries into the closed arms for the treatment condition but not for the stress condition. The interaction between the factors was significant. The post hoc analysis showed that rats treated with fluoxetine had greater percentages of entries into the closed arms when previously exposed to MCS (p < .05). The post hoc comparison also showed that animals treated with MDMA had lower percentages of entries into the closed arms when previously exposed to MCS (p < .05). The comparison of the averages of the groups also showed that both fluoxetine and MDMA decreased the percentage of entries into the closed arms for animals without stress exposure (p < .05).
Significant differences were also found for the treatment condition in the time spent in the closed arms but not for the stress condition (F[2, 26] = 9.609; p < .001; F[2, 26] = 1.334; p = .259). The interaction between the two factors was not significant (F[2, 26] = 1.503; p = .241). As a general effect, animals treated with MDMA spent less time exploring the closed arms (p < .05). ANOVA showed no significant differences for any of the factors in the time spent in the center of the maze. The interaction between them was also no significant.
Figure 1-C shows the percentages of distance traveled within the closed arms. ANOVA showed significant differences for the treatment condition but not for the stress condition (F[2, 26] = 10.186; p < .001; F [1, 26] = 1.291; p = .2 66). The interaction between the two factors was significant (F[2, 26] = 6.531; p = .005). The post hoc comparison of the averages of the groups (Student Newman-Keuls) showed that animals exposed to the stress condition and treated with MDMA had lower percentages of distance traveled within the closed arms than subjects treated with saline solution (p < .001). The post hoc analysis showed that within animals treated with MDMA those with MCS exposure traveled longer distances in the closed arms than those without MCS exposure (p < .05). The post hoc comparison also showed that animals treated with fluoxetine had higher percentages of closed arms exploration when pre-exposed to MCS (p < .05).
Figure 1-D shows the percentage of distance traveled within the open arms. ANOVA showed significant differences for the treatment condition but not for the stress condition (F[2, 26] = 8.905; p < .001; F [1, 26] =2.412; p = .113). The interaction between the two factors was significant (F[2, 26] = 5.479; p =.001). The post hoc comparison of the averages of the groups (Student Newman-Keuls) showed that animals exposed to MCS and treated with MDMA explored more the open arms than did animals treated with saline solution (p = .001). These animals also explored more the open arms than did animals treated with the same compound but without MCS pre-exposure (p = .007).
ANOVA showed significant differences for both treatment and stress condition in the time spent exploring the endings of the open arms (see, Figure 1-E; F [2, 26], = 5.420; p = .011; F [1, 26] = 4.910; p = .036). The interaction between the two factors was also significant (F[2, 26] = 5.842; p = .008). The post hoc comparison of the averages of the groups (Student Newman-Keuls) showed that animals exposed to MCS and treated with MDMA explored more the open arms endings than did animals treated with saline solution (p = .003). These animals also explored more the open arms endings than did animals treated with the same compound but without MCS pre-exposure (p = .001).
ANOVA showed no differences for any of the factors neither in frequency nor in the time spent dipping the head, grooming the body or rearing against the walls of the maze. There were found significant differences for the treatment condition in the frequency of horizontal peeping out of the border of the open arms (head out). There were no significant differences for the stress condition. The interaction between the factors was not significant (please refer to Table 1 to see the numeric data). As a general effect, animals treated with fluoxetine and MDMA showed more horizontal scanning than do animals treated with saline solution (p < .05).
There were found no significant differences for any of the factors in the frequency of the stretching behavior (F[2, 26] = 2.233; p = .127; F [1, 26] = 1.944; p = .175). However, the interaction between the factors was significant (F[2, 26] = 9.170; p < .001). Animals treated with saline solution and pre-exposed to MCS showed an increased amount of flat back approaches (p = .008).
