Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.16 no.2 Bogotá July/Dec. 2012
The geochemistry of claystone-shale deposits from the Maastritchian Patti formation, Southern Bida basin, Nigeria
Olugbenga A Okunlola and Olubunmi Idowu
Geology Department, University of Ibadan, Ibadan, Nigeria. E-mail: gbengaokunlola@yahoo.co.uk
Record
Manuscript received: 29/09/2010
Accepted for publications: 06/05/2012
ABSTRACT
An inorganic geochemical study of a claystone and shale sequence from the Patti Formation around Ahoko in the Southern Bida basin, Nigeria, was carried out to determine the basin’s depositional conditions, provenance and tectonics. Representative samples underwent mineralogical and geochemical analysis involving major, trace and rare earth element analysis.
Mineralogical studies using X-ray diffraction analysis revealed prominent kaolinite, dickite and illite peaks; accessory minerals included quartz and microcline. Major element abundance showed that the shale samples had SiO2 (61.26%), Al2O3 (16.88%) and Fe2O3 (3.75%) constituting more than 79% of bulk chemical composition whilst claystone samples contained SiO2 (67.79%), Al2O3 (17.81%) and Fe2O3 (1.67%). Higher SiO2, Ba, Sr, V, and Nb concentrations were observed in claystone samples rather than shale whereas the shale samples were observed to be more Zr-, Ni- and Zn-enriched than the claystone ones. The shale and claystone samples showed slightly light rare earth enrichment and slightly flat heavy rare earth depleted patterns having a negative Eu and Tm anomaly. High TiO2 and Rb/K2O values also indicated that the shale and claystone samples were matured. Geochemical parameters such as U, U/Th, Ni/Co and Cu/Zn ratios indicated that these shales were deposited in oxic conditions; the Al2O3/TiO2 ratio suggested that intermediate igneous rocks were probable source rocks for the shales, while mafic rocks were suggested as being source rocks for the claystone. However, the La/Sc, Th/Sc, Th/ Co ratios and shales and claystone plots revealed that they came within the range given for felsic rocks as provenance, thereby suggesting mixed provenace for the sediments. A passive-margin tectonic setting was adduced for the sedimentary sequences.
Keywords: geochemical, inorganic, shale, claystone, provenance, tectonic.
RESUMEN
Se realizó un estudio de geoquímica inorgánica de una secuencia de arcillolitas y limolitas de la Formación Patti en Ahoko, cuenca sur de Bida, Nigeria, con el fin de determinar las condiciones de sedimentación de la cuenca, la procedencia de los sedimentos y la tectónica de la zona. A las muestras representativas se les realizaron análisis mineralógicos y geoquímicos: estudio de trasas y análisis de tierras raras.
Los análisis de difracción de rayos X revelaron importantes niveles de caolinita, dickita e ilita y, minerales accesorios incluido cuarzo y microclina. En las muestras de limolita se encontró SiO2 (61,26%), Al2O3 (16,88%) y Fe2O3 (3,75%) como constituyentes de más del 79% de la composición química, mientras que las muestras de arcillolita contienen SiO2 (67,79%), Al2O3 (17.81%) y Fe2O3 (1,67%). Las concentraciones más altas de SiO2, Ba, Sr, V, Nb se observaron en las muestras de lutita mientras que en las muestras de arcillolita se observó una mayor concentración de Zr, Ni- y enriquecimiento de Zn- que en las lutitas. Las muestras de lutitas y arcillolitas presentan un ligero enriquecimiento de tierras raras y algo de tierras raras pesadas teniendo patrones de anomalía negativa de Eu y Tm. Los altos valores de TiO2 y Rb/K2O también indican que las muestras de limolita y arcillolita fueron maduradas. Los parámetros geoquímicos como la relación de U, U/Th, Ni/Co y Cu/Zn indican que las limolitas y arcillolitas fueron depositadas en condiciones aeróbicas, la relación Al2O3/TiO3 sugiere que las rocas ígneas intermedias fueron probablemente la roca fuente de las limolitas, mientras que se sugiere que las rocas máficas son las rocas fuente de las arcillolitas. Sin embargo, la relación La/Sc, Th/Sc, Th/Co y limolitas indica que la zona de procedencia está dada en el rango de rocas félsicas, lo que sugiere una fuente mixta para los sedimentos. Un ambiente tectónico de margen pasivo se adujo para las secuencias sedimentarias.
Palabras claves:Geoquímica, Inorgánica, esquisto,arcillas, procedencia, tectónica.
Introduction
Shale represents the most abundant type of sediment in sedimentary basins worldwide (Pettijohn, 1975) and are considered to represent average crustal provenance composition much better than any other detrital sedimentary rocks (McCulloch and Wasserburg, 1978).
Some researchers have suggested that sedimentary rocks’ major element geochemistry is more useful in discriminating a tectonic setting (Bhatia, 1983; Roser and Korsch, 1986); however, major trace elements, such as La, Y, Sc, Cr, Th, Zr, Hf and Nb, particularly in combination withTiO2, are best suited for provenance and tectonic setting determination studies because of their relatively low mobility during sedimentary deposition (McLennan et al., , 1983). The relative distribution of immobile elements differing in concentration in felsic and basic rocks such as La and Th (felsic rock-enriched) and Sc, Cr and Co (enriched in basic rocks relative to felsic rocks) has been used to infer felsic and basic sources’ relative contribution in shales from different tectonic environments (Wronkiewicz and Condie, 1990). Several authors have used geochemical parameters to understand ancient sediments’ paleo-oxygenation conditions (Calvert and Pedersen, 1993; Jones and Manning, 1994; Nath et al., , 1997; Cullers, 2002; Armstrong-Altrin et al., , 2003; Dobrzinski et al., , 2004).
