Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Earth Sciences Research Journal
versão impressa ISSN 1794-6190
Earth Sci. Res. J. v.15 n.2 Bogotá jul./dez. 2011
Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Madhuranthakam, Tamil Nadu, India
K. Brindha and L. Elango
Department of Geology, Anna University, Chennai- 600 025, India. Corresponding author. E-mail: elango34@hotmail.com, elango@annauniv.edu
Record
Manuscript received: 10/03/2011 Accepted for publications: 24/11/2011
ABSTRACT
Hydrochemical study was carried out in Madhuranthakam located near Chennai in Tamil Nadu, India with an objective of understanding the suitability of local groundwater quality for domestic and irrigation purposes. Twenty groundwater samples were collected in February 2002 and analysed for physical and chemical parameters. groundwater in this area was found to be within the desirable Bureau of Indian Standards and World Health Organisation limits for drinking water. Ca-HCO3 was the dominant groundwater type. groundwater in this area was assessed for irrigation purposes on the basis of sodium percentage (Na%), magnesium hazard (MH), residual sodium carbonate (RSC), sodium absorption ratio (SAR), permeability index (PI) and United States department of Agriculture (USDA) classification. Most of the groundwater samples were suitable for irrigation, except in a few locations (15%) based on MH. Overall the groundwater quality was suitable for drinking and domestic purposes and permissible for irrigation activities.
Keywords: groundwater, Madhuranthakam, drinking water quality, irrigation water quality, Tamil Nadu, India.
RESUMEN
Un estudio Hidroquímico fue llevado en la localidad Madhuranthakam cerca Chennai en Tamil Nadu, India, con el propósito de evaluar la calidad de las aguas subterráneas locales para usos domésticos y fines de riego. Veinte muestras de agua subterránea fueron recogidas y analizadas en términos de parámetros físicos y químicos en Febrero 2002. Las aguas subterráneas en esta área fueron encontradas aptas como potables según los limites sugeridos por el Consejo de Estándares de India y los límites permisibles por la Organización Mundial de la Salud para el agua potable. Ca-HCO3 es dominante en las aguas subterráneas. Las aguas subterráneas en esta área fueron asignadas para propósitos de riego sobre la base del porcentaje de sodio (Na%), magnesio peligroso (MH), carbonato sodico residual (RSC), proporción de absorción de sodio (SAR), índice permeabilidad (PI) y la clasificación del departamento de Agricultura de los Estados Unidos (USDA). La mayoría de las muestras de las aguas subterráneas son aptas para el riego, excepto en unas pocas ubicaciones (15%), basadas sobre MH. En general, la calidad del agua subterránea fue apta para el consumo, uso doméstico y permisible para las actividades de riego.
Palabras claves: Agua Subterraneas, Madhuranthakam, calidad de agua potable, calidad de agua de riego, Tamil Nadu, India.
Introduction
groundwater is a precious source of fresh water, being the most distributed form on the earth, excluding the polar icecaps and glaciers. groundwater studies are gaining more importance in the present day as it is used for almost all purposes such as domestic, industrial and agricultural activities in most parts of the world. Improper management of this replenishable resource may lead to groundwater contamination and scarcity. When some ions and minerals are present beyond the permissible limit, they become unsuitable for drinking and irrigation purposes, which may be due to natural and also anthropogenic causes. groundwater quality has been given lot of importance and studied worldwide (Lahermo and Back-man, 1999; Omo-Irabor et al., 2008; Baalousha, 2010).
Several regions in India have encountered degradation in ground-water quality too, due to rapid urbanisation and an exponential increase in population (Ramesh and Elango, 2005; Brindha and Elango, 2010; Brindha et al., 2011). The present study was carried out with the aim of understanding groundwater quality and its suitability for drinking and irrigation purposes in Madhuranthakam, located near Chennai in Tamil Nadu, India. groundwater is the major source for domestic and irrigation practices in this area. There has been an increase in the demand for groundwater due to the growth of the local population.
Groundwater is usually put to direct use in rural areas without proper monitoring and treatment. groundwater may also become contaminated by the agrochemical products used for irrigation. The groundwater quality in the nearby regions, namely Chennai, Kancheepuram and Chengalpet, has been studied earlier (Elango and Manickam, 1987; Ramesh, 1999; Rajmohan et al., 2000; Elango et al., 2003a; Elango et al., 2003b; Kumaresan and Riyazuddin, 2006). However, no studies have been carried out in the Madhuranthakam region pertaining to groundwater quality. The suitability of groundwater for domestic and irrigation purposes thus had to be determined based on the presence of major ions in the groundwater of this region. The present study, which was carried out in 2002, will serve as baseline data for comparing future groundwater quality.
