Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.16 no.2 Bogotá July/Dec. 2012
The geochemistry of lignite from the Neogene Ogwashi-Asaba Formation, Niger Delta Basin, southern Nigeria
Jude E. Ogala
Geology Department, Delta State University, P.M.B.1, Abraka, Nigeria. E-mail: etunimogala@yahoo.com
Record
Manuscript received: 12/01/2012, Accepted for publications: 06/05/2012
ABSTRACT
Major and trace element compositions of lignite from the Tertiary Ogwashi-Asaba formation, Southern Nigeria, have been investigated to determine the prevailing environmental conditions which controlled their formation. Seven samples were obtained from outcrops along river valleys, streams and springs in seven localities; these samples were subsequently analysed using fusion combined with inductively-coupled plasma (FUS-ICP) and total digestion with inductively-coupled plasma (TD-ICP). Geochemical analysis revealed the following concentration range in weight percent (wt. %) for major oxides: SiO2 (0.04-9.78); Al2O3 (0.56-6.40), Fe2O3 (0.05-1.22), MnO (0.001-0.004), MgO (0.02-0.11), CaO (0.05-0.15), K2O (0.01 - 0.04), TiO2 (0.016-0.299) and S (0.08-0.39). Trace elements indicated the following concentration range in parts per million (ppm): Be (2-5), Zr (4-63), Sr (5-22), Y (13-68), Ba (9-89), V (4-216), Zn (17- 176), Ni (8-28), Co (5-13), Cr (2-31), Cu (1-22) and Ga (1-14). The low silica (<10 wt. %) and alumina contents (<7 wt. %) were explained by very limited detrital input during coal formation. Magnesium oxide and CaO content were relatively low thereby confirming the continental nature of the peat-forming environment. Redox-sensitive trace element ratios (Ni/Co, V/Cr and V/V+Ni) indicated predominantly oxic environments for coal deposition. The low trace element concentrations determined in the lignite samples did not point to any severe environmental impact from coal use.
Keywords: Geochemistry, lignite, Ogwashi-Asaba formation, continental environment, Nigeria.
RESUMEN
Las composiciones de elementos mayores y trazas de lignita de la formación terciaria Ogwashi-Asaba, en el sur de Nigeria, han sido investigadas para determinar las condiciones ambientales prevalecientes que controlaron su formación. Siete muestras fueron tomadas de afloramientos a lo largo de valles de ríos, arroyos y manantiales en siete localidades; estas muestras se analizaron posteriormente mediante fusión combinada (FUS-ICP) y la digestión total con plasma de acoplamiento inductivo (ICP-TD). El análisis geoquímico reveló el siguiente intervalo de concentración en porcentaje en peso (% en peso) para los óxidos mayores: SiO2 (0,04 a 9,78); Al2O3 (0,56-6,40), Fe2O3 (0,05-1,22), MnO (0.001-0.004), MgO (0,02 -0,11), CaO (0.05-0.15), K2O (0,01 - 0,04), TiO2 (0.016-0,299) y S (0,08 a 0,39). Los elementos traza indican el siguiente rango de concentración en partes por millón (ppm): Be (2-5), Zr (4-63), Sr (5-22), Y (13-68), Ba (9-89), V (4-216), Zn (17-176), Ni (8-28), Co (5-13), Cr (2-31), Cu (1-22) y Ga (1-14). El bajo contenido de sílice (<10 en peso.%) y el contenido de aluminio (<7 wt.%) fueron explicados por la entrada muy limitado de detrítos durante la formación de carbón. El óxido de magnesio y el contenido de CaO son relativamente bajos, lo que confirma el carácter continental del entorno de formación de turba. La relación de elementos traza Redox-sensibles (Ni/Co, V/ Cr y V/V + Ni) indicó ambientes predominantemente óxicos para la deposición de carbón. Las bajas concentraciones de trazas de elementos hallados en las muestras de lignito no indican un impacto ambiental severo de la utilización del carbón.
Palabras claves: Geoquímica, Lignito, Formación Ogwashi- Asaba, ambientte continental, Nigeria
Introduction
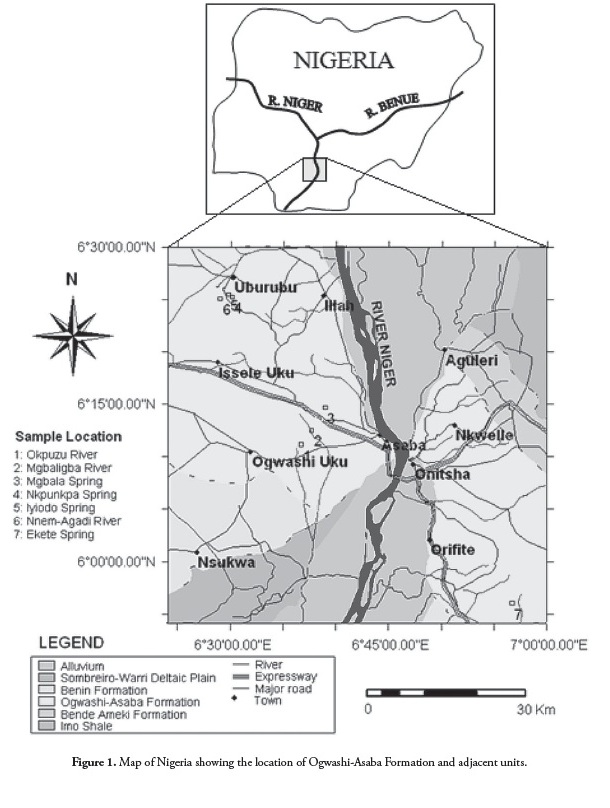
Coal accumulation in Nigeria took place mainly during upper Cretaceous and Tertiary times, resulting in the formation of extensive lignite, sub-bituminous and bituminous coal deposits in the Benue trough and Anambra basin (Simpson, 1954; Reyment, 1965; Akande et al.,, 1992; Obaje and Ligouis, 1996; Obaje and Hamza, 2000; Obaje, 2009). The Ogwashi-Asaba formation occurs extensively within the Niger Delta basin
in southern Nigeria (Figure 1); it was first described by Wilson (1925) and named the -lignite group.- Lithologically, it consists of a sequence of coarse-grained sandstones, light-coloured clays and carbonaceous shales, within which are continental lignite seam intercalations (Reyment, 1965; Kogbe, 1976; Jan du Chene et al.,, 1978). Nigeria has the largest known lignite deposits in Africa, proven reserves exceeding 300 million tons (Orajaka et al.,, 1990; MOMSD, 2007). Lignite is mined in open pits in Nigeria and is used mainly as a substitute for wood as fuel in domestic cooking. The pertinent literature on Nigerian lignite deposits’ major and trace element composition is scarce (Adedosu et al.,, 2007; Olobaniyi and Ogala, 2011).