Finally, ANOVA showed significant differences for the stress condition in the time spent stretching, but no for the treatment condition (F[2, 26] = 0.747; p = .484; F [1, 26] = 7.170; p = .013). The interaction between the factors was significant (F[2, 26] = 7.935; p = .002). The post hoc analyses showed that animals submitted to the stress condition spent less time stretching the body when received fluoxetine than when received saline solution (p = .03; see Figure 1-F).
Discussion
Our data show that subchronic administration of MDMA or fluoxetine induce different behavioral patterns of exploration in the elevated plus-maze in rats previously submitted to a MCS protocol. MDMA increased the percentage of entries into the open arms in rats both submitted and not submitted to MCS, suggesting an anxiolytic-like effect. Interestingly, this anxiolytic-like effect was more evident in subjects previously submitted to MCS.
Given the fact that the mechanism of action of MDMA involves the massive release and thus the extracellular accumulation of 5-HT, the anxiolytic-like properties of MDMA found here must depend on serotonergic mechanisms. In a relatively short time (seven days), the enhanced 5-HT levels induced by MDMA led to an anxiolytic-like effect while in the same short time fluoxetine seemed to have no effect. As earlier mentioned, the acute treatment with fluoxetine induces anxiogenic-like behaviors while the chronic treatment has the opposite effect (Abrams et al., 2005; Alves et al., 2004; Dos Santos et al., 2008; Silva & Brandao, 2000; Silva et al., 1999). Here we found that this anxiolytic-like effect of fluoxetine was absent in subjects previously exposed to MCS, suggesting that the dose used here and the amount of time of treatment were ineffective to reverse the anxiety induced by MCS. However, in animals with no exposure to MCS subchronic fluoxetine induced the expected anxiolytic effect.
Beside the number of entries into the open arms and the time spent exploring them, the pattern of exploration of the open arms is a good index of anxiety. Animals showing low levels of anxiety spent more time actively exploring the open arms (Botelho et al., 2007; Cardenas et al., 2001; Garcia et al., 2005). Our data showed that subjects submitted to MCS and treated with MDMA spent more time in the open arms and explored them more actively, as reflected by the increased distance traveled within them in comparison to the distance run within the closed arms. These data suggest that MDMA effects are not due to a general motor activity enhancement but to an intentional motivation for exploration maybe driven by the richer environment offered by the open spaces. The finding that this particular effect was present only in animals submitted to MCS suggest that the 5-HT enhancement induced by MCS (Bekris et al., 2005) is added to that induced by MDMA, maybe leading to faster changes in 5-HT receptors function and expression. This could be the mechanism by which MDMA interacts with basal anxiety. The enhanced time spent stretching showed by animals submitted to MCS suggests a clear increase in anxiety. In fact, it has been reported that increased stretching in the EPM is related to enhanced plasma corticosterone (Albrechet-Souza et al., 2007).
It has been reported that acute increases of 5-HT enhance impulsive behavior (Arce & Santisteban, 2006; Bizot et al., 1999; Dalley et al., 2002; Harrison et al., 1997). In the EPM, the exploration of the central square has been found to be a good index of impulsiveness (Albrechet-Souza et al., 2009; Carobrez & Bertoglio, 2005; Griebel et al., 1997; Setem et al., 1999; Silva & Brandao, 2000). Our data showed that neither MDMA nor fluoxetine led to changes in the time spent within the central square, indicating the absence of any impulsive behavior.
Cunningham et al. showed little anxiety related behaviors in the EPM in MDMA pretreated rats. It is possible that their stress-inducting procedure was "stronger" than ours (Cunningham et al, 2009). Their results were interpreted as "unpredictable". However, it should be noted that the dose of MDMA used by them is around three times higher than the dose used here. Moreover, there are reports showing anxiolytic-like effects in the EPM for mice when using acute MDMA in extremely high (20 mg/Kg) doses (Lin et al., 1999). These anxiolytic-like effects could represent impulsive rather than anxiolytic-like behavior and could represent an inverted-U-shaped-effect for MDMA on anxiety with low doses acting as anxiolytic, moderated doses acting as anxiogenic and extremely high doses acting as "anxiolytic" or more properly "impulsiveness- inductors".