This study was therefore aimed at identifying Patti formation claystone- shale sequence source rock characteristics, tectonic setting and paleo- environmental conditions in the Bida basin around Ahoko in central Nigeria using their major, trace and rare earth elements’ geochemistry.
Geology and stratigraphy
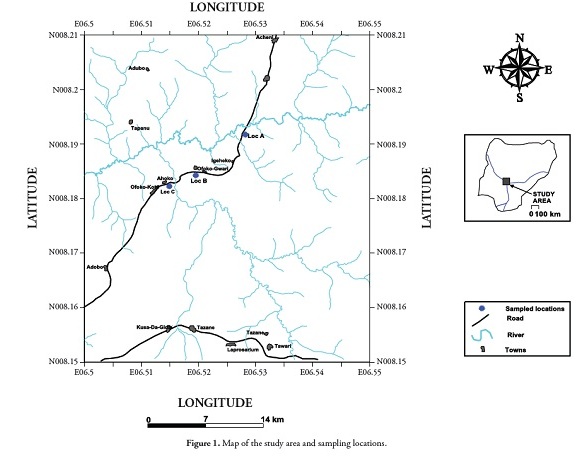
The Bida basin is a northwest-southeast-trending embayment, lying perpendicular to the main axis of the Benue trough (Figure 1). The South Atlantic Tethys seaway was routed through this basin during the Campanian to Maastrichtian age. The basin is usually regarded as a north-western extension of the Anambra basin (Whiteman, 1982; Ladipo et al., , 1994; Akande and Ojo, 2002; Akande et al., , 2005), both of them being major depocenters during this period. Adeleye (1975a,b) viewed the Bida basin as a gently down-warped trough, whose genesis may be closely connected with south-eastern Nigeria and the Benue valley’s Santonian orogenic movements, while Whiteman (1982) has suggested that the basin was formed from simple cratonic sag.
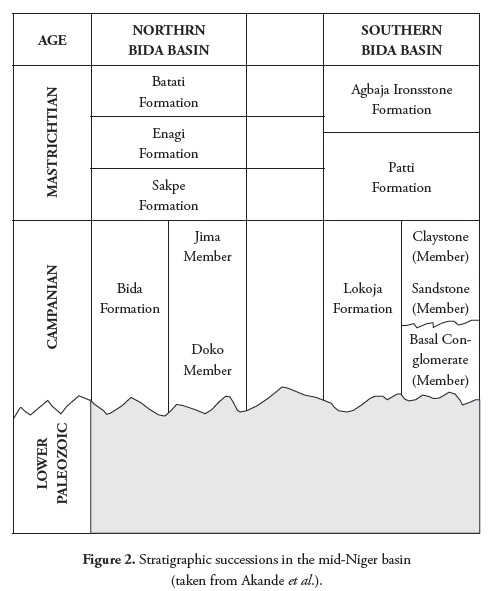
Agyingi (1991) has proposed a post-Santonian origin for the Bida basin; he reported tension build-up in the Benue trough during Africa’s separation from South-America (Aptian-Albian). The adjacent area probably sagged along a northwest-southeast orientated fracture during this period to ease tensional build-up, resulting in the Nupe depression forming a side basin. He stated that the sediments in the basin were generally undisturbed as in the case of the post-Santonian sediment of the adjacent Sokoto and Anambra basins. A maximum sedimentary pile of up to 3,300m has been recorded from aeromagnetic interpretation, suggesting a post-Santonian origin (Agyingi, 1991). The Bida basin’s stratigraphy varies, depending on the area being considered; several authors have discussed this basin’s stratigraphy in two sectors: the northern Bida basin (Adeleye, 1971) and the southern Bida basin (Agyingi, 1991; Akande and Ojo, 2002).
The northern Bida basin is successively made up of Bida sandstone, Sakpe ironstone, Enagi siltstone and Batati ironstone (Figure 2) whereas the southern Bida basin lithostratigraphy successively consists of Lokoja sandstone and the Patti and Agbaja ironstone formation. This study has concentrated on the southern Bida basin’s Patti formation.
Methodology
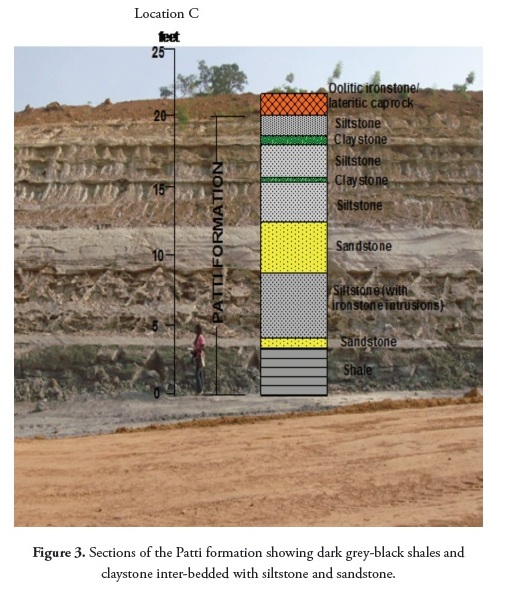
The twenty-five samples used for this study were taken from the exposed shale section in road cuttings at Ahoko, along the Koton-Karifi/ Abaji highway. The location being studied lies within the Patti formation in the southern Bida basin. Representative shale and claystone samples were collected from different vertical sections of the outcrops (Figure 3). Efforts were made to avoid weathered horizons.