Study area
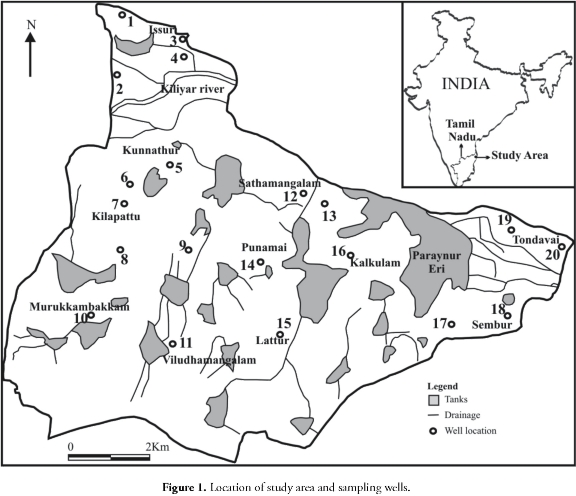
The study area is situated 76 km south of Chennai city and forms a part of Madhuranthakam taluk, Kancheepuram district, Tamil Nadu, India (Figure 1). Most of the annual rainfall of 1,206 mm is received from October to December and the rest during the southwest monsoon season from June to September. Climatic condition in the study area varies from 39oC to 40oC during summer (April to June) and 20oC to 24oC during winter (October to December). The study area is surrounded by a number of tanks which are used for drinking and agricultural purposes. This area is intensively cultivated by pumping groundwater from dug wells as well as surface water resources. geologically this area is characterised by charnockites of Precambrian era. Outcrops of these rocks are found in many parts of the study area. Sandy clay and clayey sand overlay the charnockites with thickness ranging from 1.5 to 5m. groundwater occurs under unconfined condition mainly in the weathered and fractured part of this charnockites in this area.
Sampling and analysis
A well inventory survey was carried out in February 2002 to obtain background information about the well population, lithology, well use etc. Forty-seven wells were investigated and 20 wells (Figure 1) were chosen as representative wells for groundwater sampling based on electrical conductivity (EC). The groundwater samples were collected in clean 500 ml polyethylene bottles. The sampling bottles were soaked in 1:1 diluted HCl solution for 24 hours and washed twice with distilled water before sampling. They were washed again in the field with groundwater sample filtrates.
The groundwater samples were collected from bore wells after pumping the water for about 10 minutes. Field parameters such as EC and pH were measured in the field using portable digital meters. Samples were analysed for major ions in the laboratory using the standard recommended methods (APHA, 1998). Sulphate concentration in the groundwater samples was analysed using a UV visible spectrophotometer. Sodium and potassium content was determined by using a flame photometer and calcium, magnesium, chloride, carbonate and bicarbonate by titration technique. Total ions measurement precision was checked by calculating the ion balance error (IBE). The IBE was within ±10%. Total dissolved solids (TDS) were calculated by using the formula: TDS (mg/l) = EC (µS/cm) x 0.64; total hardness (TH) was calculated by using: TH = 2.497Ca + 4.115Mg in mg/l.
Results and Discussion
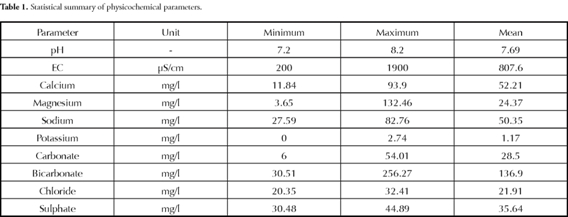
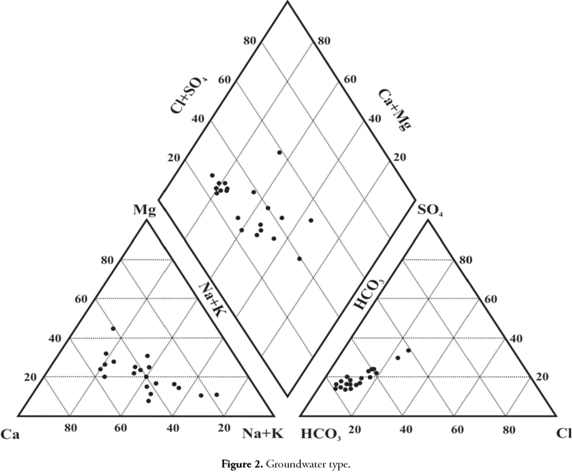
Table 1 shows the maximum, minimum and mean concentrations of various parameters. The order of cation dominance was Ca2+>Na+>Mg2+>K+ and HCO3>SO42->CO32->Cl- for anions. The general chemical nature of groundwater can be understood by plotting major cation and anion concentrations on a Piper trilinear diagram (Piper, 1944). Ca-HCO3 was the major water type dominant in this area (Figure 2). The next dominant water type was mixed Ca-Na-HCO3.
