Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Earth Sciences Research Journal
versión impresa ISSN 1794-6190
Earth Sci. Res. J. vol.17 no.1 Bogotá ene./jun. 2013
Metal speciation in sediments from crude oil prospecting in the coastal area of Ondo State, Nigeria
Olu-Owolabi Bamidele I.1, Agunbiade Foluso O.2 and Adebowale Kayode O.3
1 Department of Chemistry, University of Ibadan. Email: iromidayobamidele@yahoo.co.uk
2 Redeemer’s University, Nigeria. Email: foagunbiade@gmail.com
3 Department of Chemistry, University of Ibadan. Email: adebowale2003@yahooi.com
Recibido: 24/10/2012 - Aceptado: 04/042013
ABSTRACT
Information obtainable from metal speciation is far more valuable regarding the bioavailability, toxicity and fate of metals than information from total metal data. Metal speciation on sediment was thus carried out in this study to assess the bioavailability, fate and mobility of As, Cd, Cr, Fe, Mn, Ni, Pb, and V in the crude oil prospecting area of Ondo State in Nigeria. Five operationally-defined metal species were obtained using the sequential extraction method: exchangeable species, carbonate-bound species, iron/manganese-bound species, organic-bound species and residual species. Species’ concentration, spread and relative abundance were used to assess their fate, mobility and potential toxicity. The crude oil exploration site and the mouth of the estuary had the highest concentrations of these metals compared to other sites. The exchangeable and the organic-bound high risk species were most predominant in the crude oil exploration sites but their concentration became reduced downstream away from these sites. The residual fraction (the least in the crude oil prospecting site) was the most predominant in the most distant sites downstream. There were indications of self-management of the metals in the coastal system through favourable biogeochemical reactions partitioning metals from high risk species from low risk ones and the non-toxic residual fraction. It could, however, be concluded that sediment from the crude oil prospecting area may serve as non-point sources for metals contaminating the coastal system, they have higher metal bioavailability and higher toxicity risk potential than other sites (which should be curtailed)..
Key words: metal, fate, speciation, biogeochemical reaction, coastal sediment.
RESUMEN
La información obtenida a través de la especiación de metales es mucho más valiosa en términos de biodisponibilidad, toxicidad y destino que aquella información de sus datos generales. En este trabajo se llevó a cabo una especiación de metales en sedimentos para evaluar la biodisponiblidad, destino y movilidad de As, Cd, Cr, Fe, Mn, Ni, Pb y V en el área de explotación de crudo del estado de Ondo, Nigeria. Se obtuvieron cinco especies de metales operacionalmente definidas con el método de extracción secuencial: especies intercambiables, especies vinculadas al carbono, especies vinculadas al hierro y al manganeso, especies relacionadas a lo orgánico y especies residuales. La relativa abundancia, propagación y concentración de las especies fue utilizada para evaluar su destino, movilidad y riesgo de toxicidad. El sitio de la explotación de crudo y la boca del estuario tienen la mayor concentración de estos metales en comparación con otros lugares. Las especies intercambiables y relacionadas a lo orgánico de alto riesgo fueron más predominantes en los sitios de exploración de crudo, pero su concentración se reduce río abajo de estos lugares. La fracción residual (menos en el sitio de exploración de crudo) fue la más predominante en los lugares más distantes aguas abajo. Hubo señales de automanejo de los metales en el sistema costero a través de reacciones biogeoquímicas con particiones de metales desde especies de mayor riesgo hacia las de menor riesgo y con la fracción residual no tóxica. Podría concluirse que los sedimentos del área de explotación de crudo podrían ser fuentes no puntuales de metales contaminantes en el sistema costero, ya que tienen mayores potenciales de riesgo en biodisponibilidad y alta toxicidad a comparación de otros lugares (lo que debería ser reducido).
Palabras clave: Metales, destino, especiación, reacción biogeoquímica, sedimento costero.
Introduction
Sediment contamination poses one of the worst environmental problems in marine ecosystems around the world (Chapman et al., 1996; Sadiq, 2002; Bay et al., 2003; Valdes et al., 2005). Sediments act as sinks and sources for contaminants in aquatic systems (Adams et al., 1992; Rowlatt and Lovell, 1994; Mucha et al., 2003). Contaminants may sink in aquatic systems through the precipitation, complexation, adsorption and bioaccumulation of such contaminants which then settle by gravitational force (James, 2002). Sediments having high organic matter and fine-grained particles have been reported as showing high affinity for metal sorption, which may serve as better sinks (Ip et al., 2006; Pekey et al., 2007). However, changes in the environment’s biogeochemical and physical factors can reverse reactions and cause contaminants to be released from sediments. High magnitude contaminant concentrations and low variation regarding sediment composition have been documented in the pertinent literature (Shine et al., 1995, Beiras et al., 2003, Caccia et al., 2003; Pekey et al., 2006). These qualities have enhanced the use of sediments as preferred tools for environmental pollution monitoring in aquatic systems (Liu et al., 2003). Such monitoring always involves investigating metals, organic pollutants and other toxic substances on surface sediments or by coring through sediment depth (Szefer and Kaliszan 1993; Atgin et al., 2000). Studies of metals in sediments abound in the literature (Vogt, 1990; Zwolsman et al., 1996; Shin and Lam, 2001; Korfali and Davies, 2004). However, most studies have investigated total metal concentrations in these sediments. This approach has recently been regarded as less appropriate since not all total metal concentrations are bioavailable; only bioavailable metals are regarded as toxic in both soil and sediment (Ritchie et al., 2001; Korfali and Davies, 2004). These bioavailable metal species are the labile ones which are affected by changes in the environment’s biochemistry, thereby representing a departure from using total metals in monitoring the environmental pollution of soil and sediments. The use of metal species in now being adopted (Ianni et al., 2000; Korfali and Davies, 2004). Speciation studies of metals have been undertaken by sequential extraction, as initially proposed by Tessier et al., (1979), several modifications having mentioned in the literature. The operationally-defined species are: exchangeable species, carbonate-bound species, iron/manganese-bound species, organic-bound species and residual species. The outcome of metal speciation from sequential extraction gives the true pictures of metals’ bioavailability, their potentially adverse effects on the environment, the fate of the metals discharged into the environment and the degree of such metals’ mobility. This study was therefore aimed at evaluating the different species into which metals in coastal sediments from the crude oil exploration area of Ondo state have been partitioned. This will serve for measuring the metals’ bioavailability, potential risk, degree of mobility and fate.