The amount of time spent in the extremities of the open arms has been established as a good index of anxiety (Cardenas et al., 2001; Estanislau & Morato, 2006; Garcia et al., 2005; Martinez et al., 2007). Both the significant higher time spent into the open arms extremities and the huge amount of time spent in the whole open arms in MDMA MCS [+] subjects, suggest a very low level of apprehension. In 1999, King reported an unstable elevated plus-maze as a model of extreme anxiety. In her model, the time spent on the open arms correlates with an increased level of anxiety. She proposed that it could reflect the search for an exit of the aversive situation (King, 1999). In our case, although MDMA induced an enhancement in 5-HT function, the time spent in the open arms could not be interpreted as a behavior oriented to the search for an exit of the situation mainly because it is not correlated to any other anxiety-related responses, i.e. decreased dipping of the head or augmented stretched flat approaches, as defined by earlier works (Cruz et al., 1994). A similar exploratory profile was found in rats tested in the absence of light (Cardenas et al., 2001; Garcia et al., 2005; Martinez et al., 2002; Morato & Castrechini, 1989). This supports the idea of a specific strong anxiety release property for MDMA that could in turn improve coping strategies.
Conclusion
It could be stated that MDMA acts as an enhancer of "coping strategies" as expressed by the increased exploration of the EPM. The anxiolytic-like effect of MDMA suggests a change in the emotional valence of potential aversive stimuli. This reversion in the emotional valence was already inferred from experiments on emotional memory conducted in our laboratory (Leon et al., 2009) and could represent a coping strategy enhancing effect. It is feasible that MDMA changes the affective valence of stressors in such a way that normal aversive stimuli could be "reinterpreted" as normal or even appetitive stimuli, maybe by dopaminergic mediation (Callaway & Geyer, 1992).
Acknowledgements
This research was supported by grant FAPA from Universidad de los Andes. The authors want to acknowledge the invaluable help of Dr. J. Landeira-Fernandez (Pontifícia Univesidade Católica de Rio de Janeiro) and Dr. Isabel Riveros (Instituto Nacional de Medicina Legal, Colombia).
Disclosure
The authors declare that except for income received from primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years of research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
Abrams J. K., Johnson P. L., Hay-Schmidt, A., Mikkelsen, J. D., Shekhar, A. & Lowry, C. A. (2005). Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience, 13, 983-997. [ Links ]
Albrechet-Souza, L., Borelli, K. G., Carvalho, M. C. & Brandao M. L. (2009). The anterior cingulate cortex is a target structure for the anxiolytic-like effects of benzodiazepines assessed by repeated exposure to the elevated plus maze and Fos immunoreactivity. Neuroscience, 16, 387-397. [ Links ]
Albrechet-Souza, L., Cristina de Carvalho, M., Rodriguez France, C. & Brandao, M. L. (2007). Increases in plasma corticosterone and stretched-attend postures in rats naïve and previously exposed to the elevated plus-maze are sensitive to the anxiolytic-like effects of midazolam. Hormones & Behavior, 52, 267-273. [ Links ]
Alves, S. H., Pinheiro, G., Motta, V., Landeira-Fernandez, J. & Cruz, A. P. (2004). Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into ventral but not dorsal hippocampus. Behavioural Pharmacology, 1, 37-43. [ Links ]