Representative whole rock shale samples were studied by X-ray diffraction (Phillips –PW 1011 diffractometer). The diffractograms were recorded using a 1o 2/min/cm scanning rate with Ni-filtered Fe K alpha radiation. X -ray diffraction curves were interpreted by comparing notable intensity peaks with those for standard minerals established by Carrol (1971). Quantitative determination was made by a real method; the shale samples’ clay fraction was not separated for speciation evaluation in this study because, initially, it was necessary to establish the shale sequence’s holistic compositional features to have an unobliterated pattern of its evolution within the basin.
Geochemical characterisation involved analysing thirteen shale and two claystone samples at the Acme Analytical Laboratories Ltd for major, minor and trace element geochemistry using an ICP mass spectrometer (Perkin-Elmer, Elan 6000) and inductively coupled plasma mass spectrograph on powdered, pressed pellets prepared from 3-5g samples. It was digested by weighing 0.2g aliquot in a graphite crucible mixed with 1.5g LiBO2/LiB4O7 flux. The crucibles were placed in an oven and heated at 980ºC for 30 minutes. The cooled bead was dissolved in 5% HNO3 (ACS grade nitric acid diluted in demineralised water). Calibration standards and reagent blanks were added to sample sequences.
The basic package consisting of thirty-four elements (Ba, Co, Cs, Ga, Hf, Nb, Rb, Sn, Sr, Ta, Th, U, V, Y, Zr, La, Ce Pr, Nd, Sm, Eu, Gd, Lu, etc) was determined for the shale and claystone samples. A second 0.5g split sample was digested in Aqua Regia and analysed by ICP-MS to determine Au, Ag, As, Bi, Cd, Cu, Hg, Mo, Ni, Pb, Sb, Se and Zn. An ICP emission spectrograph (Spectro Ciros Vision or Varian 735) was used for determining major oxides and some trace elements (i.e. SiO2, Al2O3, Fe2O3, CaO, MgO, TiO2, P2O5, Cr2O5, Ba, Nb, Ni, Sr, Sc, Y and Zr). Loss on ignition (LOI) was determined for both packages by measuring the weight loss after heating a 1g split sample at 95ºC for 90 minutes.
Results and discussion
Mineralogy
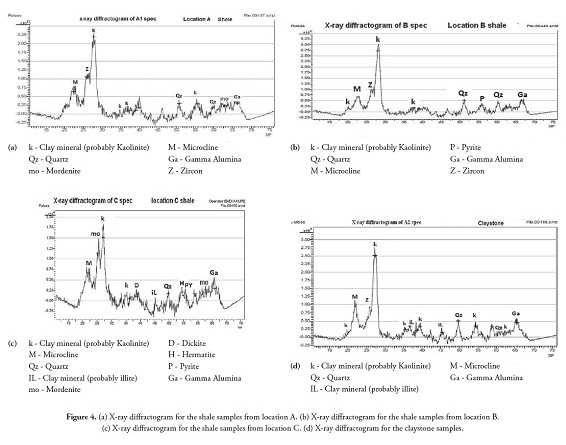
The X-ray diffractograms (XRD) for the whole rock Bida shale samples from location A revealed minerals such as kaolinite, quartz, mordenite, microcline, zircon and gamma alumina (Figure 4a); kaolinite was the dominant clay mineral.
The XRD results for shale in location B also revealed the predominance of kaolinite, quartz, microcline, pyrite and zircon present in subordinate amounts (Fig. 4b) and whole at location C. Kaolinite, illite, quartz, microcline, dickite, mordenite, gamma alumina and iron rich minerals, such as pyrite and hematite, constituted the mineral makeup. The percentage of kaolinite in the shale in location C was 46.91%. Typical diffractograms of the claystone revealed kaolinite as the dominant mineral (66%), while illite, dickite, quartz, microcline and zircon were present in subordinate amounts (Figure 4d).
Geochemistry
Major element composition
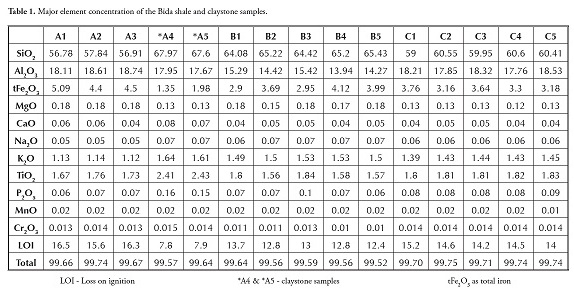
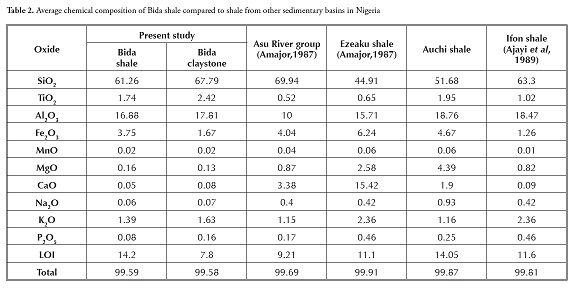
Table 1 gives the major oxide analysis of thirteen shale and two claystone samples; Table 2 summarises the average major element oxide (wt %) data for the samples being studied. These were compared to average shales worldwide (Pettijohn, 1957), NASC (Gromet et al., , 1984), Turekan & Wedephol (1961) and shales from other parts of Nigeria (Table 3). It was apparent that most shale samples in Table 1 varied slightly regarding SiO2 (56.78-65.43%), had moderately high Al2O3 (13.94 -18.74%) and a small variation in Fe2O3 values (2.90%-5.09%); they were, however, low in TiO2, CaO, Na2O and K2O. Such low K2O content indicated the low amount of illite or K-feldspar present (Akpokodje et al., , 1991). The claystone was relatively rich in Al2O3, TiO2 and SiO2 compared to the shale, even though the shale had higher Fe2O3 content than the claystone.