Methodology
Background
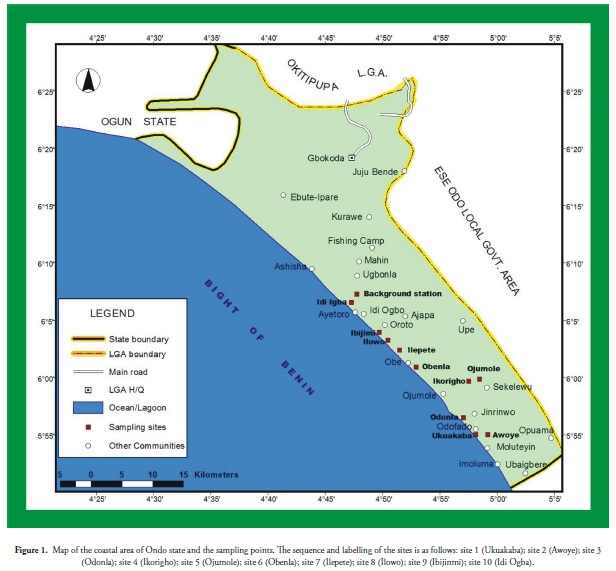
This study was conducted in Mahin, in the Ilaje area of Ondo State (Lat. 5o 50'N - 6o 09'N and Long. 4o 45'E - 5o 05'E); it is an estuary adjacent to the Atlantic Ocean. The area covered by the study has emerging communities dispersed along throughout the coastal area. The population of the entire state is about 3 million, population growth rate being 2.2% annually. The communities are dependent on water for their economic activities, being a fishing community. Several settlements are scattered around the river tributaries that empty into the ocean through this estuary. The area also falls within Nigeria’s oil-prospecting states, the so-called the Niger Delta region, because site 1 (Ukua) is an oil-prospecting site hosting the crude oil exploration platform of one of the operators of the Nigerian National Petroleum Corporation (NNPC) joint venture. Diverse river courses and streams traverse different settlements and discharge into the ocean (Bright of Benin, Atlantic Ocean) through this estuary. Ten sampling points were selected (Figure 1) along the estuarine part of the coast for evaluation in this study; sediment samples were collected at these sampling points. Sampling points were chosen after considering anthropogenic activities in the area representing potential sources of contaminants having a negative impact on the zone. Site 2 (Awoye), which is the estuary discharge point, also plays a significant role in the chemistry of the coast. Several crude oil exploration platforms are located within the vicinity of the discharge point but located in the Atlantic Ocean. The other eight sites were close to different settlements, at increasing distances from sites 1 and 2. Trading and fishing are the major occupations of the people along the coast. All domestic waste is directly discharged via river tributaries onto the coast; there is no waste treatment system in the area. Transportation of heavy goods and people in the area and fishing activities are carried out by motorboat, which is a potential non-point source of pollution.
Sampling and chemical analysis
A Van Veen grab sampler was used for randomly sampling sediments at each sampling site before being composited and kept in nylon. Samples were spread on plastic bags and air dried at the laboratory. The samples were ground after air drying and sieved through a 2mm sieve and analysed for As, Cd, Cr, Fe, Mn, Ni, V and Pb.