Arce, E. & Santisteban, C. (2006). Impulsivity: a review. Psicothema, 1, 213-220. [ Links ]
Bambico, F. R., Nguyen, N. T. & Gobbi, G. (2009). Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. European Neuropsychopharmacology, 1, 215-228. [ Links ]
Bekris, S., Antoniou, K., Daskas, S. & Papadopoulou-Daifoti, Z. (2005). Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behavioural Brain Research, 16, 45-59. [ Links ]
Bizot, J., Le Bihan, C., Puech, A. J., Hamon, M. & Thiebot, M. (1999). Serotonin and tolerance to delay of reward in rats. Psychopharmacology, 14, 400-412. [ Links ]
Bondi, C. O., Rodriguez, G., Gould, G. G., Frazer A. & Morilak, D. A. (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology, 3, 320-331. [ Links ]
Botelho, S., Estanislau, C. & Morato, S. (2007), Effects of under- and overcrowding on exploratory behavior in the elevated plus-maze. Behavioural Processes, 7, 357-362. [ Links ]
Braida D. & Sala, M. (2002). Role of the endocannabinoid system in MDMA intracerebral self-administration in rats. British Journal of Pharmacology, 136, 1089-1092. [ Links ]
Callaway, C. W. & Geyer, M. A. (1992). Stimulant effects of 3,4- methylenedioxy-methamphetamine in the nucleus accumbens of rat. European Journal of Pharmacology, 214, 45-51. [ Links ]
Cardenas, F., Lamprea, M. R. & Morato, S. (2001). Vibrissal sense is not the main sensory modality in rat exploratory behavior in the elevated plus-maze. Behavioral Brain Research, 12, 169-174. [ Links ]
Carobrez, A. P. & Bertoglio, L. J. (2005). Ethological and temporal analyses of anxiety-like behavior:the elevated plus-maze model 20 years on. Neuroscience & BiobehavioralReviews, 2, 1193-1205. [ Links ]
Colado, M. I., Camarero, J., Mechan, A. O., Sanchez, V., Esteban, B., Elliott, J. M. & Green, A. R. (2001). A study of the mechanisms involved in the neurotoxic action of 4-methylenedioxymethamphetamine (MDMA, 'ecstasy') on dopamine neurones in mouse brain. British Journal of Pharmacology, 13, 1711-1723. [ Links ]
Cruz, A. P., Frei, F. & Graeff, F. G. (1994). Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacology, Biochemistry, & Behavior, 4, 171-176. [ Links ]
Cunningham, J. I.; Raudensky, J.; Tonkiss, J. & Yamamoto, B. K. (2009). MDMA pretreatment leads to mild chronic unpredictable stress-induced impairments in spatial learning. Behavioral Neuroscience, 12, 1076-1084. [ Links ]
D'Aquila, P. S.; Brain, P. & Willner, P. (1994). Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiology & Behavior, 5, 861-867. [ Links ]
Dalley, J. W., Theobald, D. E., Pereira, E. A., Li, P. M. & Robbins, T. W. (2002). Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology, 16, 329-340. [ Links ]
Dos Santos, J. Jr, de Andrade, T. G. & Graeff, F. G. (2010). Social separation and diazepam withdrawal increase anxiety in the elevated plus-maze and serotonin turnover in the median raphe and hippocampus. Journal of Psychopharmacology, 2, 725-731. [ Links ]
Dos Santos, J. Jr, de Andrade, T. G. & Zangrossi, J. H. (2008). 5-HT1A receptors in the dorsal hippocampus mediate the anxiogenic effect induced by the stimulation of 5-HT neurons in the median raphe nucleus. European Neuropsychopharmacology, 1, 286-294. [ Links ]
Ducottet, C., Griebel, G. & Belzung, C. (2003). Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2, 625-631. [ Links ]
Dunn, R. W., Corbett, R. & Fielding, S. (1989). Effects of 5-HT1A receptor agonists and NMDA receptor antagonists in the social interaction test and the elevated plus maze. European Journal of Pharmacology, 16, 1-10. [ Links ]