Drinking water quality
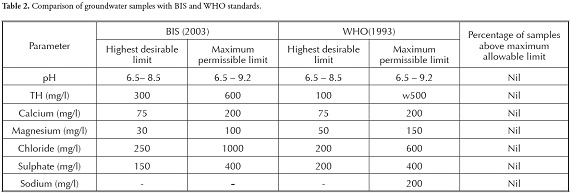
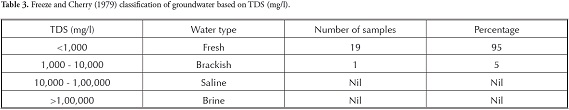
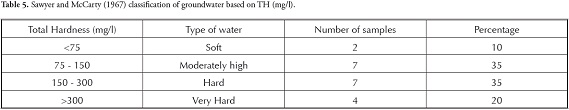
groundwater used for domestic purposes, such as drinking and cooking, should be free from toxic chemicals and pathogens. Domestic water quality indicates that a particular parameter at a given concentration may be suitable for the human body beyond which it is unsuitable. The concentration of various ions in the groundwater samples was compared with Bureau of Indian standards (BIS, 2003) and World Health Organisation (WHO, 1993) standards, which are given in Table 2 wherein all the groundwater samples were found to be within the suitable limits. The groundwater in this area was thus seen to be fit for domestic consumption, based on the major ion analysis carried out in this study. The groundwater samples were classified regarding TDS and TH (Tables 3, 4 and 5). Most of the groundwater was fresh and suitable for drinking purposes based on TDS (Freeze and Cherry, 1979; Davis and DeWeist, 1966). groundwater in this study area varies from soft to very hard (Sawyer and McCarty, 1967).
Irrigation water quality
good quality irrigation water is essential for achieving maximum crop productivity. groundwater suitability for irrigation purpose in this study area was assessed using sodium percentage (Na%), magnesium hazard (MH), residual sodium carbonate (RSC), sodium absorption ratio (SAR), permeability index (PI) and United States Department of Agriculture (USDA) classification. groundwater in most of the study area was found to be suitable for irrigation.
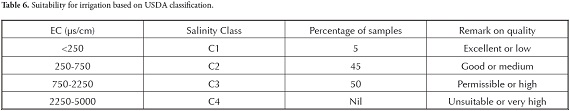
Irrigation water having high EC content will affect root area and water flow. groundwater in this area was grouped according to the guidelines established by the United States Salinity Laboratory (Freeze and Cherry, 1979) based on EC (Table 6). This showed that groundwater in this area
was permissible for irrigation. Sodium is an important parameter for irrigation water and is denoted as Na% which was calculated from the formula given in equation 1 (Wilcox, 1955) and all concentrations were expressed in meq/l.
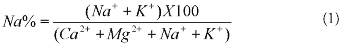
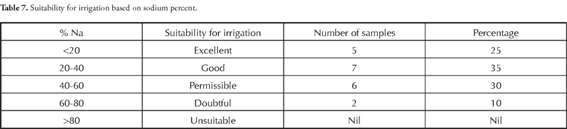
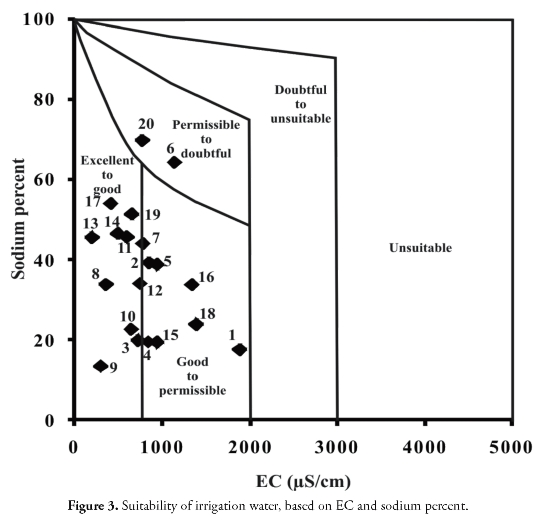
The Na% in this area is given in Table 7. groundwater was suitable for irrigation in 60% of the samples while 40% were permissible to doubtful. EC and Na% are plotted in Figure 3 which showed that most of the groundwater samples were good for agriculture.