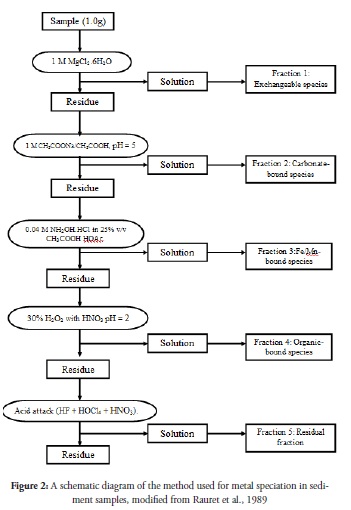
Figure 2 gives a schematic representation of the sequential extraction used for this analysis modified from the method proposed by Rauret et al., (1989). Fractions were shaken in conditions specified in the schematic diagram for each extraction (Figure 2) and centrifuged before decanting the supernatants. The exchangeable fraction was obtained after equilibrating for 1 hr at 25oC, after which the residue was used for extracting carbonate-bound metals by shaking the reaction mixture at 25oC for 5 hrs. Fe/Mn oxide-bound metals were then extracted from the residue with 6 hr equilibration of extractants and sediment samples at 100oC in a hot water-bath shaker. After extracting Fe/Mn oxide-bound metal species, the organic-bound fractions were obtained by shaking the reaction mixture at 25oC for 30 min. The final fraction (i.e. residual fraction) was obtained by wet oxidation at 100oC for 2hours. The chemicals used for the extraction are represented in the flow-chart (Figure 2). All reagent used were analytical grade and the necessary quality assurance steps were observed. The supernatants so obtained were quantitatively transferred into volumetric flasks and made up to 25ml each with 1M HNO3 before analysing for heavy metals using an atomic absorption spectrophotometer (AAS, Buck Scientific Model 205A). Analysis was carried out in triplicate and the mean values calculated.
Results and Discussion
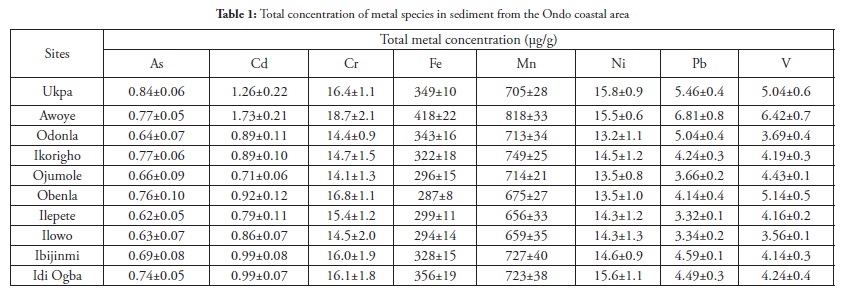
Metal concentration in sediments
Table 1 presents total metal species concentration results for the sediment samples from the ten sites in the coastal area. The results showed Mn to be the most abundant metal followed by Fe, this being consistent with earlier studies carried out along this coast (Adebowale et al., 2008a; Agunbiade et al., 2010). Ni and Cr ranked next in concentration. Metal concentrations had the highest values at the discharge point into the Atlantic Ocean at Awoye, followed by the crude-oil prospecting site (Ukpua) which had the highest As and Ni concentration. This trend may have been attributed to crude oil exploration in this region. Studies have shown that metal concentrations in the mouth of an estuary are generally higher than in the middle estuary (Grabemann et al., 1997; Guieu et al., 1998; Ip et al., 2007). The spread of metals in the different species obtained from sequential extraction is useful in understanding coastal sediments’ potential toxicity and the impact of activities along the coast, the fate and mobility of metals rather than information obtainable from total metal concentrations. Box and whisker presentation of data was used for assessing the spread of the following operationally-defined species: exchangeable-species, carbonate-bound species, Fe/Mn-bound species, organic-bound species and residual species.
Exchangeable metal species
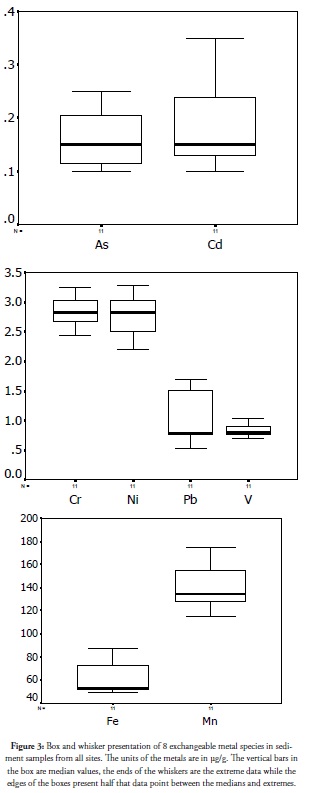
Exchangeable metal species as presented by the extraction from sediment method was the most loosely-bound and mobile metal species. It could be extracted from samples having aqueous solutions at pH 7 with only a change in extractant ionic strength; it was therefore the most bioavailable one. At high concentration it is an indicator of high impact, fresh discharge and increased environmental risk for the ecosystem (Ãlvarez et al., 2002). The box and whisker presentation of this specie is presented in Figure 3. As and Cd average concentration were almost equal, but their spread was different; Cd had the wider spread. The two metals ranged from 0.4μg/g to 0.1μg/g in this fraction. Ni and Cr were other metals having close concentration ranges and nearly equal median values. Ni varied more widely than Cr, both metals having values ranging from above 2μg/g to less than 3.5μg. The plot for Pb and V also showed nearly equal median values for both metals. Pb plots skewed towards the lower concentration and had a clearly wider range than V. The plot for Fe and Mn indicated that their concentration and variation was high in the exchangeable fractions in the coastal sediments from the coastal sediments. Exchangeable fraction concentrations of the studied metals indicated increased exposure risk potential from the sediments. The spread was wide and caused by high values at crude oil exploration sites, estuary discharged points and the reduction as distance increased away from these points. This was consistent with the results of earlier studies revealing the role of the environment’s biogeochemistry which has self- purification ability (Agunbiade et al., 2010). Such self-purification lowers the concentration of the highly mobile, more toxic fractions from the sediments as distance increases away from the aforementioned sites.