Coal is heterogeneous sediment having both organic and inorganic fractions; it contains variable amounts of almost all the elements included in the periodic table (Christanis et al.,, 1998; Orem and Finkelman, 2003). Most elements contained in coal usually show a close association with organic matter or with the coal’s inorganic fraction. Changes in the elements’ affinity with either the organic or inorganic fraction may also occur during coalification (Christanis et al.,, 1998). Several researchers have attempted to study the geochemical features of trace elements contained in coal to understand and evaluate trace elements’ mode of occurrence as well as their behaviour during combustion (Gluskoter et al.,, 1977; Swaine, 1990; Finkelman, 1994; Meij, 1995; Spears and Zheng, 1999; Davidson, 2000; Vassilev et al.,, 2001). Previous studies on trace elements in Nigerian coal have revealed their distribution (Olajire et al.,, 2007; Ogala et al.,, 2010a), composition (Ndiokwere et al.,, 1983; Olabanji, 1991; Ewa et al.,, 1996; Adedosu et al.,, 2007; Ogala et al.,, 2010b), characteristics (Nwadinigwe, 1992; Sonibare et al.,, 2005) and association ( Ewa, 2004; Ogala et al.,, 2009).
Several researchers have studied trace element content in lignite and their behaviour during combustion (Foscolos et al.,, 1989; Goodarzi et al.,, 1990; Georgakopoulos et al.,, 1994; Filippidis et al.,, 1996; Gentzis et al.,, 1990; Gerouki et al.,, 1996; Sakorafa et al.,, 1996; Christanis et al.,, 1998; Chatziapostolou et al.,, 2006; Adamidou et al.,, 2007). Trace elements are of great importance in ascertaining the depositional environment and maturity of geological samples within a particular basin (Adedosu et al.,, 2007).
The present study was aimed at investigating the chemical composition (major and trace elements) of lignite samples from the Ogwashi- Asaba formation, southern Nigeria, to predict the prevailing geochemical environmental conditions as well as the processes responsible for their distribution.
The study area’s geology and stratigraphy
The Ogwashi-Asaba formation (Reyment, 1965) occurs extensively within the Niger Delta basin in southern Nigeria, covering a 4,900 km2 area (Figure 1). The formation was originally referred to as a -lignite group- (Wilson, 1925; Wilson and Bain, 1928), -lignite series- (Simpson, 1949; 1954), and -lignite formation- (De Swardt and Casey, 1963). Lignite layers have also been encountered in the lowermost strata of the Ameki group Fig. 1) and the uppermost strata of the Benin formation in drill holes, streams/river banks and road-cut outcrops (Okezie and Onuogu, 1985).
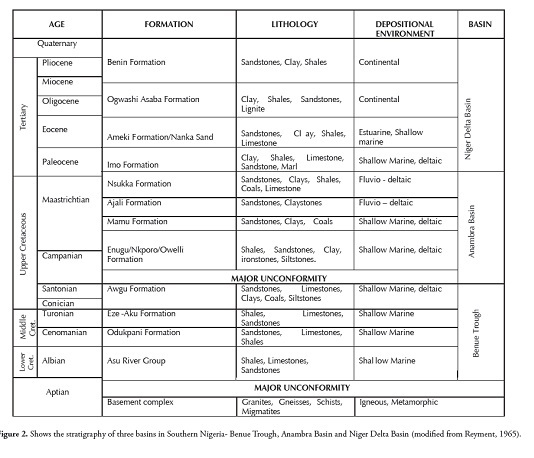
The southern Nigerian sedimentary basin’s formation began during the early Cretaceous period (Albian) following basement subsidence along the Benue and Niger troughs (Nwachukwu, 1972; Olade, 1975). Folding and uplift occurred during the Santonian along a northeast-southwest axis in the Abakaliki-Benue area. The Anambra platform, lying to the west and southwest of the Abakaliki folded belts, subsided to form the Anambra basin (Reyment, 1965; Short and Stauble, 1967; Murat, 1972; Benkhelil, 1989).
The upper Cretaceous stratigraphic succession in the Anambra basin began with the deposition of sediment from the marine Campanian-Maastrichtian Enugu/Nkporo shales and their lateral equivalent - deltaic Owelli sandstone (Fig. 2). These base units were successively overlain by the lower-middle Maastrichtian deltaic coal-bearing Mamu formation (lower coal measures) and middle Maastrichtian tidal Ajali sandstone (false-bedded sandstones) and overlain by the fluvial-deltaic Nsukka formation (upper coal measures) . The Imo and Nsukka formations marine shales were deposited during the Paleocene era and overlain by the regressive Ameki formation (Eocene); the paralic Ogwashi-Asaba formation (Oligocene- Miocene) was capped by the continental Benin formation (Miocene-recent) constituting the tertiary succession (Figure 1)and Fig. 2. The Ogwashi- Asaba formation consists of a succession of coarse-grained sandstone, clay and carbonaceous shale, containing continental lignite seam intercalations (Kogbe, 1976; Jan du Chene et al.,, 1978). Reyment (1965) suggested an Oligocene-Miocene age for the Ogwashi-Asaba formation, but palynological study by Jan du Chene et al., (1978) proposed a late Eocene age for the base part. The lignite seams found within the Ogwashi-Asaba formation are usually brownish to black, varying in thickness from a few millimetres to a maximum of 6 meters. They are thinly laminated and fissile, having leaf and woody fragments on fresh cleats. The average overburden to lignite ratio is 6:1, thereby appearing to rule out open-cast mining (Da Swardt and Piper, 1957).
Ogwashi-Asaba lignite outcrops along river valleys and streams/ springs in Ibusa (Okpunzu and Mgbiligba River), Okpanam (Mgbala Spring), Obomkpa (Nnem-Agadi River, Iyiodo and Nkpunkpa Springs) and Nnewi (Ekete Spring) (Fig. 1).
Analysis methods
Field work and sampling
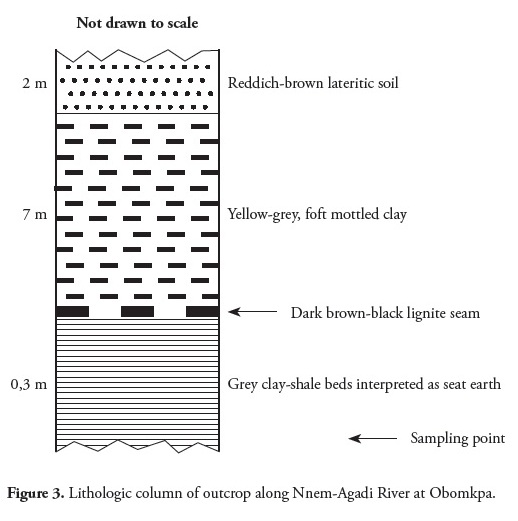
Field work for the present study took place in outcrops along river valleys, streams and springs in seven localities (Fig. 1). Lignite seams do not easily outcrop in the field but good exposure was found along the Mgbiligba and Okpuzu Rivers in Ibusa and at the Mgbala and Ekete Springs in Okpanam and Nnewi, respectively. Lignite seams are also exposed along river valleys at the Nkpunkpa and Iyiodo Springs as well as Nnem-Agadi River, all occurring in Obomkpa. The lignite beds are brownish to black and vary in thickness from a few millimetres to a maximum of 2 meters. They are thinly laminated and fissile with leaf and woody fragments on fresh cleats. The lithological sequence of lignite seam outcropping along the Nnem-Agadi River was logged (Fig. 3); seven lignite samples were collected: three samples from Obomkpa, two from Ibusa and one each from Okpanam and Nnewi.