The claystone (17.67-17.95%) had less variation in Al2O3 content than the shale (13.94-18.74%). Most samples had low P2O5 content; P2O5 depletion could have been due to the lower amount of accessory phases, such as apatite and monazite, compared to PAAS (Ramasamy et al., , 2007).
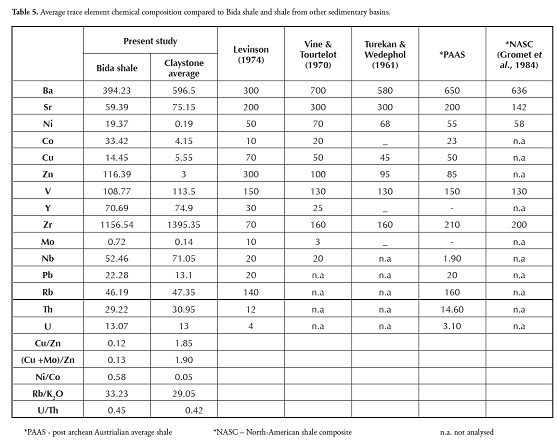
The MgO, MnO and Na2O content values were very low in both samples, collectively only accounting for less than 2%. The shale and claystone MgO and CaO content was low; the samples thus did not indicate associated carbonates or dolomitisation. The shale and claystone alumina to silica ratio was low (0.27). The 14.28% average loss on ignition (LOI) for shale was high, showing great shale potential for carbonaceous compounds. The 3.63 silica-alumina ratio showed that that shale and claystone samples were highly siliceous. The average SiO2 (61.26%) and Al2O3 (16.88%) chemical composition in shale constituted about 78% of the samples’ total chemical composition. The other chemical impurities in shale samples from the Patti formation were Fe2O3 (3.75%) and TiO2 (1.74%). The Patti formation shale and claystone samples had higher TiO2 values than post-Archean Australian average shale (PAAS; Taylor and Mclennan, 1985).
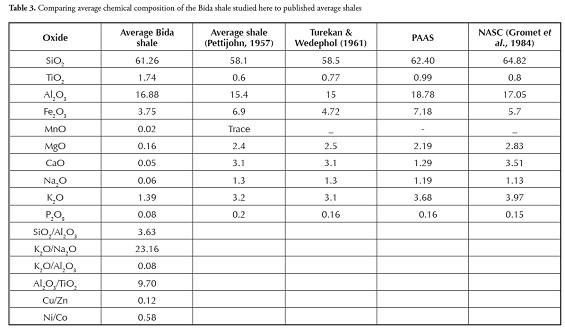
Sediments’ K2O/Al2O3 ratio can be used as an indicator of ancient sediments’ original composition. The K2O/Al2O3 ratios for clay minerals and feldspars are different (0.0 to 0.3, 0.3 to 0.9, respectively) according to Cox ( Cox et al., , 1995). The average K2O/Al2O3 ratio for shale varies from 0.06 to 0.1 and is 0.09 for claystone. The K2O/Al2O3 ratios were closer to the lower clay mineral range limit in both the shale and claystone samples. Comparing the chemical composition of the shale and claystone samples from Bida with that of shales from other parts of Nigeria revealed that the shale and claystone samples were relatively rich in SiO2, Al2O3, TiO2 and Fe2O3 comparable with black lignite shale from Ifon and shale from the Auchi area (Akpokodje et al., ,). The present study’s CaO and MnO values also had values comparable with Ifon shale. The samples were rich in SiO2, Al2O3 and TiO2 compared to Ezeaku shales; however, the Ezeaku shale had higher Fe2O3 and CaO compared to Bida shale and claystone. The Asu river group had higher SiO2, Fe2O3 and CaO values but lower TiO2 and Fe2O3 values compared to the Bida shale and claystone samples from the Patti formation. The shale’s MgO and CaO contents were generally low by contrast with values reported by Emofurieta et al., , (1994) for Ewekoro and Gombe shales. When compared to shales from other parts of the world (Table 4), the Bida basin shale had similar averages to those reported by Pettijohn (1957), Turekian and Wedephol (1961) and NASC (Gromet et al., , 1984) in terms of SiO2, Al2O3 and Fe2O3; the TiO2 composition of the Bida shale was perhaps slightly higher than that of the other shales. The Bida shale samples were, on average, relatively rich in SiO2, Al2O3, TiO2 and P2O5 comparable with PAAS and NASC (Gromet et al., ,1984). However, the Bida shale had depleted MgO, CaO, Na2O, K2O composition compared to world shale averages.
Provenance, tectonic setting and depositional environment
The geochemical signatures of clastic sediments have been used to ascertain provenance characteristics (Taylor and Mclennan, 1985; Condie et al., , 1992; Cullers, 1995; Armstrong-Altrin el al., 2004).
Most clastic rocks’ Al2O3/TiO2 ratios are essentially used to infer source rock composition because Al2O3/TiO2 ratios increase from 3 to 8 for mafic igneous rocks, from 8 to 21 for intermediate rocks and from 21 to 70 for felsic igneous rocks (Hayashi et al., , 1997). The Al2O3/TiO2 ranged from 8.38 to 10.84 in the Ahoko shales (Patti formation) and 7.27 to 7.44 in the claystone samples; hence, this study’s Al2O3/TiO2 ratio suggested intermediate rocks as being probable source rocks for the shale samples while mafic rocks were suggested as being source rocks for the claystone samples.