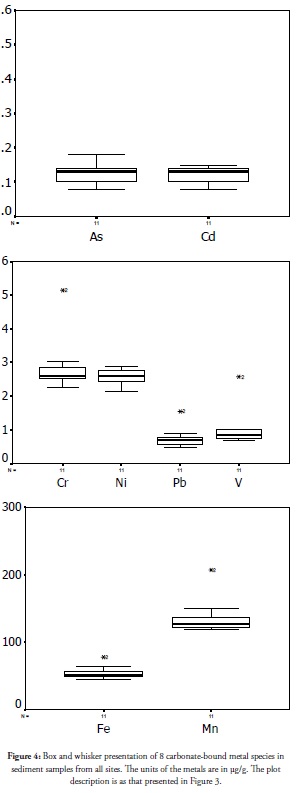
Carbonate-bound species
The extraction procedure for carbonate species in sediments requires a reduction in pH to 5 from the residue after exchangeable species extraction. pH reduction liberates CO2 and enhances metal species’ mobility and bioavailability in this matrix. It implies that the fate of carbonate-bound species metals is dependent on an ecosystem’s prevailing pH. Jarvie et al., (1997) documented that partitioning metals in the carbonate phase is enhanced by increased pH and the possibility of having a river with underlying carbonate, sedimentary rock. Reduced ecosystem pH will increase this species’ mobility and bioavailability and the risk of affecting an ecosystem without which a metal is safe. Jain (2004) reported that metals in carbonate species are in the medium risk range compared to high risk metal species. The outcome of carbonate-bound species extraction and their spread across this coast is presented in Figure 4. As and Cd results were closely related, as witnessed in exchangeable fractions, only their concentration and spread being lower than the result for exchangeable species. The lower concentration implied that As and Cd were less scavenged by the carbonate. Outlier values were observed at Awoye (site 2) for the metals which were in the upper extreme, indicating the estuary discharge point’s positive role in cleaning-up metals from the coastal region by partitioning Cd discharge into the carbonate fraction and reducing its bioavailability. Carbonate species in sediment were less affected by environmental changes such as naturally and commonly occurring microbial activities (Korfali and Davies, 2004). This species was therefore scavenging some metal contaminants and would stabilise them for a long time once the system’s pH was not lowered. Comparing the carbonate species (Figure 4) to the exchangeable species (Figure 3) showed lower concentrations (median value) and spread of carbonate species than exchangeable ones. However, upper extreme outliers witnessed for the carbonate bound metals at Awoye (site 2) represent positive results. It is therefore very evident that the chemistry of the Awoye coastal environment (the biogeochemical reactions in it) significantly influenced contaminant status by events occurring in this estuary and were favourable for reducing metal mobility, their consequent bioavailability and toxicity, thereby further confirming this coastal environment’s self-purification ability.
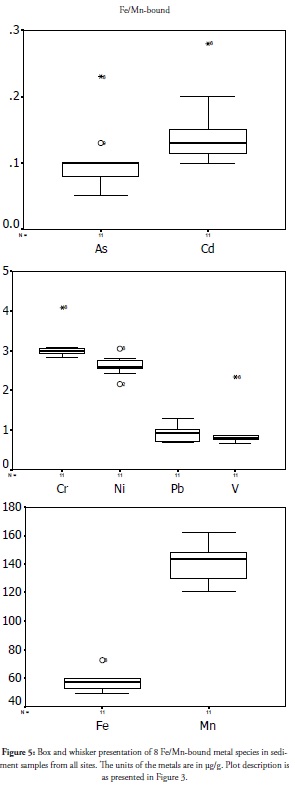
Fe/Mn-bound species
Metal affinity for Fe and Mn oxides by the adsorption of such metals to precipitate Fe and Mn oxides has been documented in the pertinent literature (Dekov et al., 1997; Guieu et al., 1998; Korfali and Davies, 2004). Fe and Mn oxide precipitates have active sites adsorbing metals and reducing their mobility and availability for living tissues (Ip et al., 2007). The extraction procedure also reflected a condition which may not occur naturally and frequently, indicating this species’ degree of stability in the ecosystem, medium range exposure risk and their reduced toxicity. The Fe/Mn-bound metal species trend in this study area’s coastal sediment is presented in Figure 5. Extreme upper concentration outliers were witnessed for most metals at site 6 (Obenla), except for Mn, Pb and Ni. Arsenic and nickel had a high concentration outlier at site 9 (Ibijinmi), therefore implying that the geochemistry for sites 6 and 9 was enhancing the scavenging of these metals from the ecosystem into this less bioavailable and relatively safe Fe/Mn bound fraction. However, Fe/ Mn bound metal species and the organic-bound ones in sediment could be remobilised by microbial activity (Jones and Turki, 1997; Korfali and Davies, 2004). Micro-organisms having reducing ability in sediment were able to reduce insoluble Fe (III) oxides to soluble Fe (II) oxides, thereby releasing the associated metals into solution; this metal fraction’s fate and mobility and its resulting toxicity was thus determined by these microbes’ activities and other prevailing biogeochemical reactions. The concentration and spread of the metal followed a trend similar to that of other fractions but geochemical activity at site 6 and 9 complimented the role played by the estuary discharge point to ensure contamination management in this coastal zone.
