Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.16 no.2 Bogotá July/Dec. 2012
The impacts of alkaline mine drainage on Ba, Cr, Ni, Pb and Zn concentration in the water resources of the Takht coal mine, Iran
Behnaz Dahrazma1* and Mehdi Kharghani2
1* Environmental Geology Department, Earth Science Faculty, Shahrood University of Technology, P.O. Box 316, Shahrood, 36199-95161, Iran.
E-mail: behnaz_dahrazma@yahoo.com
2 Mining Department, Islamic Azad University Shahrood branch, Shahrood, Iran. E-mail: kharghani.m@gmail.com
* Corresponding author: Behnaz Dahrazma, Environmental Geology Department, Earth Science Faculty, Shahrood University of Technology, P.O.
Box 316, Shahrood, 36199-95161, Iran. Phone: + 98 (912) 373 9562 Fax: +98 (273) 339 6007. E-mail: behnaz_dahrazma@yahoo.com
Record
Manuscript received: 17/02/2012 Accepted for publications: 06/05/2012
ABSTRACT
The release of heavy metals into the environment represents one of the most important environmental effects involved in extracting coal; it needs to be studied more fully. The present research investigated the effects of coal-mining in an alkaline environment and alkaline mine drainage in the Takht coal mine regarding the distribution of selected heavy metals (Zn, Pb, Ni, Cr and Ba) on the region’s surface and ground water. The mine is located 12 Km southeast of Minoodasht, in Golestan province in northern Iran. Samples were collected from groundwater and surface water resources upstream and downstream of the mine. The elements’ concentrations were measured by the inductively-coupled mass spectrometry (ICPMS) method. The results showed that an alkaline environment was responsible for producing alkaline mine drainage due to the presence of limestone; this caused high pH (8.41) in the area’s groundwater resources. Mining activities increased Ba, Cr, Ni, Pb and Zn concentration in the groundwater from 3.39, 0.5, 0.2, 0.5, 9.2 ppb to 83.52, 2.2, 0.6, 2.6, 48.3 ppb and from 68.7, 0.5, 1.3, 0.8, 172.6 ppb to 91, 1.2, 4.5, 1.3, 27.6 ppb in surface water, respectively. Due to the basic environment, heavy metal accumulation in the bed sediment for both tunnel effluents and runoffs was higher than during the soluble phase. pH was the main controlling factor in elements’ solubility and their distribution in the environment. Increased Ba concentration in water resources was due to high Ba concentration in the coal, coal tailing and in quarry tailings.
Keywords: heavy metals, groundwater, surface water, sediment, alkaline mine drainage, coal-mining.
RESUMEN
La liberación de metales pesados en el medio ambiente representa uno de los efectos ambientales más importantes en la extracción de carbón, lo cual necesita ser estudiado con mayor profundidad. El presente estudio investigó los efectos de la minería del carbón en un ambiente alcalino y en el drenaje de la mina de carbón Takht, con respecto a la distribución de determinados metales pesados (Zn, Pb, Ni, Cr y Ba) en superficie y en las aguas subterráneas de la región. La mina está ubicada a 12 km al sureste de Minoodasht, en la provincia de Golestán en el norte de Irán. Se recogieron muestras de aguas subterráneas y aguas superficiales aguas arriba y aguas abajo de la mina. Las concentraciones de los elementos se midieron mediante la espectrometría de masa de acoplamiento inductivo (ICP-MS). Los resultados indican que un ambiente alcalino produjo drenaje de minas alcalinas debido a la presencia de la piedra caliza; esto causo altos niveles de pH (8,41) en las fuentes de aguas subterráneas de la zona. La actividad minera incrementó los niveles de concentración de Ba, Cr, Ni, Pb y Zn en las aguas subterráneas de 3,39, 0,5, 0,2, 0,5, 9,2 ppb a 83,52, 2,2, 0,6, 2,6, 48,3 ppb y de 68,7, 0,5, 1,3, 0,8, 172,6 ppb a 91, 1,2, 4,5, 1,3, 27,6 ppb en el agua de la superficie. Debido al ambiente básico, la acumulación de metales pesados en el lecho sedimentario, tanto para los efluentes del tunel como para las aguas de escorrentia, fueron mayores que durante la fase soluble. El pH fue el principal determinante en la solubilidad de los elementos y su distribución en el medio ambiente. El aumento de la concentración de Ba en los recursos hídricos se debió a la alta concentración de Ba en el carbón, carbón de relaves y colas en cantera.