Cr and Ni abundance in siliciclastic sediments was considered a useful provenance tool. Cr and Ni concentration was low in the shale and claystone samples; a low Cr concentration indicates felsic provenance, according to Wrafter and Graham (1989), and high Cr and Ni content is mainly found in ultramafic rock-derived sediment (Armstrong-Altrin et al., , 2004). The Cr and Ni content in these shales was low, therefore suggesting felsic provenance.
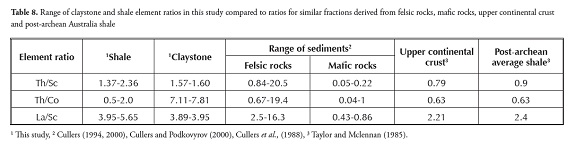
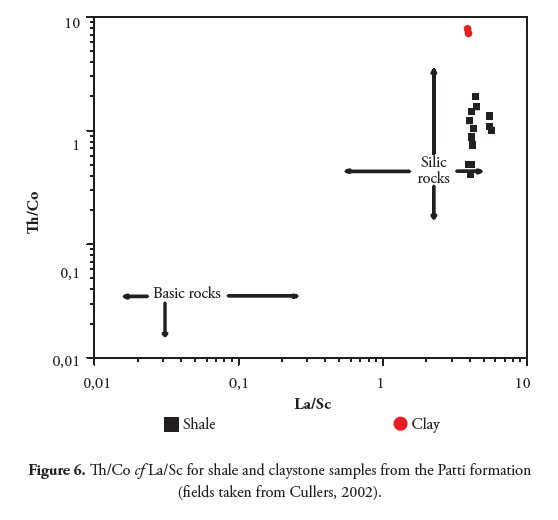
Ratios such as La/Sc, Th/Sc and Th/Co are significantly different in felsic and basic rocks and may lead to constraints on average provenance composition (Wronkiewicz and Condie, 1990; Cox et al., , 1995; Cullers, 1995). The Th/Co, Th/Sc and La/Sc ratios for shale and claystone samples from this study were compared to those of felsic and basic rock-derived sediment (fine fraction) upper continental crust (UCC) and PAAS values (Table 8). These comparisons also indicated that such ratios came within the range of felsic source rocks.
The Th/Co compared to La/Sc plot (Figure 5; Cullers, 2002) also suggested that the Patti formation shale and claystone samples were both derived from felsic source rocks.
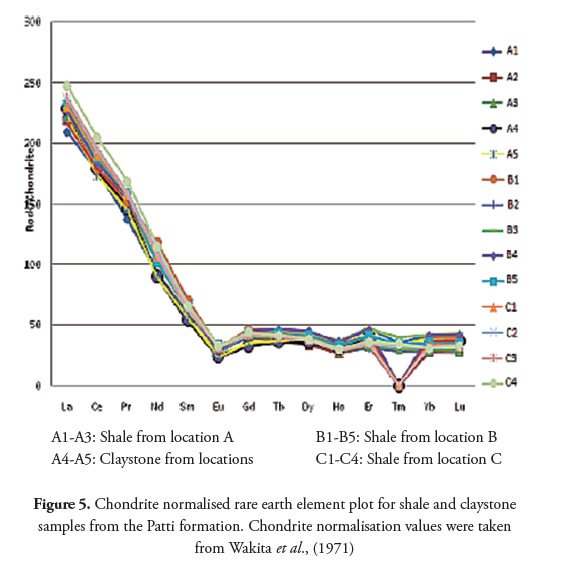
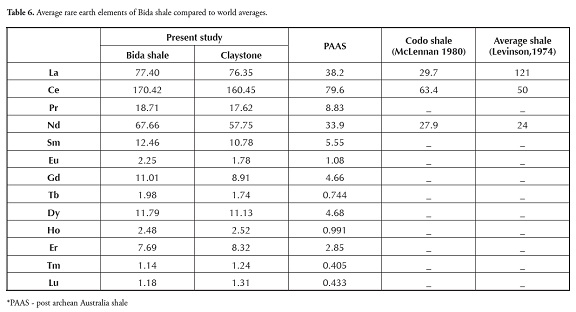
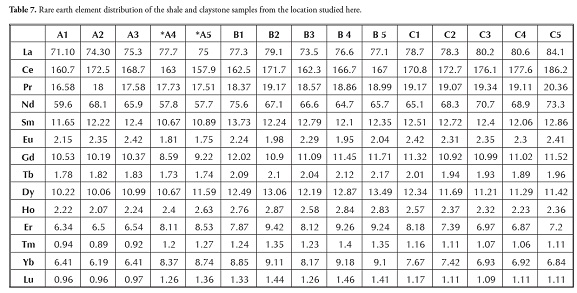
Table 7 shows the shale and claystone samples’ rare earth element (REE) concentration; a slight variation in ΣREE content was observed between the shale (361.18 to 422.75) and claystone samples (365.83 to 371.04). Bulk REE normally reside in the fine fraction (silt or clay) and it has also been inferred that trivalent REE is readily accommodated in most clay minerals enriched with alumina and ferric iron (Cullers et al., , 1987, 1988). The chondrite-normalised REE plot (Figure 5) showed that shale and claystone samples from the Patti formation were LREEenriched and had an almost flat HREE pattern, with negative Eu and Tm anomalies.
The sedimentary rocks’ REE pattern and Eu anomaly also help in providing important clues regarding source rock characteristics (Taylor and Mclennan, 1985). Higher LREE/HREE ratios and negative Eu anomalies are generally found in felsic rocks, whereas mafic rocks have lower LREE/ HREE ratios and no or small Eu anomalies (Cullers, 1995). Higher LREE/ HREE ratios and negative Eu anomalies further confirmed the felsic source rock characteristics of the shale and claystone samples studied here.