Analytical procedure
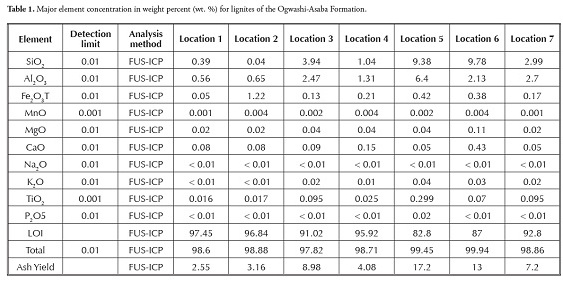
Samples (0.25 g) were powdered in an agate mortar and determined by a combination of methods to investigate lignite chemical composition (major and trace elements), i.e. fusion with inductively-coupled plasma (FUS-ICP) and total digestion with inductively-coupled plasma (TDICP). Loss on ignition (LOI) caused by escaping volatiles was measured after heating to about 1,050oC. Limits of detectable measurement (LDM) for major elements were 0.01 for SiO2, Al2O3, Fe2O3(T), MgO, CaO, Na2O, K2O and P2O5, 0.001 for MnO and TiO2 and 0.01% for S.
LDM for trace elements were 2 ppm for Ba, Sr, Zr, Bi, Te, 1 ppm for Y, Sc, Be, Co, Cr, Cu, Ga, Hg, Ni and Zn, 5 ppm for V, Sb, Tl and W, 0.3 ppm for Ag and Cd, 10 ppm for U, 3 ppm for As and Pb and 4 ppm for Sc Coal data was calculated on an ash basis (value in ash = element value in coal à 100/ash yield, where ash yield = 100 –LOI).
Major oxides and selected trace elements were analysed by the fusion technique (Table 1). Samples were mixed with a flux of lithium metaborate and lithium tetraborate and fused in an induction furnace. The molten melt was immediately poured into a 5% nitric acid solution containing an internal standard and mixed continuously until becoming completely dissolved (c. 30 minutes). The samples were run for major oxides and selected trace elements using a combination fusion technique (FUS-ICP) followed by inductively-coupled plasma-optical emission spectrometry (ICP-OES) analysis using simultaneous/sequential Thermo Jarrell-Ash Enviro II ICP.
A 0.25 g sample was digested with four acids for trace element analysis (Table 2), beginning with hydrofluoric acid (HF), followed by a mixture of nitric (HNO3) and perchloric acid (HClO4), heated using precise programmer-controlled heating in several ramping and holding cycles thus drying the samples. After dryness was attained, samples were brought back into solution using hydrochloric acid. The sample solution was then analysed for element concentration using Varian Vista Pro inductively-coupled plasma-optical emission spectrometry (ICP-OES). USGS and CANMET certified reference materials were used for calibration. The Activation Laboratories in Ancaster, Canada, performed the chemical analysis.
Statistical analysis
The elements were subjected to univariate (minimum, maximum, mean and standard deviation, correlation coefficient) and multivariate statistical analysis (factor and principal component) using statistical package for social sciences (SPSS) software (version 16.0). Since most parameters measured here were not normally distributed, Spearman’s rank correlation was used to examine the correlation between elements (the correlation coefficient matrix measures how well each constituent’s variance can be explained by its relationship to the others). Factor analysis (FA)/principal component analysis (PCA) was applied to the experimental data standardised through z-scale transformation to avoid misclassification due to differences in the units of measurement. Standardisation tends to increase the influence of variables whose variance is small and reduce the influence of variables whose variance is large (Liu et al.,, 2003). Factor analysis was applied to the data set to study the association of the trace elements and extract the principal factors governing trace element distribution (Lu et al.,, 1995). Components having > 1 eigenvalue were selected to explain association amongst the measured variables.
Cluster analysis (CA) established site segregation and desegregation; such analysis is an unsupervised pattern recognition technique which uncovers a data set’s intrinsic structure or underlying pattern without previous knowledge concerning the data being available. This is to enable classification being made based on measured objects’ nearness or similarity, thereby helping to establish relationships among sites in the form of a dendrogram. Hierarchical agglomerative CA involved normalising the data set by means of Ward’s method (i.e. using Euclidean distances as a measurement of similarity). CA was applied to the data set to group similar sampling sites (spatial variation) spread over the region.
Results and discussion
The chemical composition of lignite
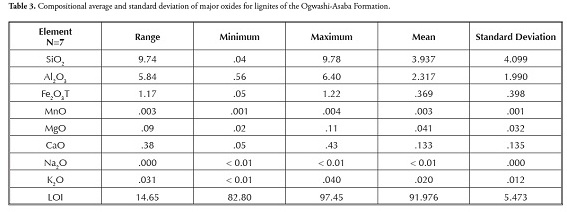
The chemical composition of the lignite samples is presented in Tables 1 and 2. Thirty-seven parameters were measured, consisting of ten major elements, loss on ignition (LOI) and 26 trace elements. SiO2 (0.04-9.78%), Al2O3 (0.56-6.40%) and Fe2O3 (0.05-1.22%) were the dominant oxides in most lignite samples; Mgo, MnO, CaO, TiO2, Na2O, K2O and P2O5 all occurred in traces. LOI had high values (82.80- 97.45%). The low to moderate SiO2, Al2O3 and Fe2O3 concentration was due to quartz, feldspar and pyrite constituting the lignite samples’ main mineral phase (Adamidou et al.,, 2007; Kalaitzidis et al.,, 2010). The low SiO2 (< 10%) and Al2O3 content (< 7%) suggested very limited detrital input during coal formation. The relatively low CaO (0.05-0.43%) and MgO content (0.02-0.11%) implied a continental origin for the lignite (Olobaniyi and Ogala, 2011); this agreed with the Ogwashi-Asaba formation’s continental setting (Reyment, 1965; Kogbe, 1976; Jan du Chene et al.,, 1978).