Palabras claves: metales pesados , aguas subterráneas, aguas superficiales, sedimentos, drenaje alcalino en minería, minería de carbón.
Introduction
Coal is a heterogeneous material which is formed through plant decomposition in a variety of conditions regarding moisture, temperature and pressure. The diversity of elements in coal’s compositions as well as the complex process of the coal forming are responsible for the existence and accumulation of light elements (e.g. Li, Be), non-metallic elements (e.g. Se, As, Bi) and heavy metals (e.g. Cu, Pb, Co, Cr) in coal. These elements can be released into the environment during coal extraction, its preparation, processing, transportation and combustion (mainly in power plants) thereby causing environmental pollution (Finkelman et al. ., 2002; Swaine and Goodarzi, 1995; Yiwei et al.., 2007).
Geochemical characteristics of coal in the Alborz region of Iran have been thoroughly investigated. The findings have shown that aluminium silicates and sulphide minerals are heavy metals’ main hosts. According to the chemical and physical weathering of excavated materials (coal and its wastes), elements are released from various complexes and then transported or precipitated due to their geochemical properties. After tunnel effluent transportation from a reduced to an oxidised environment, the elements’ oxidation state becomes changed and some heavy metals become precipitated (i.e. Pb, Ni and Zn). Reaction oxidation and reduction potential, pH, colloid materials in transporting media and transferred materials’ ion potential are the main factors controlling materials’ transportation and precipitation (Northeast Alborz Mining Co., 2009).
The Takht coal mine is located at 37° 2’ 10- N and 37° 10’ 35- N latitude and 55° 22’ 30- E and 55° 27’ 40- E longitude, lying 12 km southeast of Minoodasht, Golestan, in Iran (Fig. 1). Extraction started from 2001 onwards with horizontal tunnels being bored on the south side of the area (Minoodasht Water Office, 2003a). Summer in the area is warm and humid whilst the winter involves a large amount of precipitation, mostly in the form of snow in the mountainous areas. The temperature varies from -10°C to 35°C. The basin’s annual average rainfall is about 500 mm. Runoff feeds the Chehel-Chay River which is located downstream of the mine (Minoodasht Water Office, 2003b).
This research investigated the effects of alkaline mine drainage on heavy metals’ distribution (namely Ba, Cr, Ni, Pb and Zn) on groundwater and surface water in the vicinity of the Takht coal mine in Minoodasht, Iran, regarding environmental concerns.
Materials and methods
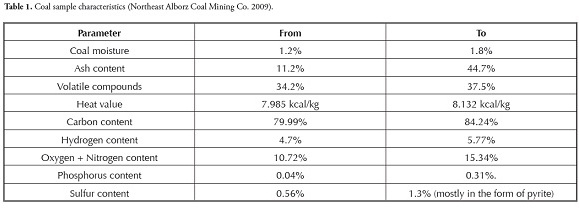
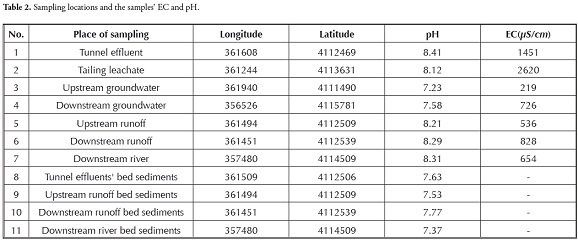
Triplicate samples were collected from coal, coal and quarry tailings tunnel effluent, tailing leachate and surface and ground water as well as bed sediments from tunnel effluent and runoff to assess the effects of coal mining on heavy metals’ distribution in surface and ground water. Sampling was done in October 2009 at the end of the dry season to find the worst conditions. Table 1 gives the coal samples’ characteristics. Sediments were air dried at room temperature and passed through #80 mesh (180 µm opening size) to measure the heavy metals’ concentrations. Samples were analysed at the ACME Lab, Vancouver, Canada using inductively-coupled mass spectrometry (ICP-MS) techniques. The water’s pH and EC were measured on site using a Jenway pH meter (model 3510) and Jenway EC meter, respectively. For the sediments, pH was measured referring to the EPA SW-846 Method 9045. Table 2 shows sampling locations, pH and EC.