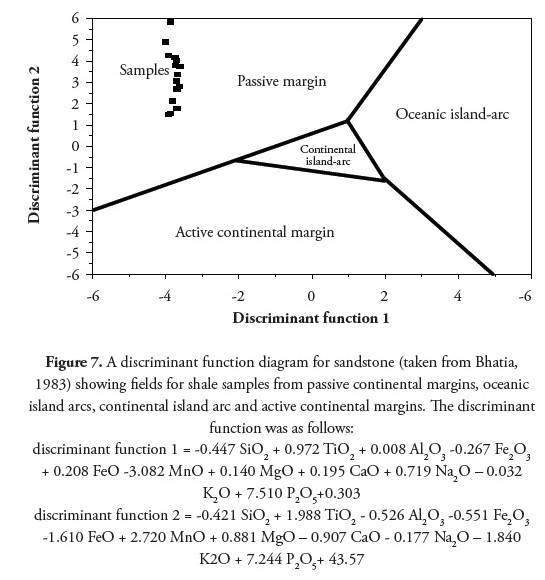
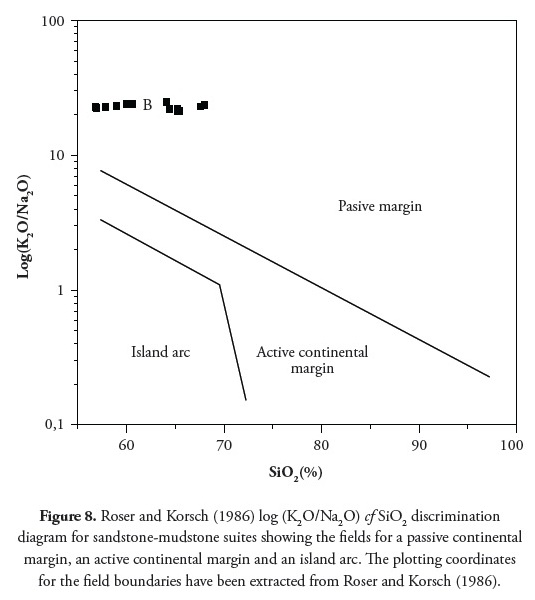
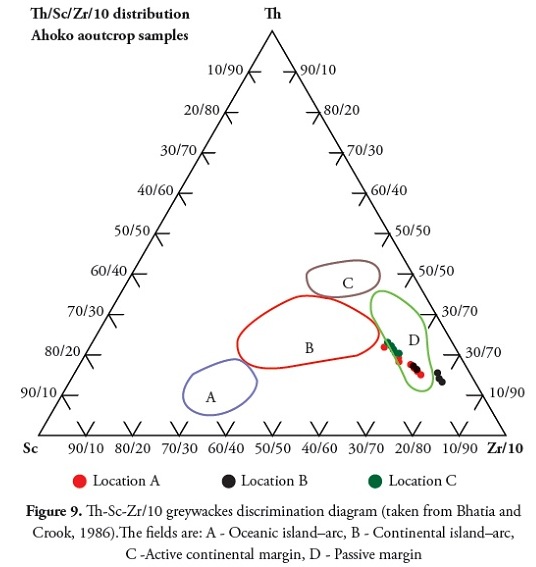
Bhatia and Crook (1986) identified La, Th, Zr, Nb, Y, Sc, Co, and Ti as being the most useful elements in discriminating different tectonic settings. Distinctive field for four environments (Oceanic island arc, continental island arc, active continental margin and passive margin) are recognised on La-Th-Sc and Th-Sc-Zr/10 trivariate plots. The tectonic setting identified in the present study was passive-margin type. Figure 7 shows the shale and claystone units plot within the passive margin using a discriminant function diagram (Bhatia 1983). The Roser and Korsch (1986) plot of log (K2O/Na2O) cf SiO2 discrimination diagram and Th-Sc-Zr/10 greywackes discrimination diagram (after Bhatia and Crook, 1986) indicated a passive-margin tectonic setting (Figure 8 and 9).
The passive-margin comprised Atlantic-type rifted continental margins developed along the edges of the continent, remnant, ocean basins adjacent to collision orogens, and inactive or extinct convergent margins. Intra-cratonic and rift-bounded grabens (e.g. the Benue trough) were formed on a thick continental crust which are included in the passive-margin type tectonic setting. The Bida basin is an intra-cratonic sedimentary basin.
Passive-margin type sandstone is generally enriched in SiO2 and depleted in Na2O, CaO and TiO2, suggesting their highly recycled and mature nature (Bhatia, 1983). Major element analysis of the studied shale and claystone samples confirmed this, as the samples were also enriched in SiO2 but depleted in Na2O, CaO and TiO2, respectively.
Paleo-oxygenation condition
Cu/Zn and (Cu+Mo)/Zn ratios have been put forward by Hallberg (1976) as redox parameters. According to Hallberg (1976), high Cu/Zn ratios indicate reducing depositional conditions, while low Cu/Zn ratios suggest oxidising conditions; he showed that high values should indicate more reducing conditions in the depositional basins than lower values, which may indicate more oxidising conditions. In this present study, the shale and claystone samples had ~0.03-1.15 and ~1.7-2.0 Cu/Zn ratios, respectively (Table 5), indicating more oxidising conditions. Wedephol (1968) noted that average shales reflect shallow marine sediments accumulating in oxidising conditions. The U/Th ratio may be used as a redox indicator with U/Th ratio being higher in organic-rich mudstones (Jones and Manning, 1994). A U/Th ratio below 1.25 suggests oxic deposition conditions whereas values above 1.25 suggest suboxic and anoxic conditions (Nath et al., , 1997). In this study, the shale and claystone samples had low U/Th ratios (Table 5: ~0.37-0.58, ~0.41-0.44, respectively), indicating that both shale and claystone samples were deposited in an oxic environment.