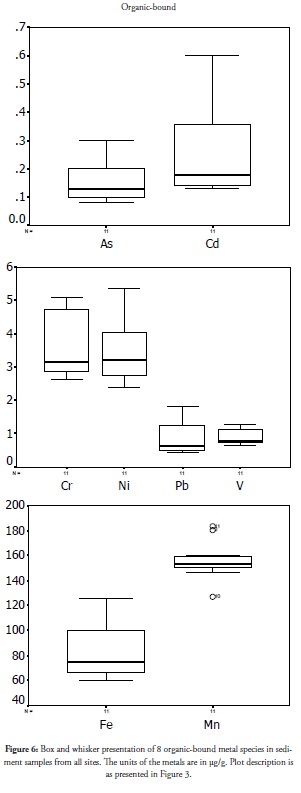
Organic-bound specie
Figure 6 presents the outcome for organic-bound metal species in coastal sediments. Concentration and variation were higher than that for the other species studied thus far. The highest set of organic species was observed at site 1 (Ukpa) and site 2 (Awoye), such wide variation being due to the reduction in value with increasing distance at these two sites. This would imply that metals discharging into the coast may have formed chelates with organic ligands possibly arising from Ukpa crude oil exploration and partition into the sediment phase as organic-bound metal species. The bioavailability of metals bound to organic complexes is affected by microbial activity in the sediment. Oxidising micro-organisms can oxidise the organic moiety into carbon dioxide and release associated metals, thereby increasing their mobility and toxicity. Likewise, organic complex formation by metals can increase their solubility in a lipophilic system and enhance their transport into living tissues for bioactivity. Organic-bound species’ concentration and spread pattern are thus indicators of high risk factors associated with anthropogenic activities in an area, especially in regions associated with the discharge of organic ligands. These organic and exchangeable fractions should be controlled, regardless of a coastal environment’s absorbing capacity so as not to over-burden it.
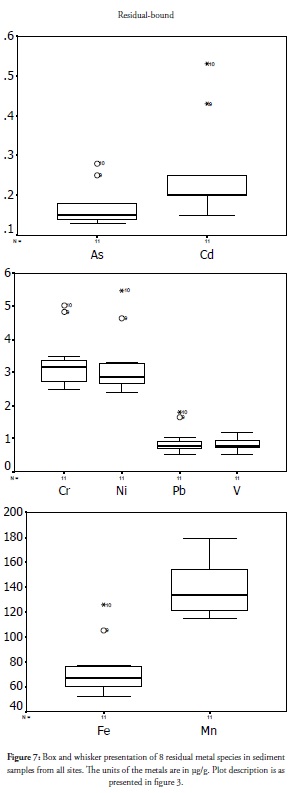
Residual fraction
The residual metal species is an indicator of metals natural to a site and is neither bioavailable nor mobile; it is thus not a toxic fraction. This fraction is stable and can only be obtained from samples by rigorous acid oxidation which does not occur in nature. This species’ high value relative to that of others is a positive development, indicating safe, non-toxic environmental media and could also be an expression of long-term metal deposition. Metals in the non-residual fraction may be indicators of temporary fresh discharge as against distant time release (Ip et al., 2007). The results of residual fraction for metals in Ondo coastal sediment is presented in Figure 7. The concentrations of the metals’ residual fractions increased away from sites 1 and 2. The plot shows higher concentration outliers at sites 9 and 10 except for V and Mn. It is evident that the metals in distant sites 9 and 10 were not freshly discharged and were less associated with anthropogenic activity. Site 9 and 10 were relatively safer than other sites studied. This was reflected in earlier studies on this coast (Adebowale et al., 2008a, Adebowale et al., 2008b). The increasing concentration of residual fraction away from sites 1 and 2 lend supported the reported self-management structure found in the coastal environment.
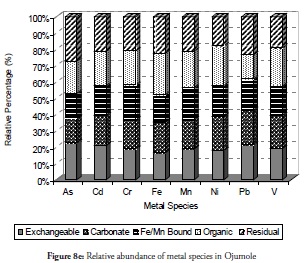
Metal species’ relative abundance
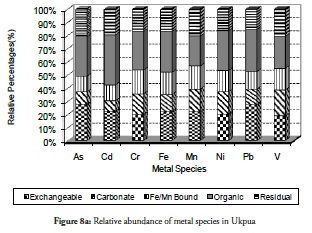
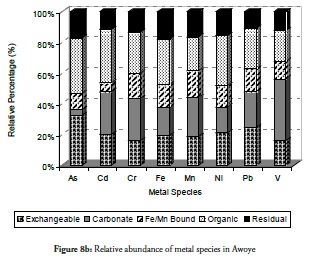
The trend of metal species relative to each other at the sites was evaluated with percentage-based bar charts. Figure 8a - 8j presents the results for the relative abundance of metal species within the five fractions investigated at the ten sites. It is evident that the organic bound (oxidisable species) and the exchangeable fraction were the most dominant species relative to others in sites 1 to 3 (Figure 8a - 8c) while the residual was the lowest in sites 1 and 2 but increased slightly at site 3. This would imply that metals in sediment from sites 1 to site 3 were highly mobile and could cause a high risk to the ecosystem. It indicated fresh or recent discharge of the metals. These two sites have been reported as playing significant roles in the chemistry of the area and the coast’s environmental status (Adebowale et al., 2008b). Crude oil exploration at site 1 (Ukpa) was therefore established as point source of contaminants affecting the coastal environment and the metals from this particular site were found in the free ion state in the water column while the sediment was the readily available exchangeable fraction. All the above increased the toxicity tendency of the metals at this site. The organic fraction was found abundant in these three locations, even the exchangeable fraction which was likely associated with formation of complexes (ligands) between the organic compounds from the crude-oil with metals. The organic fraction was also a potentially risky species to the biota because the formation of complexes by metals would increase the metals’ solubility and their mobility into lipophilic systems which are common in living tissues and enhance the formation of bonds in living tissues by the complexes so formed. Thus, organic fraction formation increases metal mobility into living tissues, the food chain and consequently their bioaccumulation and bioactivity.