Results and Discussion
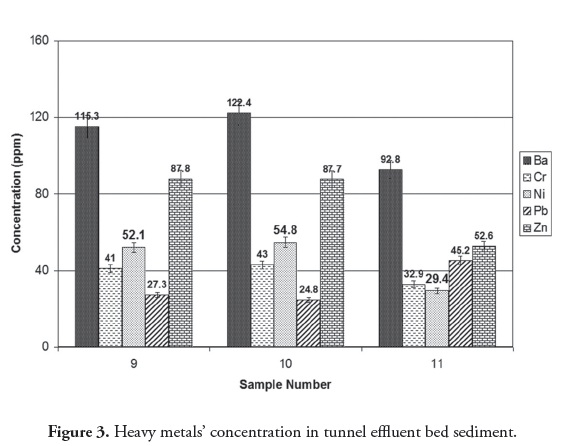
Alkaline mine drainage has resulted from the neutralisation of acidic mine drainage (AMD) by carbonate minerals and low grade pyrite rocks. The basic tunnel effluent (pH 8.4) indicated the presence of alkaline mine drainage (Table 2) due to the highly alkaline environment. This alkalinity was responsible for the sedimentation of most elements in tunnel effluent bed sediments.
EC tended to increase downstream of the mine which could have been due to the high concentration of heavy metals and ions. Sample 4 (taken from groundwater 7 km downstream of the mine) had an EC which was 3.3 times higher than that of sample 3 (taken from groundwater upstream of the mine). This increase could have resulted from tunnel effluent and tailing leachate entering the environment (1,451 and 2,620 µS/cm EC, respectively) and causing an increased EC in surface water (from 536 µS/cm in upstream runoff to 828 µS/cm in downstream runoff).
A rise in surface and ground water pH was also observed (Table 2). The results indicated that sulphate concentration in surface water increased from zero to 48 mg/L due to pyrite oxidation and sulphate complex entering the environment which facilitated the retention of heavy metals in solution phase. The present research’s findings agreed with Anderson et al.., (2005) who noted that concentrations of sulphate, iron, manganese and other constituents can increase, even in the presence of alkaline mine drainage.
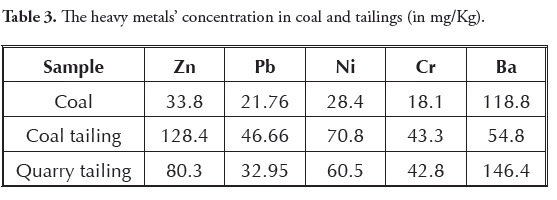
Coal, coal tailing and quarry tailing analysis revealed high concentrations of heavy metals (especially Ba) in all three contaminant sources. Comparing these three contaminant sources indicated that coal tailings had the maximum Zn, Pb, Ni and Cr concentrations while maximum Ba concentration occurred in quarry tailings (Table 3). Washing and the consequent leaching of coal, coal tailings and quarry tailings were the main factors for contaminants entering water.