Dypvik (1984) and Dill (1986) have used the Ni/Co ratio as a redox indicator. Jones and Manning (1994) have suggested that Ni/Co ratios below 5 indicate oxic environments, whereas ratios above 5 indicate suboxic and anoxic environments. Thus, the low Ni/Co ratio for shale (~0.34- 0.70) and claystone (~0.01-0.07) samples indicate that Patti formation shale and claystone was deposited in well-oxidising conditions.
Conclusions
Field studies have indicated that Patti formation shales are found at the base of an outcrop section at Ahoko along the Koton-Karfi and Abaji axis, occurring alongside siltstones, claystones and sandstone inter-bedded with bioturbated ironstone.
The mineralogical composition of the whole rock shale samples based on X-ray diffraction analysis identified clay mineral constituents as probably being kaolinite and illite, although, subsequent detailed clay fraction determination is needed to further confirm this. Other non-clay minerals included microcline, quartz, zircon, pyrite and hematite. The proportions of minerals identified in the shale and claystone samples varied.
The Patti formation’s claystone and shale samples showed considerable variation regarding their major, trace and rare earth elements. Major element abundance showed that shale samples had SiO2 (61.26%), Al2O3 (16.88%) and Fe2O3 (3.75%), constituting more than 79% of the bulk chemical composition while claystone samples contained SiO2 (67.79%), Al2O3 (17.81%) and Fe2O3 (1.67%). There was close correlation between the major element and rare earth element composition of shale and claystone samples in the Bida basin. Higher SiO2, Ba, Sr, V and Nb concentrations were observed in claystone than shale, whereas shale samples were more Zr-, Ni- and Zn-enriched than the claystone samples. Zr was extremely high in both samples.
The discrimination diagrams (Bhatia, 1983) for distinguishing the tectonic setting mostly indicated a passive-margin type. The provenance indicated a non-homogenous source rock. Analysis and interpretation using geochemical parameters, such as La/Sc, Th/Sc and Th/Co ratios and La/Sc cf Th/Co, suggested that the shale and claystone samples might have been derived from felsic source rock rather than intermediate or mafic source rocks, such provenance being indicated from interpretation using the Al2O3/ TiO2 ratio. Geochemical parameters like U, U/Th, Ni/Co and Cu/Zn ratios strongly implied that these shales were deposited in an oxic environment.
References
Adeleye, D.R. 1971. Stratigraphy and sedimentation of the Upper Cretaceous strata around Bida, Nigeria. Unpublished Ph.D. Thesis, Department of Geology, University of Ibadan, 297 pp. [ Links ]
Adeleye, D.R. 1975a. A fauna from the ironstones of the middle Niger valley, NIGERIA. Journal of Minning Geology, Vol. 8. pp. 45-48. [ Links ]
Adeleye, D.R. 1975b. Nigeria Cretaceous stratigraphy and paleography. American Association of Petroleum Geologists, Vol. 59, pp. 2302- 2313. [ Links ]
Adeleye, D.R. 1976. The Geology of the Middle Niger Basin. In: C.A. Kogbe (ed) The Geology of Nigeria, Univ. Ife., pp. 283 287. [ Links ]
Agyingi, C.M., 1991. Geology of Upper Cretaceous rocks in the eastern Bida, Nigeria. Unpublished Ph.D. Thesis. Department of Geology, University of Ibadan, 501pp. [ Links ]
Akande, S.O. and Ojo O.J., 2002. Organic Petrology and Rock-Eval studies on source rock facies of Champion-Maastrichtian Patti formation, southern Bida basin, Nigeria. Nigerian Association of Petroleum Explorationists Bulletin, No. 16, pp. 21-39. [ Links ]
Akande, S.O., Ojo O.J. and Ladipo, K.O., 2005. Upper Cretaceous sequences in the southern Bida Basin, Nigeria. A field guide book (Nigerian Association of Petroleum Explorationists), 60pp. [ Links ]
Akpokodje, E.G., Etu-Efeotor, J.O and Olorunfemi, B.N. 1991. The composition and physical properties of some ceramic and pottery clays of South Eastern Nigeria. Journal. Mining and Geology. 27,pp 9-15. [ Links ]
Amajor ,L.C, 1987. Major and Trace element geochemistry of Albian and Turonian shales from the Southern Benue Trough Nigeria, jour .Afric Earth Sci 6 pp633-641. [ Links ]
Armstrong-Altrin, J.S., Lee, Y.I., Verma, S.P., Ramasamy, S., 2004. Geochemistry of sandstones from the upper Miocene Kudankulam Formation, southern India: Implications for provenance, weathering, and tectonic setting: Journal of Sedimentary Research, 74(2), 285- 297. [ Links ]
Bhatia, M.R., 1983, Plate tectonics and geochemical composition of sandstones: Journal of Geology, 91, 611-627. [ Links ]
Bhatia, M.R., Crook, K.A.W., 1986. Trace element characteristics of greywackes and Tectonic setting discrimination of sedimentary basins. Contrib. Mineral. Petrol. 92, 181 193. [ Links ]
Calvert, S.E., Pedersen, T.F., 1993, Geochemistry of Recent oxic and anoxic marine sediments, implications for the geological records: Marine Geology, 113(1-2), 67-88. [ Links ]