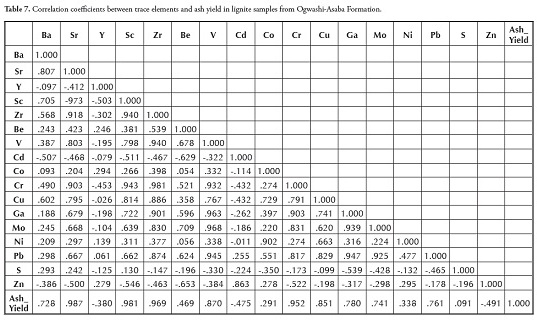
The most abundant trace element was Zn (average 67 ppm), followed by V, Ba, Y and Zr (50.71 ppm, 45.43 ppm, 37.86 ppm and 24.29 ppm average values, respectively) (Tables 2 and 6). The average mean values for Sr, Sc, Be, Cd, Co, Cr, Cu, Ga, Mo, Ni and Pb ranged from 0.2 ppm to 18 ppm, Ag, As, Bi, Hg, Sb, Te, Tl, U and W concentration being below the detection limit in all samples. Compared to average worldwide coal concentration (Seredin and Finkelman, 2008), most trace element content was lower than world average values for low-ranking coal due to low ash yield (Table 2). Y, Be, V, Co and Zn were the only trace elements having relatively higher concentrations in the coal samples used here than the world coal average.
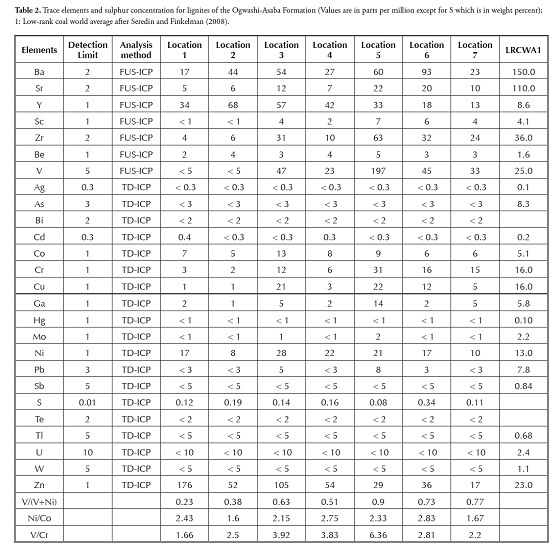
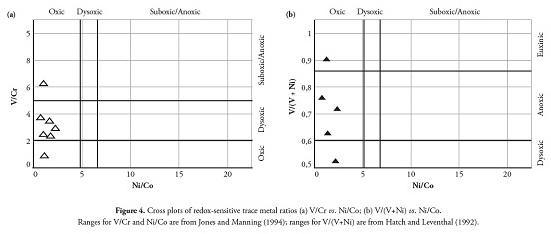
Redox-sensitive trace element ratios (Ni/Co, V/Cr and V/V+Ni) are usually considered powerful geochemical indicators for environmental discrimination (Lewan, 1984; Hatch and Leventhal, 1992; Jones and Manning, 1994; Hoffman et al.,, 1998; Rimmer, 2004; Rimmer et al.,, 2004; Algeo and Maynard, 2004; Johnson et al.,, 2010; Saez et al.,, 2011). Jones and Manning (1994) suggested that < 5 Ni/Co ratios inferred oxic conditions, 5-7 dysoxic conditions and > 7 suboxic to anoxic conditions. They also used < 2 V/Cr ratios to infer oxic conditions, 2-4.25 for dysoxic conditions and > 4.25 for suboxic to anoxic conditions. Lewan (1984) showed that V/V+Ni should be greater than 0.5 for organic matter accumulated in euxinic conditions.
Hatch and Leventhal (1992) compared V/V+Ni ratios to other geochemical redox indicators, including degree of pyritisation, and suggested that ratios greater than 0.84 showed euxinic conditions, 0.54-0.82 anoxic water and 0.46-0.60 for dysoxic conditions. According to Calvert and Pedersen (1993), Jones and Manning (1994), Hoffman et al (1998) and Algeo and Maynard (2004) anoxic environments are characterised by high V/Cr ratios and V/V+Ni values lying between 0.5 and 0.9 because of the disparate behaviour of V, Ni and Cr during redox processes in marine environments characterised by fine-grained detritic sedimentation.
All the samples analysed and shown on the V/Cr cf Ni/Co and V/ V+Ni cf Ni/Co diagram (Fig. 4) plotted within the oxic ranges for the four redox-sensitive trace element ratios proposed by Jones and Manning (1994).
Table 3 shows the composition average and standard deviation for the lignite samples from the Ogwashi-Asaba formation analysed here.
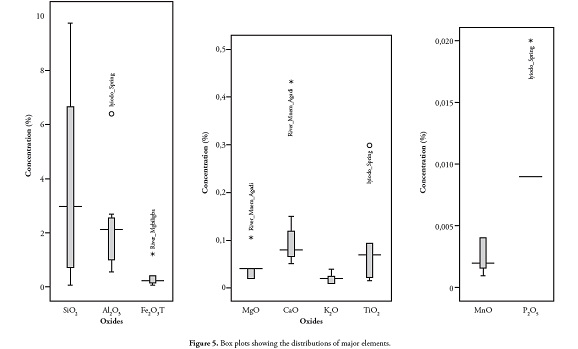
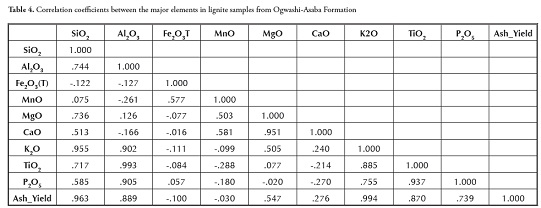
The data set was screened to identify outliers (Figure 5). The other elements were not normally distributed, except for SiO2, K2O and MnO; a non-parametric Spearman test was thus used which applies variables’ ranking order in determining the correlation coefficient. Table 4 shows major elements’ correlation coefficients. There was a strong positive relationship between MgO, K2O, TiO2 and P2O5 with SiO2, Al2O3 and ash yield, suggesting a common detrital source for these elements; the strong positive statistical correlation between Al2O3 and TiO2 (r= 0.993) was typical of elements which probably came from clastic rocks.
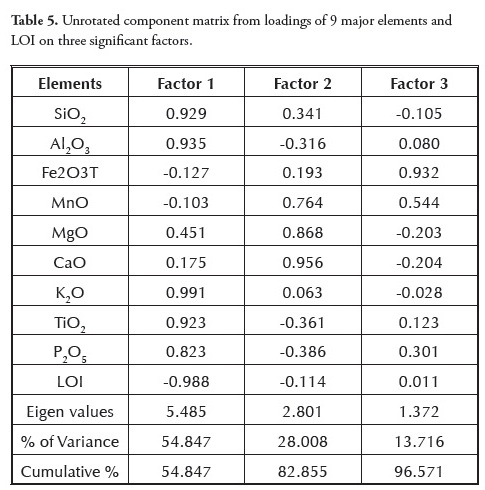
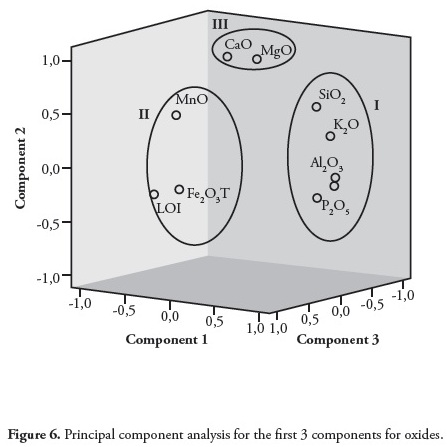
Factor analysis of the major elements studied here gained statistical information on the grouping and hence the processes responsible for their formation. The variance/covariance and factor loadings of the variables having eigenvalues were computed; factor analysis results are summarised in a factor matrix (Table 5). A 3-factor model was adopted, covering 96.6% of total variance.