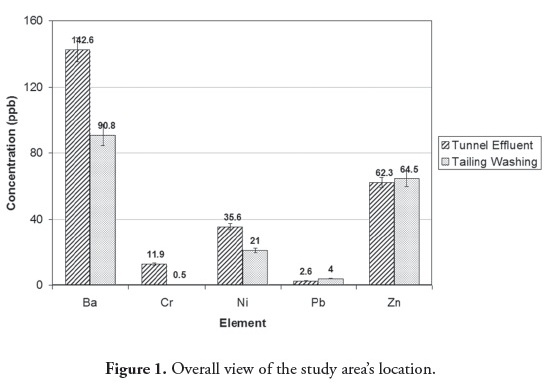
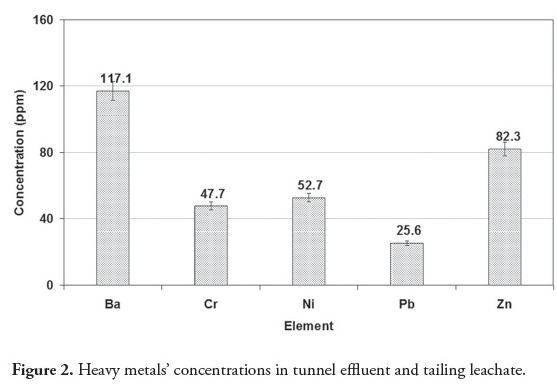
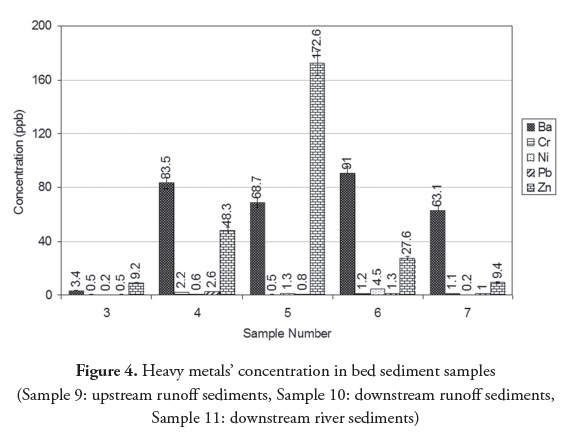
Tunnel effluent and drainage of tailing washing were the main sources of pollution, containing heavy metals Ba, Cr, Ni, Pb and Zn. Ba had the highest concentration due to its high solubility in an alkaline environment (Figure 2). Barium also had the maximum concentration in tunnel effluent bed sediment compared to the other heavy metals (Figure 3). Regarding pH 8.4, the heavy metals in the bed sediment had much higher concentrations than in the tunnel effluent. In other words, heavy metals’ concentrations in solid phase were higher than in the aqua phase when pH was 8.4 (Figures (Fig. 2) Fig. 3 , (Table 2)). Figure 4 shows that heavy metals’ concentration in bed sediments from upstream and downstream runoff had the following order: Ba > Zn > Ni > Cr > Pb. Ion exchange, adsorption and complexation are the main known mechanisms for heavy metals’ accumulation in sediment (Dahrazma and Mulligan, 2007). Smith (1999) mentioned that increased pH enhances cation sorption and decreases anion sorption. The high Ba concentration in upstream runoff bed sediment could have been due to a carbonated environment and Ca exchange with Ba; this finding agreed with that of Hem (1985). Concentrations of heavy metals in downstream river bed sediments (except for Pb) became decreased, due to drained water becoming diluted in the river.
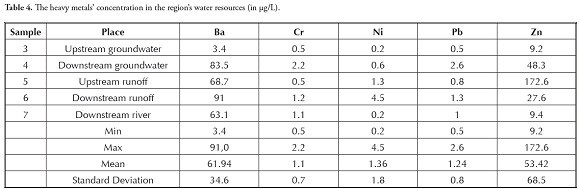
Contaminants entering the environment through mining activities (as mentioned above) changed the area’s heavy metals’ distribution in groundwater and surface water (Table 4). Ba concentration in downstream groundwater was 24.6 times greater than upstream due to high Ba concentration in tunnel effluent (142.6 mg/L) and tailing leachate (90.8 mg/L). Mining activities also caused increased Ba concentration in downstream runoff compared to upstream (Table 4).