The concentration of bicarbonate and carbonate higher than calcium and magnesium will influence the suitability of water for irrigation purposes. The RSC value was computed using the following formula (equation 2) where ions were expressed in meq/l.
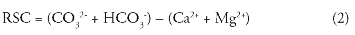
However, regarding RSC, all samples fall within the safe category for irrigation.
SAR is another important parameter for determining the desirability of irrigation water. The SAR values were calculated by using equation 3 (Richards, 1954),
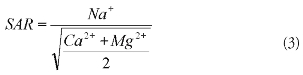
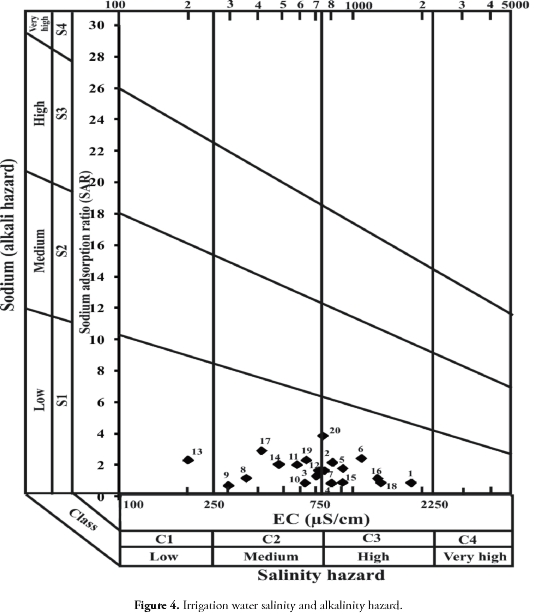
where all the concentrations were given in meq/l. All the groundwater samples collected from this area were excellent on the basis of SAR. Water used for irrigation can be classified into four types- C1, C2, C3 and C4 based on salinity hazard and S1, S2, S3 and S4 based on sodium hazard. Figure 4 shows the plot of groundwater samples grouped on the above basis. Most groundwater samples fall under C1S1 and C2S1 which are suitable for irrigation. Comparatively few samples were grouped under C3S1 (which is permissible for irrigation). Thus the groundwater in this area was seen to be suitable for irrigation based on salinity hazard and sodium hazard.
MH for irrigating water was calculated using the formula MH = Mg/ (Ca + Mg) x 100, where concentrations were given in meq/l (Szabolcs and Darab, 1964). MH above 50 is considered to be unsuitable for irrigation. groundwater had an MH above 50 in 15% of the samples, thus not being fit for irrigation.
The suitability of groundwater for irrigation based on PI was calculated using equation 4,
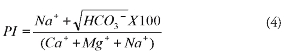
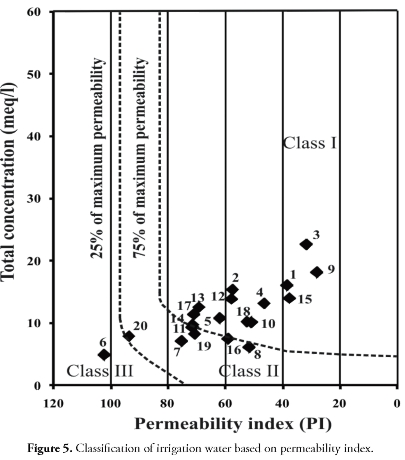
where concentrations were in meq/l. Class I and class II waters are considered to be good and suitable for irrigation while class III water is unsuitable for irrigation (Doneen, 1964). Figure 5 shows that one ground-water sample was not suitable for irrigation based on PI whereas the rest of the samples were good. In general, the groundwater fall within the permissible category for irrigational use, except for a few locations where it was unsuitable based on MH.
Conclusion
groundwater quality of an area must be studied to understand its suitability for domestic and irrigation purposes. All the groundwater samples collected from the Madhuranthakam area, Tamil Nadu, India, showed that the major ions fall within the permissible range. The dominant groundwater type was Ca-HCO3. Based on TDS, 95% of the groundwater was fresh and permissible for drinking. The groundwater varied from soft to very hard on the basis of TH. The suitability of groundwater for irrigation was assessed from Na%, EC, RSC, SAR, MH, PI and USDA classification. The groundwater in this area was seen to be good and suitable for drinking and domestic purposes. However, the groundwater was unsuitable for irrigation in a few places, based on MH. Overall, groundwater in the Madhuranthakam area remains usable. The present groundwater quality status must be maintained by taking precautionary measures such as rainwater harvesting, less use of chemical fertilisers and the ongoing monitoring of groundwater quality in this region.