Estanislau, C. & Morato, S. (2005). Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behavioural Brain Research, 16, 70-77. [ Links ]
Estanislau, C. & Morato, S. (2006). Behavior ontogeny in the elevated plus-maze: Prenatal stress effects. International Journal of Developmental Neuroscience, 2, 255-262. [ Links ]
Esteban, B., O'Shea, E., Camarero, J., Sánchez, V., Green, A. R. & Colado, M. L. (2001). 3,4-Methyle.nedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a periphe- rally injected neurotoxic dose. Pharmacology, 154, 251-260. [ Links ]
Fokos, S. & Panagis, G. (2010). Effects of Delta 9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. Journal of Psychopharmacology, 2, 767-777. [ Links ]
García, A. M., Cárdenas, F. P. & Morato, S. (2005). Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiology & Behavior, 8, 265-270. [ Links ]
García, A. M., Martínez, R., Brandao, M. L. & Morato, S. (2005). Effects of apomorphine on rat behavior in the elevated plus-maze. Physiology & Behavior, 8, 440-447. [ Links ]
Green, A. R., Cross, A. J. & Goodwin, G. M. (1995). Review ofthe pharmacology and clinical pharmacology of 4-methylenedioxymethamphetamine (MDMA or "Ecstasy"). Psychopharmacology, 11, 247-260. [ Links ]
Griebel, G., Rodgers, R. J., Perrault, G. & Sanger, D. J. (1997). Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacology, Biochemistry, & Behavior, 5, 817-827. [ Links ]
Harrison, A. A., Everitt, B. J. & Robbins, T. W. (1997). Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology, 13, 329-342. [ Links ]
Hata, T., Nishikawa, H., Itoh, E. & Funakami, Y. (2001). Anxiety-like behavior in elevated plus-maze tests in repeatedly cold-stressed mice. Japanese Journal of Pharmacology, 8, 189-196. [ Links ]
Iravani, M. M., Asari, D., Patel, J., Wieczorek, W. J. & Kruk, Z. L. (2000). Direct effects of 4-methylenedioxymethamphetamine (MDMA) on serotonin or dopamine release and uptake in the caudate putamen, nucleus accumbens, substantia nigra pars reticulata, and the dorsal raphe nucleus slices. Synapse, 3, 275-285. [ Links ]
Kakui, N., Yokoyama, F., Yamauchi, M., Kitamura, K., Imanishi, T., Inoue, T., et al. (2009). Anxiolytic-like profile of mirtazapine in rat conditioned feastress model: Functional significance of 5-hydro-xytryptamine 1A receptor and alpha1-adrenergic receptor. Pharmacology Biochemistry and Behavior, 9, 393-398. [ Links ]
Khan, A. & Haleem, D. J. (2006). 5-HT-1A receptor responsiveness following subchronic administration of buspirone. Pakistan Journal of Pharmaceutical Sciences, 1 , 333-337. [ Links ]
King, S. M. (1999). Escape-related behaviours in an unstable elevated and exposed environment. I. A new behavioural model of extreme anxiety. Behavioural Brain Research, 9, 113-126. [ Links ]
Li, H., Zhang, L., Fang, Z., Lin, L., Wu, C. & Huang, Q. (2010). Behavioral and neurobiological studies on the male progeny of maternal rats exposed to chronic unpredictable stress before pregnancy. Neuroscience Letters, 46, 278-282. [ Links ]
Lin, H. Q., Burden, P. M., Christie, M. J. & Johnston, G. A. (1999). The anxiogenic-like and anxiolytic-like effects of MDMA on mice in the elevated plusmaze: A comparison with amphetamine. Pharmacology, Biochemistry, & Behavior, 6, 403-408. [ Links ]
Maldonado, E. & Navarro, J. F. (2000). Effects of 4-methylenedioxy-methamphetamine (MDMA) on anxiety in mice tested in the light-dark box. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2, 463-472. [ Links ]
Martínez, J. C., Cárdenas, F., Lamprea, M. & Morato, S. (2002). The role of vision and proprioception in the aversion of rats to the open arms of an elevated plus-maze. Behavioural Processes, 6, 15-26. [ Links ]