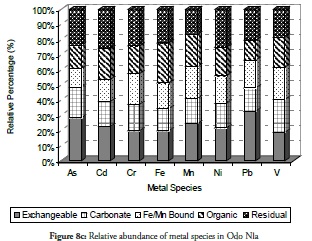
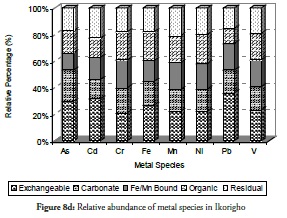
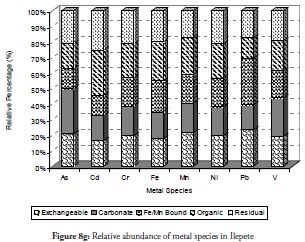
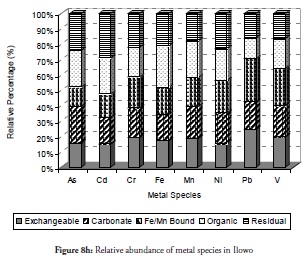
Overall, the relative abundance results for metal species at sites 1 to 3 revealed that the sediment from these sites had a negative impact on the coastal area. Despite this result, however, notable amounts of carbonate-bound fractions of V, Cd, Cr and Mn were witnessed at site 2. This would definitely have reduce these metals’ mobility, bioavailability and resultant toxicity. By contrast, carbonate species of extremely toxic metals like As, Cd, and Pb were least common at site 1 followed by their Fe/Mn-bound species. These three metals being toxic could have been preferred in carbonate- and Fe/Mn-bound species, especially at site 1 for discharged metal management. Site 3 witnessed a slight increase in residual fraction compared to other sites, thus far discussed particularly for As, Cd, Cr, Fe and Ni which were above 25% of total metal concentration. The exchangeable fraction predominated at site 4 (Figure 8d), especially for metals like As, Cd, Fe and Pb while the organic fraction was more reduced than the ratio obtained in earlier sites but relatively higher than that for other fractions. These trends regarding exchangeable and organic fractions were both risk-creating situations for this site. The residual fraction in this site was further reduced than that at earlier sites. There was a very high likelihood that there were recent discharges of metals from domestic waste disposal or other related anthropogenic activities. Closer distribution of relative abundance of other metal species was witnessed for other fractions at this site than at other sites. A similarly close relationship concerning the relative abundance of metal species was witnessed at site 5 (Figure 8e) where the five metals species were closely related for all the metals. The residual fraction however was more increased than at the preceding site (site 4) and was so at all other sites as the distance increased away from the crude-oil exploration site and the estuary discharge point.
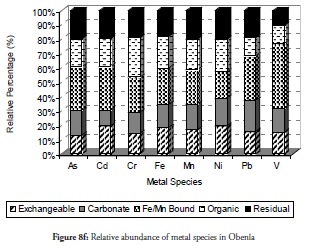
The trend of the species at site 6 (Figure 8f) was completely different from all other sites. The less common fraction at most other sites (Fe/Mn bound fraction) was the predominant specie at this site. The relative abundance of As, Cd, Pb and V was notably high in this fraction while that of Cr, Mn, Fe and Ni increased more than at other sites discussed earlier. It was therefore evident that this site’s biogeochemistry favoured these metals precipitation into the reducible fraction with resultant favourable reduction of metal mobility, bioavailability and toxicity. The organic and the exchangeable fractions reduced at this site while the carbonate-bound fraction was the least observed for most metals. The residual fraction only increased slightly in the site to more than 20%, except for V. This residual quantity was maintained in site 7 (Figure 8g) with increased residual fraction for V over the previous site. Organic fractions for Cd, Cr, Fe, Mn and Ni predominated at the site while As and V existed in the carbonate bound fraction which was positive for managing these metals at that site. The exchangeable fraction in site 7 was more reduced than that observed in earlier sites except for Pb which increased more than the preceding site. Pb in this site exhibited a preference for the Fe/Mn-bound at this site while the other metals had the lowest concentration in the Fe/Mn-bound fraction.