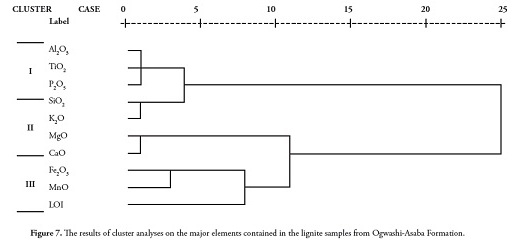
The first factor expressed 54.9% of total data variance and showed high positive loading for SiO2, Al2O3, K2O, TiO2 and P2O5 and negative loading for LOI (Table 6). The strong positive correlations between SiO2, Al2O3 and TiO2 were typical of clay minerals. The loadings in factor 1 reflected sediments believed to have their source probably in the mafic basement complex (Oban Massif ) on the north-eastern flank of the Niger Delta basin (Edet et al.,, 2003; Elueze et al.,, 2009). The presence of TiO2 and P2O5 was indicative of basaltic rocks from an oceanic environment (Ogala et al.,, 2009); such interpretation was supported by the strong positive correlation between TiO2 and P2O5 (r=0.937). The second factor expressed 28% of total variance and showed high positive loadings with MnO, MgO and CaO. The grouping in factor 2 suggested a lithogeochemical input representative of weathering and the composition of carbonate sediment. The close proximity of the limestone bands/nodules in the Ameki formation within the Anambra basin may have been the explained by organic affinity and these elements’ association with sulphide phases. The poor correlation of S with Cu (r = - 0.099), Zn (r = - 0.196), Cd (r = - 0.224), Ga (r = - 0.539), Pb (r = - 0.465), Sr (r = 0.242), Sc (r = 0.130), Y (r = -0.125), Zr (r = - 0.147), Be (r = - 0.196), V (r = - 0.330), Co (r = - 0.350), Cr (r = - 0.173), Ni (r = - 0.132) and Mo (r = - 0.428) indicated that sulphur was not only present in its sulphide form but also as an organic form (Finkelman, 1995).
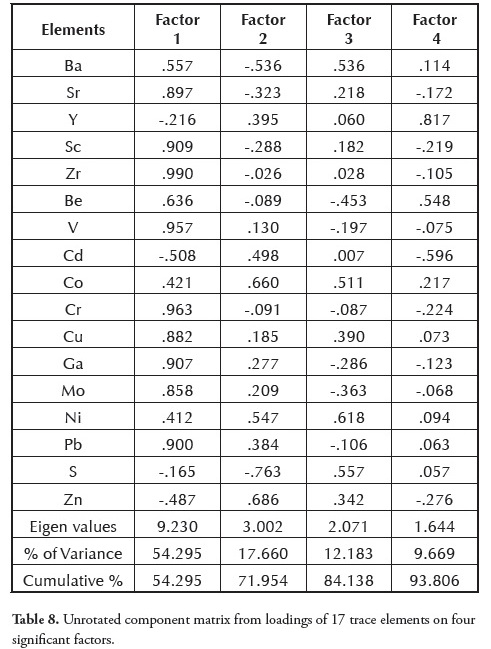
There were highly significant correlations (r ⥠0.90) between the elements in the following couples: Sr and Sc, Sr and Zr, Sr and Cr, Sc and Zr, Sc and Cr, Zr and V, Zr and Cr, Zr and Ga, V and Cr, V and Ga, V and Mo, V and Pb, Co and Ni, Cr and Ga, Ga and Mo, Ga and Pb and Mo and Pb (Table 7). This result was consistent with that recorded by Spears and Zheng (1999) and was interpreted as being essentially associated with detrital minerals and clay minerals.
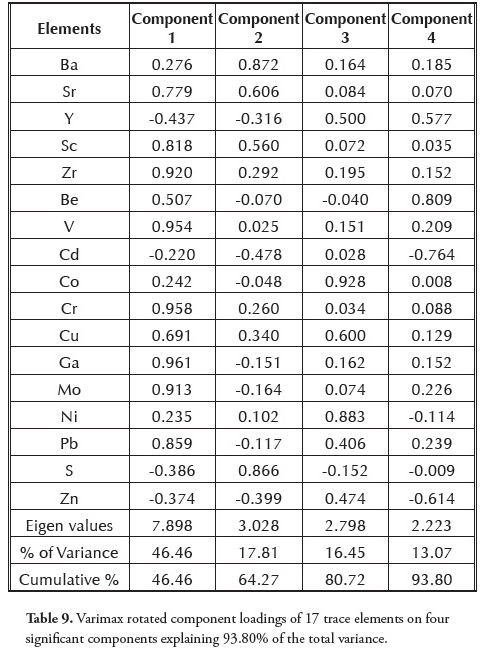
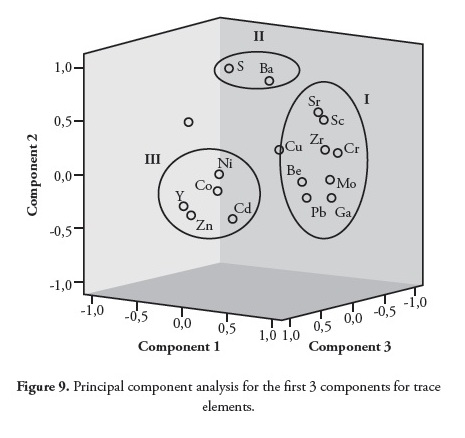
FA applied to the correlation matrix initially produced overlaps in Ba, Be, Co, Ni and Cd (Table 8), thereby necessitating further analysis. This was achieved by rotating the axis, thereby producing a new set of factors; this was attained after 11 iterations (Table 9). Each factor involved a sub-set of the original variables with as much minimal overlap as possible (Table 9). Since the differences between the unrotated and rotated components were significantly different, the rotated option was used for further analysis. A 4-factor model having 93.8% total cumulative variance was obtained (Table 9). Principal component 1 (PC1) expressed 46.5% of total data variance and showed high positive loadings with Sr, Sc, Zr, V, Cr, Cu, Ga, Mo and Pb (Table 9). The inferred host phases for PC1 were a mixture of lithophile (Sr, Sc, Zr, V and Cr; r = 0.803-0.973) and chalcophile (Cu, Ga, Mo and Pb; r = 0.620-0.829). The high positive loading of Pb in PC1 may have resulted from processes which led to the emplacement of Pb-Zn mineralisation in the Lower Benue trough (Akande and Erdtmann, 1998; Ogala et al.,, 2010b). The positive loading of Cu in both PC1 and PC3 indicated dual sources contributing to the presence of Cu. Further analysis using CA (Figure 10) confirmed that Cu belonged to PC1. PC1 had a high positive loading with Zr (Table 9), indicating a concentration of resistant heavy minerals, such as zircon.