Condie, K.C., Wronkiewicz, D.J., 1990. A new look at the Archean-Proterozoic boundary: Sediments and the tectonic setting constraint, in Naqvi, S.M. (ed.), Precambrian Continental Crust and its Economic Resources: Elsevier, Amsterdam, 61-84. [ Links ]
Condie, K.C., Boryta, M.D., Liu, J., Quian, X., 1992. The origin of khondalites: geochemical evidence from the Archean to Early Proterozoic granulitic belt in the North China Craton: Precambrian Research, 59(3-4), 207-223. [ Links ]
Cullers, R.L., 1995. The controls on the major and trace element evolution of shales, siltstones and sandstones of Ordovician to Tertiary age in the Wet Mountain region, Colorado, U.S.A: Chemical Geology, 1231-4, 107-131. [ Links ]
Cox, R., Lowe, D.R., Cullers, R.L., 1995. The influence of sediment recycling and basement composition on evolution of mudrock chemistry in the southwestern United States: Geochimica et Cosmochimica Acta, 59(14), 2919-2940. [ Links ]
Dill, H., 1986. Metallogenesis of early Paleozoic graptolite shales from the Graefenthal Horst (Northern Bavaria-Federal Republic of Germany): Economic Geology, 81, 889-903. [ Links ]
Dobrzinski, N., Bahlburg, H., Strauss, H., Zhang, Q.R., 2004. Geochemical climate proxies applied to the Neoproterozoic glacial succession on the Yangtze Platform, South China, in Jenkins, G. et al., (eds.), The extreme Proterozoic: Geology, Geochemistry and Climate: American Geophysical Union Monograph Series, 146, 13-32. [ Links ]
Dypvik, H., 1984. Geochemical compositions and depositional conditions of Upper Jurassic and Lower Cretaceous Yorkshire clays, England:Geological Magazine, 121(5), 489-504. [ Links ]
Emofurieta, W.O.,Ogundimu, T.O and Imeokpara, E.C.1994. Mineralogical geochemical and economic appraisal of some clay and shale deposits in southwestern Nigeria. Nig. Jour. Min.Geol.Vol. 30, p151- 159. [ Links ]
Hallberg, R.O., 1976, A geochemical method for investigation of palaeoredox conditions in sediments : Ambio, Special Report, 4,139-147. [ Links ]
Hayashi, K., Fujisawa, H., Holland, H., Ohmoto, H., 1997, Geochemistry of ~1.9 Ga sedimentary rocks from northeastern Labrador, Canada: Geochimica et Cosmochimica Acta, 61(19), 4115-4137. [ Links ]
Gromet, L.P., Dymek,R.F., Haskin,L.A.,andkorotev,R.L,1984. The North American shale composite. Its compilation, major and trace element characteristics. Geochim.Cosmochim.Acta 48,2469-2482 [ Links ]
Jones, B., Manning, D.C., 1994. Comparison of geochemical indices used for the interpretation of paleo-redox conditions in Ancient mudstones: Chemical Geology, 111(1-4), 111-129. [ Links ]
Ladipo, K.O., Akande, S.O. and Mucke, A., 1994. Genesis of ironstones from Middle Niger sedimentary basin, evidence form sedimentologisedimentological ore microscopic and geochemical studies. Journal of Mining and Geology, vol. 30, pp. 161-168. [ Links ]
Levinson, A.A. 1974. Introduction to Exploration Geochemistry, Applied Publishing, Illnois. [ Links ]
Mclennan,S .M ,and Taylor,S.R, 1980. Geochemical standards for sedimentary rocks: Trace elements data for USGS standards SCOI, MAG-I AND SGR-I.Chem.Geol.29,333-343. [ Links ]
McCulloch, M.T., Wasserburg, G.J., 1978, Sm-Nd and Rb-Sr chronology of continental crust formation: Science, 200, 1003-1011. [ Links ]
Nath, B.N., Bau, M., Ramalingeswara Rao, B., Rao, Ch.M., 1997, Trace and rare earth elemental variation in Arabian Sea sediments through a transect across the oxygen minimum zone: Geochimica et Cosmochimica Acta, 61(12), 2375-2388. [ Links ]
Pettijohn, F.J. 1957. Sedimentary Rocks, 2nd edn. Harper & Row, New York [ Links ]
Roser, B.P., Korsch, R.J., 1986. Determination of tectonic setting of sandstonemudstone suites using SiO2 content and K2O/Na2O ratio. J. Geol. 94, 635 650. [ Links ]
Roser, B.P., Korsch, R.J., 1988. Provenance signature of sandstone-mudstone suite determined using discriminant function analysis of major element data. Chem. Geol. 67, 119 139. [ Links ]
Taylor, S.R., McLennan, S., 1985.The Continental Crust: Its Composition and Evolution: Blackwell, Oxford, 312 p. [ Links ]
Turekian, K.K., and Wedepohl, K.H., 1961. Distribution of the elements in some major. Units of the Earth s crust: Geological Society of America Bulletin, v, 72, p. 175-191. [ Links ]
Vine, J.D. and Tourtelot, E.B.1970. Geochemistry of black shale deposits - a summary report. Econ. Geol. Vol.65, p253-272. [ Links ]
Whiteman, A. 1982. Nigeria: Its Petroleum Geology, Resources and Potentials. Vol. 1 & 2. Graham and Trotman Ltd. London. UK. [ Links ]
Wrafter, J.P., Graham, J.R., 1989. Ophiolitic detritus in the Ordovician sediments of South Mayo Ireland: Journal of the Geological Society, London, 146, 213-215. [ Links ]
Wronkiewicz, D.J., Condie, K.C., 1990. Geochemistry and mineralogy of sediments from the Ventersdorp and Transvaal Supergroups, South Africa: Cratonic evolution during the early Proterozoic: Geochimica et Cosmochimica Acta, 54(2), 343-354. [ Links ]