The reduction in the trend of exchangeable fraction concentration witnessed at site 7 became further reduced at site 8 (Figure 8h) except for Pb as was the case with site 7. The residual fraction also increased especially for As, Cd, Cr, and Ni. Pb exhibited a high preference for the reducible fraction similar to that obtained at site 7 while the carbonate specie of As in this site was similar to that of site 7. It was evident that this coastal site’s alkalinity was essential for management of As present in the area.
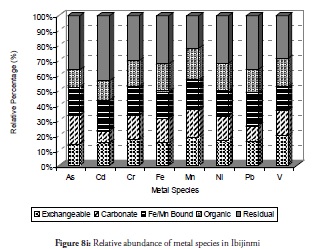
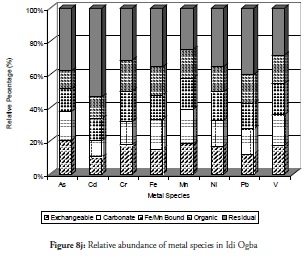
The relative percentage abundance of the metal species at sites 9 and 10 (Figure 8i and 8j respectively) clearly revealed the favourable roles played by the biogeochemical reactions natural to this environment. The metals discharged into the environment have been demobilised into the residual fraction at these two sites. This showed why the safe, non-bioavailable residual fraction predominated at these coastal sites. The trends observed for all the metal fractions in the sediment from these sites agreed perfectly with the results expressed earlier in this study which supported the self-purification hypothesis. Of particular interest was Cd and Pb management at these two sites as they have been found in high quantities in other sites referred to by previously discussed results. The organic and exchangeable fractions which were higher at other sites reduced in these sites while the carbonate- and Fe/Mn-bound species maintained their relative abundance, except in Cd. Thus, the relative abundance of the metals at these two sites indicated that the sites were more risk-averse zones on the entire coast and less stressed in the ecosystem.
Conclusion
This study has assessed five operationally-defined metal species: exchangeable species, carbonate-bound species, iron/manganese-bound species, organic-bound species and the residual species in sediments from the Ondo coastal area using sequential extraction. The crude oil exploration site and the mouth of the estuary had the highest concentrations of these metals compared to other sites. Mn and Fe had the highest concentrations of all the eight metals investigated. The high risk, exchangeable and organic-bound species were the most predominant metal species in the crude oil exploration and estuary discharge sites. There were reductions in the concentrations of highly mobile species away from these two sites to other sites downstream. The reduction was complimented by increased low risk species. The residual fraction occurring least in the crude oil prospecting site was the most predominant in the farthest downstream sites. This would thus indicate self-management of metals in some of the sites within the costal system through favourable biogeochemical reactions partitioning the metals from the high risk species to the low risk ones and the non-toxic residual fraction. It could thus be concluded that the fate of the metals in the sediments from this coastal sediment is from highly mobile, bioavailable species, exchangeable and organic-bound, to the less bioavailable, less toxic species, carbonate-bound and residual species. The crude oil prospecting area may serve as a non-point source of metal contaminant to the coastal system. It has higher metal bioavailability and higher toxicity risk potential than the other sites and should be curtailed.
References
Adams, W.J., Kimerle, R.R., Barnett, J.W., 1992. Sediment quality and aquatic life assessment. Environmental Science and Technology 26 (10), 1864-1875. [ Links ]
Adebowale, K. O., Agunbiade, F. O., Olu-Owolabi, B. I. 2008a. Impacts of natural and anthropogenic multiple sources of pollution on the environmental conditions of Ondo State coastal water, Nigeria. Electronic Journal of Environmental, Agriculture and Food Chemistry 7(4): 2797 - 2881. [ Links ]
Adebowale, K. O., Agunbiade, F. O., Olu-Owolabi, B. I. 2008b. Fuzzy Comprehensive Assessment of Metal Contamination of Water and Sediments in Ondo Estuary, Nigeria. Nigeria. Chemistry and Ecology 24 (4), 269 - 283. [ Links ]
Agunbiade, F. O., Olu-Owolabi, B. I., Adebowale, K. O. 2010. Seasonal and spatial variations analysis of pollution status of Ondo coastal environment Nigeria using principal component analysis. Geochemical Journal 44 (2), 89 - 98. [ Links ]
Alvarez, E.A., Mochón, M.C., Sánchez, J.C.J. and Rodríguez, M.T. 2002. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 47(7), 765-775 [ Links ]
Atgin, R.S., El-Agha, O., Zararsiz, A., Kocatas, A., Parlak, H., Tuncel, G., 2000. Investigation of the sediment pollution in Izmir Bay: trace elements. Spectrochimica Acta Part B 55, 1151-1164. [ Links ]
Bay, S. M., Zeng, E. Y., Lorenson, T. D., Tran, K. D., Alexander, C. 2003. Temporal and spatial distributions of contaminants in sediments of Santa Monica Bay, California. Marine Environmental Research 56, 255 - 276. [ Links ]