Principal component 2 (PC2) expressed 17.8% of total data variance and had the highest loadings with Ba, Sr and S. According to Finkelman (1995) and Christanis et al.,, (1998), Ba and S have a strong organic affiliation, while Sr indicates a carbonate origin.
Principal component 3 (PC3) expressed 16.5% of total data variance and showed positive loading with Co, Cu and Ni. It could be inferred from the data obtained that this group of elements was associated with Fe-sulphide (pyrite). The reported occurrence of chalcopyrite (CuFeS2) in the Abakaliki area of the Lower Benue trough cannot be ruled out due to the presence of Cu (Elueze et al.,, 2009).
Principal component 4 (PC4) expressed 13% of total variance and showed positive loading with Y and Be and negative loading for Cd and Zn. PC4 was made up of a mixture of lithophile (Be and Y) and chalcophile (Cd and Zn), suggesting that the elements were associated with silicates and sulphides, respectively.
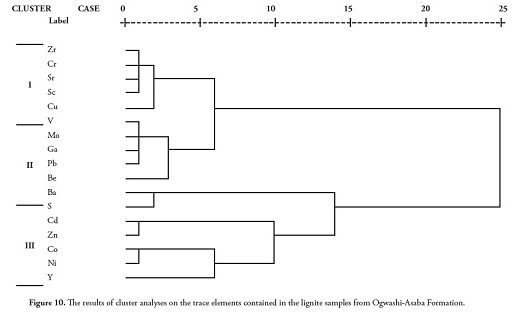
The trace elements were plotted in rotated space yielding three components (Figure 9). CA established trace element association and origin (Figure 10); this clustering (Fig. 10) coincided with the results obtained from studying the correlation coefficient matrix (Table 7) and PCA (Figure 9).
Cluster 1 consisted of elements associated with detrital input (i.e. Zr, Cr, Sr, Sc, Cu, V, Mo, Ga, Pb and Be) including quartz, clay and accessory minerals such as zircon and rutile commonly representing inorganic constituents (Finkelman, 1995). These elements’ strong positive correlation with each other (Table 7) and with ash yield indicated a common terrigenous origin.
Cluster 2 was made up of Ba and S, suggesting a strong organic association. The positive statistical correlation between Ba and S (r = 0.693) also supported an organic association for these elements. Cluster 3 consisting of Cd, Zn, Co, Ni and Y, indicated a strong association with sulphides such as pyrite (FeS2), sphalerite (ZnS), chalcopyrite (CuFeS2) and galena (PbS). The occurrence of polymetallic sulphide lodes of PbS, ZnS and CuFeS2 within the Lower Benue trough cannot be ruled out for these elements (Elueze et al.,, 2009).
Conclusion
The geochemical characteristics of the ignite seams within the Ogwashi- Asaba formation were investigated; the main mineral phases contained in the lignite were quartz, feldspar and pyrite. The relatively low silica (SiO2) and alumina (Al2O3) content suggested very limited detrital input during coal formation.
The elements’ geochemical association was examined through statistical correlation and principal component analysis (PCA) and cluster analysis (CA) of major and trace elements. The three components derived from FA corresponded to the three major elements’ cluster groups. The first group consisted of clastic rock-derived elements, whilst the second and third groups consisted of elements having carbonate and sulphide affinity, respectively. The concentrations for most trace elements were lower than world average values for low-rank coals. This study has also shown that silicate and sulphide minerals were responsible for the occurrence and distribution of most trace elements in the lignite samples studied here.
Acknowledgement
The author is grateful to Activation Laboratories, Ancaster, Canada, for performing the geochemical analysis.
References
Adamidou, K., Kassoli-Fournaraki, A., Filippidis, A., Christanis, K., Amanatidou, E., Tsikritzis, L., and Patrikaki, O., 2007. Chemical investigation of lignite samples and their ashing products from Kardia lignite field of Ptolemais, Northern Greece. Fuel, 86, 2502-2508. [ Links ]
Adedosu, T.A., Adedosu, H.O., and Adebiyi, F.M., 2007. Geochemical and mineralogical significance of trace metals in Benue Trough coals, Nigeria. Journal of Applied Sciences, volume 7, number 20, pp. 3101-3105. [ Links ]
Akande, S.O., and Erdtmann, B.D., 1998. Burial metamorphism (thermal maturation) in Cretaceous sediments of the southern Benue Trough and Anambra Basin, Nigeria. American Association of Petroleum Geologists Bulletin, volume 82, number 6, pp. 1191-1206. [ Links ]
Akande, S.O., Hoffknecht, A., Erdtmann, B.D., 1992. Rank and petrographic composition of selected Upper Cretaceous and Tertiary coals of southern Nigeria. International Journal of Coal Geology 71, 209- 224. [ Links ]
Algeo, T.J., and Maynard, J.B., 2004. Trace-element behaviour and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems. Chemical Geology 206, 289-318. [ Links ]
Benkhelil, J., 1989. The evolution of the Cretaceous Benue Trough, Nigeria. Journal of African Earth Sciences, 8, 251-282. [ Links ]
Calvert, S.E., and Pedersen, T.F., 1993. Geochemistry of recent oxic and anoxic marine sediments: implications for the geological record. Marine Geology, 113, 67-88. [ Links ]
Chatziapostolou, A., Kalaitzidis, S, Papazisimou, S, Christanis, K., and Vagias, D., 2006. Mode of occurrence of trace elements in the Pellana lignite (SE Peloponnese, Greece). International of Coal Geology, 65, 3-16. [ Links ]
Christanis, K., Georgakopoulos, A., Fernandez-Turiel, J.L., and Bouzinos, A., 1998. Geological factors influencing the concentration of trace elements in the Philippi peatland, eastern Macedonian, Greece. International Journal of Coal Geology, 36, 295-313. [ Links ]
Davidson, R.M., 2000. Mode of occurrence of trace elements in coal; Results from an International Collaborative Program. IEA Coal Research, London. [ Links ]
De Swardt, A.M.J., and Piper, H., 1957. The Lignites of Asaba Division, Benin Province. Records of the Geological Survey of Nigeria, pp. 5-23. [ Links ]
De Swardt, A.M.J., and Casey, O.P., 1963. The Coal Resources of Nigeria. Bulletin of Geological Survey of Nigeria, number 28. [ Links ]
Du Preez, J.W., 1945. Geology of Oba and Nnewi lignite deoposits, Onitsha Province. Annual Report of Geological Survey of Nigeria, 26-29. [ Links ]