Martínez, R. C., García, A. M., Lamprea, M. R. & Morato, S. (2007). Thermal stress decreases general motor activity of rats in the elevated plus-maze but does not alter aversion to the open arms. Behavioural Brain Research, 18, 135-139. [ Links ]
Matuszewich, L., Filon, M. E., Finn, D. A. & Yamamoto, B. K. (2002). Altered forebrain neurotransmit-ter responses to immobilization stress following 4-methylenedioxymethamphetamine. Neuroscience, 11, 41-48. [ Links ]
Matuszewich, L., Karney, J. J., Carter, S. R., Janasik, S. P., O'Brien, J. L. & Friedman, R. D. (2007). The delayed effects of chronic unpredictable stress on anxiety measures. Physiology & Behavior, 9, 674-681. [ Links ]
Mitra, R., Vyas, A., Chatterjee, G. & Chattarji, S. (2005). Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neuroscience Letters, 38, 278-283. [ Links ]
Mlinar, B. & Corradetti, R. (2003). Endogenous 5-HT, released by MDMA through serotonin transporter- and secretory vesicle-dependent mechanisms, reduces hippocampal excitatory synaptic transmission by preferential activation of 5-HT1B receptors located on CA1 pyramidal neurons. European Journal of Neuroscience, 1, 1559-1571. [ Links ]
Morato, S. & Castrechini, P. (1989). Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Brazilian Journal of Medical & Biological Research, 2, 707-710. [ Links ]
Morley, K. C. & McGregor, I. S. (2000). -3,4-Methylenedioxymethamphetamine (MDMA, "ecstasy") increases social interaction in rats. European Journal of Progress in Neuro-Psychopharmacology & Biological Psychiatry, 2, 1151-1154. [ Links ]
Ossowska, G., Nowa, G., Kata, R., Klenk-Majewska, B., Danilczuk, Z. & Zebrowska-Lupina, I. (2001). Brain monoamine receptors in a chronic unpredictable stress model in rats. Journal of Neural Transmission, 10, 311-319. [ Links ]
Paxinos, G. & Watson, C. (2007). The rat brain in stereotaxic coordinates. New York: Academic Press. [ Links ]
Papp, M., Moryl, E. & Willner, P. (1996). Pharmacological validation of the chronic mild stress model of depression. European Journal of Pharmacology, 29, 129-136. [ Links ]
Setem, J., Pinheiro, A. P., Motta, V. A., Morato, S. & Cruz, A. P. (1999). Ethopharmacological analysis of 5-HT ligands on the rat elevated plus-maze. Pharmacology Biochemistry and Behavior, 6, 515-521. [ Links ]
Silva, M. T., Alves, C. R. & Santarem, E. M. (1999). Anxiogenic-like effect of acute and chronic fluoxetine on rats tested on the elevated plus-maze. Brazilian Journal of Medical and Biological Research, 3, 333-339. [ Links ]
Silva, R. C. & Brandao, M. L. (2000). Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: An ethological analysis. Pharmacology Biochemistry and Behavior, 6, 209-216. [ Links ]
Vaidya, A. H., Rosenthal, D. I., Lang, W., Crooke, J. J., Benjamin, D., Ilyin, S. E. & Reitz, A. B. (2005). Oral buspirone causes a shift in the dose-response curve between the elevated-plus maze and Vogel conflict tests in Long-Evans rats:relation of brain levels of buspirone and 1-PP to anxiolytic action. Methods and Findings in Experimental and Clinical Pharmacology, 2, 245-255. [ Links ]
Vyas, A. & Chattarji, S. (2004). Modulation of different states of anxiety-like behavior by chronic stress. Behavioral Neuroscience, 11, 1450-1454. [ Links ]
Zhang, Y., Raap, D. K., García, F., Serres, F., Ma, Q., Battaglia, G. & Van de Kar, L. D. (2000), Longterm fluoxetine produces behavioral anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Research, 85, 58-66. [ Links ]