The results showed that Cr, Ni, Pb and Zn concentrations in groundwater increased 4.4, 2, 5.3, and 5.5 times, respectively, from upstream to downstream. In terms of runoff, Cr, Ni and Pb concentrations from 0.5, 1.3 and 0.8 upstream reached 1.2, 4.5 and 1.3 (all in µg/L) downstream, respectively. Zinc did not increase in downstream runoff (comparing Figure 2 and (Table 4)) since its concentration in tunnel effluent and tailing leachate (62.3 and 64.5 µg/L, respectively) was lower than its concentration in upstream runoff (172.6 µg/L). It should be noted that the concentration of heavy metals in the river was generally lower than in downstream runoff due to dilution. This was clearly an indication of the adverse effect of mining activities on the region’s groundwater and surface water. It can thus be concluded that alkaline mine drainage being released into the environment increased Ba, Zn, Pb, Cr and Ni concentration (in descending order) in groundwater and Ni, Cr, Pb and Ba in surface water.
Conclusion
Alkaline mine drainage rarely occurs in coal mines throughout the world. The Takht coal mine is one of the few coal mines where alkaline mine drainage occurs. The mining activities started in 2002 and have already had adverse effects on the quality of water resources in the area since the concentration of heavy metals in downstream groundwater is higher than in upstream water. Tunnel effluent and tailing leachate entering the environment were the main causes of such increases. An alkaline environment was responsible for the heavy metals’ solubility and distribution in surrounding water resources. Heavy metals’ concentration was much higher in tunnel effluent bed sediments and upstream and downstream runoff than in the aqua phase due to the alkaline environment reducing solubility.
Mining activities increased Ba, Cr, Ni and Pb concentration in the area’s surface water and groundwater. Alkaline mine drainage has led to increased Zn concentration in downstream groundwater compared to upstream groundwater (5.2 times greater) while it decreased Zn concentration in surface water. The rate of coal-mining-related alkaline mine drainage effects on the environment should be investigated more fully to prevent further damage in the foreseeable future.
References
Anderson, R. M., Beer, K. M. et al. 2005. Water Quality in the Allegheny and Monongahela River Basins Pennsylvania, West Virginia, New York, and Maryland, 1996-98. U.S. Geological Survey Circular 1202. [ Links ]
Dahrazma, B., Mulligan, C. N. (2007). Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration, Chemosphere, 69, 705-711. [ Links ]
Finkelman R. B., Orem W., et al. (2002). Health impacts of coal and coal use: possible solutions, International Journal of Coal Geology, 50, 425-443. [ Links ]
Hem, J. D. (1985). Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd Ed. Water Supply, US Geological Survey, Paper 2254, 263pp. [ Links ]
Minoodasht Water Office. (2003a). Quality Control Report on Minoodasht Water Samples (in Persian). [ Links ]
Minoodasht Water Office. (2003b). Takht Basin Report (in Persian). [ Links ]
Northeast Alborz Mining Co. (2009). Detailed exploration of Takht region, in Annual Exploration Report (in Persian). [ Links ]
Smith, K. S. (1999). Sorption of trace elements by earth materialsâan overview with examples relating to mineral deposits. In: Plumlee, G. S., Logsdon, M. J. (Eds). The environmental geochemistry of mineral deposits. Part A. Processes, techniques, and health issues: society of economic geologists, Reviews in Economic Geology 6A, 61 182. [ Links ]
Swaine, D. J., Goodarzi, F. (1995). Environmental Aspects of Trace Elements in Coal. Kluwer Academic Publishers, Dordrecht. [ Links ]
Yiwei, C., Guijian, L., Yanming, G., Jianli, Y., Cuicui, Q., Lianfei, L. (2007). Release and enrichment of 44 elements during coal pyrolysis of Yima coal, China, Journal of Analytical and Applied Pyrolysis, 80(2), 283-288. [ Links ]