Du Preez, J.W., 1946. Further observation on the lignites of Onitsha Province. Annual Report of Geological Survey of Nigeria, 25-26. [ Links ]
Edet, A.E., Merkel, B.J., and Offiong, O.E., 2003. Trace element hydrogeochemical assessment of the Calabar coastal plain acquifer, southeastern Nigeria, using statistical methods. Environmental Geology, volume 44, pp. 137-149. [ Links ]
Elueze, A.A., Ekwere, A.S., and Nton, M.E., 2009. Geoenvironmental assessment of the environs of the Aluminium Smelting Company in Ikot Abasi, southeastern Nigeria. Journal of Mining and Geology, volume 45, number 2, pp. 115-128. [ Links ]
Ewa, I.O.B., 2004. Data evaluation of trace elements determined in Nigerian coal using cluster procedures. Applied Radiation and Isotopes, volume 60, pp. 751-758. [ Links ]
Ewa, I.O.B., Adetunji, J., and Elegba, S.B., 1996. Determination of trace elements in Nigerian coal ash by Instrumental Neutron Activation Analysis. Journal of Environmental Science and Health, Part A, volume 31, pp. 1089-1100. [ Links ]
Filippidis, A., Georgakopoulos, A., Kassoli-Fournaraki, A., Misaelides, P., Yiakkoupis, P., Broussoulis, J., 1996. Trace element contents of three lignite seams from the central part of the Drama lignite deposit, Macedonia, Greece. International Journal of Coal Geology 29, 219-234. [ Links ]
Finkelman, R.B., 1994b. Modes of occurrence of potentially hazardous elements in coals: levels of confidence. Fuel Processing Technology, volume 29, pp. 21-34. [ Links ]
Finkelman, R.B., 1995. Modes of occurrence of environmentally sensitive trace elements of coal. Environmental aspects of trace elements of coal. Kluwer Academic Publishers, The Netherlands, 24-50 pp. [ Links ]
Foscolos, A.E., Goodarzi, F., Koukouzas, C.N., and Hatziyiannis, G., 1989. Reconnaissance study of mineral matter and trace elements in Greek lignites. Chemical Geology 76, 76-130. [ Links ]
Gentzis, Th., Goodarzi, F., and Broussoulis, Y., 1990. Petrographic characteristics and depositional environment of Greek lignites: II. Ioannina Basin, Western Greece. International Journal of Coal Quality 9(2), 53-61. [ Links ]
Georgakopoulos, A., Filippidis, A., and Kassoli-Fournaraki, A., 1994. Morphology and trace element contents of the fly ash from Main and Northern lignite fields, Ptolemais, Greece. Fuel 73, 1802-1804. [ Links ]
Gerouki, F., Foscolos, A.E., and Dimitroula, M., 1996. Environmental impact of trace elements encountered in fly ashes from power stations located in the wider area of Ptolemaes Basin. In: Anagnostopoulos, A. (editor), Proceedings of 3rd International Conference of Environmental Pollution, Thessaloniki, pp. 214-218. [ Links ]
Gluskoter, H.J., Ruch, R.R., Miller, W.G., Cahill, R.A., Dreher, G.B., and Kuhn, J.K., (1977): Trace elements in coal: occurrence and distribution. Illinois State Geological Survey Circular, volume 499, 154p. [ Links ]
Goodarzi, F., Gentzis, Th., and Yiakkoupis, P., 1990. Petrographic characteristics and depositional environment of Greek lignite: I. Drama Basin, Northern Greece. Journal of Coal Quality 9(1), 26-37. [ Links ]
Hatch, J.R., and Leventhal, J.S., 1992. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) stark shale member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chemical Geology, volume 99, pp. 65-82. [ Links ]
Hoffman, D.L., Algeo, T.J., Maynard, J.B., Joachimski, M.M., Hower, J.C., and Jaminski, J., 1998. Regional stratigraphic variation in bottom water anoxia in offshore core shales of Upper Pennsylvanian cyclothems from Eastern Midcontinent Shelf (Kansas), U.S.A. In: Schieber, J., Zimmerle, W., and Sethi, P., (editors), Shales and Mudstones, I.E. Schweizerbart sche Verlagsbuchhandlung, Stuttgart, pp. 243-269. [ Links ]
Horner, T.C., and Krissek, L.A., 1992. Statistical analysis of geochemical patterns in fine-grained Permian clastics from the Beardmore Glacier region, Antarctica. Recent Progress in Antarctic Earth Science. TERRAPUB, Tokyo, Japan, pp. 241-248. [ Links ]
Jan du Chêne, R., Onyike, M.S., Sowumi, M.A., 1978. Some new Eocene pollen of the Ogwashi-Asaba Formation, Southeastern Nigeria. Revista Española de Micropaleontología 10, 285 322. [ Links ]
Johnson, C.L., Hudson, S.M., Rowe, H.D., Efendiyeva, M.A., 2010. Geochemical constraints on the Palaeocene-Miocene evolution of eastern Azerbaijan, with implications for the South Caspian Basin and Eastern Paratethys. Basin Research 22, 733-750. [ Links ]
Jones, B., and Manning, D.A.C., 1994. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chemical Geology, volume 111, pp. 111-129. [ Links ]
Kogbe, C.A., 1976. Paleogeographic history of Nigeria from Albian times. In: Kogbe, C.A. (Ed.), Geology of Nigeria. Elizabethan Publishers, Lagos, pp. 237 252. [ Links ]
Lewan, M.D., 1984. Factors controlling the proportionality of vanadium to nickel in crude oils. Geochimica Cosmochimica Acta, volume 48, pp. 2231-2238. [ Links ]
Liu, C.W., Lin, K.H., and Kuo, Y.M., 2003. Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Science of the Total Environment, v o l u m e 313, numbers 1-3, pp. 77-89. [ Links ]
Lu, X., Zeng, H., Xu, T. and Yan, R., 1995. Chemometric studies of distribution of trace elements in seven Chinese coals. Fuel, volume 74, number 9, pp. 1382-1386. [ Links ]
Meij, R., 1995. The distribution of trace elements during the combustion of coal. In: Swaine, D.J., Goodarzi, F., (Editors), Environmental Aspects of Trace Elements in Coal. Kluwer Academic Publishing, The Netherlands, pp. 111-127. [ Links ]
Ministry of Mines and Steel Development of Nigeria (MOMSD), 2007. http://msmd.gov.ng/aboutmsmd/ProfileMinistry.asp. [ Links ]
Murat, R.C., 1972. Stratigraphy and paleogeography of the Cretaceous and lower Tertiary in southern Nigeria. In: African Geology (ed. by T.J.F. Dessauvagie and A.J. Whiteman), University of Ibadan Press, pp. 251-266. [ Links ]
Ndiokwere, Ch.L., Guinn, V.P., and Burtner, D., 1983. Trace elemental composition of Nigerian coal measured by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry, 79(1), pp. 123-128. [ Links ]