Acknowledgements
The authors would like to thank the Department of Science and Technology-Funds for Improvement in Science and Technology (DST-FIST) (grant No. SR/FST/ESI-106/2010) and the University grants Commission-Special Assistance Programme (UGC-SAP) (grant No. UGC DRS II F.550/10/DRS/2007(SAP-1)) for providing financial support which helped in creating facilities to carry out this work.
References
APHA (1998). (1998). Standard methods for the examination of water and wastewater, 20th edn, American Public Health Association, Washington DC, USA. [ Links ]
Baalousha, H. (2010). Assessment of a groundwater quality monitoring network using vulnerability mapping and geostatistics: A case study from Heretaunga Plains, New Zealand, Agricultural Water Management. 97, 240-246. [ Links ]
BIS (2003). Bureau of Indian Standards Specification for drinking water, IS: 10500:91. Revised 2003, Bureau of Indian Standards, New Delhi. [ Links ]
Brindha, K. and Elango L. (2010). Study on bromide in groundwater in parts of Nalgonda district, Andhra Pradesh, Earth Science India, 3, no. 1, 73-80. [ Links ]
Brindha, K., Rajesh, R., Murugan, R. and Elango, L. (2011). Fluoride contamination in groundwater in parts of Nalgonda district, Andhra Pradesh, India, Environmental Monitoring and Assessment, 172, 481-492. [ Links ]
Davis, S. N. and DeWiest, R. J. (1966). Hydrogeology, Wiley, New York. [ Links ]
Doneen, L. D. (1964). Water quality for Agriculture, Department of Irrigation, University of Calfornia, Davis, 48 pp. [ Links ]
Elango, L. and Manickam, S. (1987). Hydrogeochemistry of the Madras aquifer, India - Spatial and temporal variation in chemical quality of groundwater, geological Society of Hong Kong Bulletin, no. 3, 525-534. [ Links ]
Elango, L., Kannan, R. and Senthilkumar, M. (2003a). Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram district, Tamil Nadu, India, Environmental geosciences, 10, no. 4, 1-10. [ Links ]
Elango, L., Kumar, S. S. and Rajmohan, N. (2003b). Hydrochemical studies of groundwater in Chengalpet region, Indian Journal of Environmental Protection, 23, no. 6, 624-632. [ Links ]
Freeze, R. A. and Cherry, J. A. (1979). groundwater, Prentice Hall Inc. Englewood Cliffs, New Jersey, 604 pp. [ Links ]
Kumaresan, M. and Riyazuddin, P. (2006). Major ion chemistry of environmental samples around sub-urban of Chennai city, Current Science, 91, no. 12, 1668-1677. [ Links ]
Lahermo, P. and Backman, B. (1999). Nitrates in groundwater in Finland: the most endangering quality problem, Hydrogeology and Land Use Management, 329-333. [ Links ]
Omo-Irabor, O.O., Olobaniyi, S.B., Oduyemi K. and Akunna, J. (2008). Surface and groundwater water quality assessment using multivariate analytical methods: A case study of the Western Niger Delta, Nigeria, Physics and Chemistry of the Earth, Parts A/B/C, 33, no. 8-13, 666-673. [ Links ]
Piper, A. M. (1944). A graphical procedure in the geochemical interpretation of water analysis, Transactions- American geophysical Union, 25, 914-928. [ Links ]
Rajmohan, N., Elango, L., Ramachandran, S. and Natrajan, M. (2000). Major ion correlation in groundwater of Kancheepuram region, south India, Indian journal of Environmental Protection, 20, no.3, 188-193. [ Links ]
Ramesh, K. and Elango, L. (2005). groundwater quality assessment in Tondiar basin, Indian Journal of Environmental Protection, 26, no. 6, 497 -504. [ Links ]
Ramesh, R. (1999). groundwater quality management: pollution perspectives impacts of urban growth on surface water and groundwater quality, Proceedings of IUGG 99 Symposium HS5, Birmingham, July, IAHS Publ. no. 259, 47-55. [ Links ]
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils, US Department of Agriculture, Hand Book 60. [ Links ]
Sawyer, g. N. and McCarty, D. L. (1967). Chemistry of sanitary engineers, 2nd edn, McGraw Hill, New York, 518 pp. [ Links ]
Szabolcs, I. and Darab, C. (1964). The influence of irrigation water of high sodium carbonate content of soils, Proceedings of 8th ISSS, Trans vol. II , 802-812. [ Links ]
WHO (1993). guidelines for drinking water quality, vol. 1, 2nd edn, Recommendations, WHO, geneva, 130 pp. [ Links ]
Wilcox, L. V. (1955). Classification and use of irrigation waters, USDA, circular 969, Washington DC, USA. [ Links ]