Nwachukwu, S.O., 1972. The tectonic evolution of the southern portion of the Benue Trough, Nigeria. Geological Magazine, 109, 411-419. [ Links ]
Nwadinigwe, C.A., 1992. Wax and resin characteristics of Nigeria s lignites and sub-bituminous coals. Journal of Mining and Geology 28(1), 75-80. [ Links ]
Obaje, N.G., 2009. Geology and mineral resources of Nigeria. Springer Verlag, Berlin, 221 pp. [ Links ]
Obaje, N.G., Hamza, H., 2000. Liquid hydrocarbon source-rock potential of mid-Cretaceous coals and coal measures in the Middle Benue Trough of Nigeria. Journal of African Earth Sciences 89, 130-139. [ Links ]
Obaje, N.G., Ligouis, B., 1996. Petrographic evaluation of the depositional environments of the Cretaceous Obi/Lafia coal deposits in the Benue Trough of Nigeria. Journal of African Earth Sciences 22, 159- 171. [ Links ]
Ogala, J.E., Akaegbobi, I.M., Omo-Irabor, O.O., and Finkelman, R.B., 2009. Statistical analysis of geochemical distribution of major and trace elements of the Maastrichtian coal measures in the Anambra Basin, Nigeria. Petroleum and Coal, volume 54, number 4, pp. 261-270. [ Links ]
Ogala, J.E., Omo-Irabor, O.O., Finkelman, R.B., and Akaegbobi, I.M., 2010a. Major and trace element distributions in coal and coaly shale seams in the Enugu Escarpment of southeastern Nigeria. Global Journal of Geological Sciences, volume 8, number 2, pp. 175-186. [ Links ]
Ogala, J.E., Finkelman, R.B., Akaegbobi, I.M., and Henry, F., 2010b. Evaluation of the trace element contents of some Nigerian coal samples. Journal of Mining and Geology, volume 46, number 2, pp. 151-161. [ Links ]
Okezie, C.N., Onuogu, S.A., 1985. The lignites of Southeastern Nigeria. A summary of available information. Geological Survey of Nigeria Occasional Paper No 10, 28 pp. [ Links ]
Olade, M.A., 1975. Evolution of the Nigeria s Benue Trough (aulacogen): a tectonic model. Geological Magazine, 112, 575-583. [ Links ]
Olabanji, S.O., (1991): Nigerian coal analysis by PIXE and RBS techniques. Journal of Radioanalytical and Nuclear Chemistry ,149(1), pp. 41-49. [ Links ]
Olajire, A.A., Ameen, A.B., Abdul Hammed, M., and Adekola, F.A., 2007. Occurrence and distribution of metals and porphyrins in Nigerian coal minerals. Journal of Fuel Chemistry and Technology, volume 35, pp. 641-647. [ Links ]
Olobaniyi, S.B., Ogala, J.E., 2011. Major and trace element characteristics of the Tertiary lignite series within the Ogwashi-Asaba Formation, southern Nigeria. Proc. 23rd Colloquium of African Geology/14th Conference of the Geological Society of Africa (Johannesburg, South Africa, January 8-14, 2011), abstract book p. 323. [ Links ]
Orajaka, I.P., Onwemesi, G., Egboka, B.C.E., Nwankor, G.I., 1990. Nigerian Coal. Mining Magazine 162, 446-451. [ Links ]
Orem, W.H. and Finkelman, R.B., 2003. Coal formation and geochemistry. In: F.T. Mackenzie (editor), Sediments, Diagenesis, and Sedimentary Rocks (volume 7), Treatise on Geochemistry, Elsevier- Pergamon, Oxford, pp. 191-222. [ Links ]
Reyment, R.A., 1965. Aspects of geology of Nigeria. University of Ibadan Press. 145 pp. [ Links ]
Rimmer, S.M., 2004. Geochemical paleoredox indicators in Devonian- Mississippian blacks shales, Central Appalachian Basin (U.S.A.). Chemical Geology 206, 373-391. [ Links ]
Rimmer, S.M., Thompson, J.A., Goodnight, S.A., and Robl, T.L., 2004. Multiple controls on the preservation of organic matter in Devonian- Mississippian marine blacks shales: geochemical and petrographic evidence. Palaeogeography, Palaeoclimatology, Palaeoecology 215, 125-154. [ Links ]
Saez, R., Moreno, C., Gonzalez, F., and Almodovar, G.R., 2011. Black shales and massive sulfide deposits: causal casual relationships? Insights from Rammelsberg, Tharsis, and Draa Sfar. Mineralium Deposita, 46, 585-614. [ Links ]
Sakorafa, V., Michailidis, K., and Burragato, F., 1996. Mineralogy, geochemistry and physical properties of fly ash from the Megalopolis lignite fields, Peloponnese, Southern Greece. Fuel 75, 419-423. [ Links ]
Seredin, V.V., Finkelman, R.B., 2008. Metalliferous coals: a review of the main genetic and geochemical types. International Journal of Coal Geology 76, 253-289. [ Links ]
Short, K.C., and Stauble, A.J., 1967. Outline of geology of Niger Delta. American Association of Petroleum Geologists Bulletin, 51, 761-779. [ Links ]
Simpson, A., 1949. The lignite seams of Asaba Division, Benin Province. Annual Report of Geological Survey of Nigeria, 6-14. [ Links ]
Simpson, A., 1954. The Nigerian coal field: The geology of parts of Onitsha, Owerri, and Benue provinces. Geological Survey of Nigeria Bulletin, No 24, 85 pp. [ Links ]
Sonibare, O.O., Ehinola, O.A., Egashira, R. and Lim, K., 2005. An investigation into the thermal decomposition of Nigerian coal. Journal of Applied Science, 5(1): 104-107. [ Links ]
Spears, D.A., and Zheng, Y., 1999. Geochemistry and Origin of elements in some UK coals. International Jour. Coal Geol., 38, pp. 161-179. [ Links ]
Swaine, D.J., 1990. Trace elements in coal. Butterworth and Co. (Publishers) Ltd. 278p. [ Links ]
Vassilev, S.V., Eskenazy, G.M., and Vassileva, C.G., 2001. Behaviour of elements and minerals during preparation and combustion of the Pemik coal, Bulgaria. Fuel Processing Technology 72, 103-129. [ Links ]
Wilson, R.C., 1924. Brown coal in Nigeria. Occassional Paper Number 1 of Geological Survey of Nigeria, 1-15. [ Links ]
Wilson, R.C., 1925. The geology of the Eastern Railway: Section I, Port- Harcourt to Enugu. Brown coal in Nigeria. Bulletin of Geological Survey of Nigeria, number 8, pp. 13-86. [ Links ]
Wilson, R.C., and Bain, A.D., 1928. The Nigerian Coalfield Section II, Parts of Onitsha and Owerri Provinces. Bulletin of Geological Survey of Nigeria, number 12, pp. 12-50. [ Links ]
Yazdi, M., and Shiravani, A.E., 2004. Geochemical properties of coals in the Lushan coalfield of Iran. International Journal of Coal Geology 60, 73-79. [ Links ]