Beiras, R., Bellas, J., Fernández, N., Lorenzo, J.I., Cobela-García, A., 2003. Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula); metal concentrations in seawater, sediments and mussels (Mytilus galloprovincialis) versus embryo-larval bioassays using Paracentrotus lividus and Ciona intestinalis. Marine Environmental Research 56, 531-553. [ Links ]
Caccia, V.G., Millero, F.J., Palanques, A., 2003. The distribution of trace metals in Florida Bay sediments. Marine Pollution Bulletin 46, 1420 - 1433. [ Links ]
Chapman, P. M., Paine, M. D., Arthur, A. D., Taylor, L. A., 1996. A triad study of sediment quality associated with a major untreated marine sewage discharge. Marine Pollution Bulletin 32, 47 - 64. [ Links ]
Dekov, V.M.; Komy, Z.; Araujo, F. and Van Grieken, R., 1997. Chemical composition of sediments, suspended matter, river water and ground water of the Nile (Aswan-Sohag traverse). Science of the Total Environment 201, 195 - 210. [ Links ]
Grabemann, I., Uncles, R. J., Krause, G., and Stephens, J. A., 1997. Behaviour of turbidity maxima in the Tamar (U.K.) and Weser (F.R.G.) estuaries. Estuarine, Coastal and Shelf Science, 45, 235-246. [ Links ]
Guieu, C., Martin, J.M., Tankere, S.P.C., Mousty, F., Trincherini, P., Bazot, M., 1998. On trace metal geochemistry in the Danube River and Western Black Sea. Estuarine Coast. Shelf Sci. 47, 471 - 485. [ Links ]
Ianni, C., Magi, E., Rivaro, P., Ruggieri, N., 2000. Trace metals in Adriatic coastal sediments: distribution and speciation pattern. Toxicol. Environ. Chem. 78, 73-92. [ Links ]
Ip, C. C. M., Li, X. D., Zhang, G., Wai, O. W. H., Li, Y. S., 2007. Trace metal distribution in sediments of the Pearl River Estuary and thesurrounding coastal area, South China. Environmental Pollution 147 (2), 311-323. [ Links ]
Jain, C.K. 2004. Metal fractionation study on bed sediments of River Yamuna, India. Water Research 38(3), 569-578 [ Links ]
James, I.D. 2002. Modelling pollution dispersion, the ecosystem and water quality in the coastal waters: a review. Environmental Modelling and Software 17, 363 - 385. [ Links ]
Jarvie, H.P., Neal, C., Leach, D.V., Ryland, G.P., House, W.A., Robson, A.J., 1997. Major ion concentrations and the inorganic carbon chemistry of the Humber rivers. Sci. Total Environ. 194y195, 285-302. [ Links ]
Jones, B., Turki, A., 1997. Distribution and speciation of heavy metals in surficial sediments from the Tees Estuary, Northeast England. Mar. Pollut. Bull. 34, 768-779. [ Links ]
Korfali, S. I., Davies, B. E. 2004. Speciation of metals in sediment and water in a river underlain by limestone: role of carbonate species for purification capacity of rivers. Advances in Environmental Research 8; 599-612. [ Links ]
Liu, W.X., Li, X.D., Shen, Z.G., Wang, D.C., Wai, O.W.H., Li, Y.S., 2003. Multivariate statistical study of heavy metal enrichment in sediments of the Pearl River Estuary. Environmental Pollution 121, 377- 388. [ Links ]
Mucha, A.P., Vasconcelos, M.T.S.D., Bordalo, A.A., 2003. Macro benthic community in the Douro Estuary: relations with trace metals and natural sediment characteristics. Environmental Pollution 121, 169-180. [ Links ]
Pekey, H. 2006. The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Marine Pollution Bulletin 52; 1197-1208. [ Links ]
Rauret, G., Rubio, R., Lopez-sanchez, J. F., Casassas, E. 1989. Specific Procedure for Metal Solid Speciation in Heavily Polluted River Sediments. International Journal of Environmental Analytical Chemistry. 35(2), 89-100. [ Links ]
Ritchie, J.M.; Cresser, M. and Cotter-Howells, J. 2001. Toxicological response of a bioluminescent microbial assay to Zn, Pb and Cd in an artificial soil solution: relationship with total metal concentrations and free ion activities. Environmental Pollution 114, 129 -136. [ Links ]
Rowlatt, S.M., Lovell, D.R., 1994. Lead, zinc and chromium in sediments around England and Wales. Marine Pollution Bulletin 28 (5), 324-329. [ Links ]
Sadiq, M. 2002. Metal contamination in sediments from a desalination plant effluent outfall area. The Science of the Total Environment 287, 37 - 44. [ Links ]
Shin, P.K.S., Lam, W.K.C., 2001. Development of a marine sediment pollution index. Environ. Pollut. 113, 281-291. [ Links ]
Shine, J.P., Ika, R.V., Ford, T.E., 1995. Multivariate statistical examination of spatial and temporal patterns of heavy metal contamination in New Bedford Harbour marine sediments. Environmental Science and Technology 29, 1781- 1788. [ Links ]
Szefer, P., Kaliszan, R., 1993. Distribution of elements in sediment cores of the Southern Baltic from the point of view of principal component analysis. Studia 1 Materialy Oceanogeczne NR 64. Mar. Pollut. 1, 95- 102. [ Links ]
Tessier, A.; Campbell, P.G.C. and Bisson, M., 1979. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51, 844 - 851. [ Links ]
Valdes, J., Vargas, G., Sifeddine, A., Ortlieb, L., Guinez, M. 2005. Distribution and enrichment evaluation of heavy metals in Mejillones Bay (23oS), Northern Chile: Geochemical and statistical approach. Marine Pollution Bulletin 50, 1558 - 1568. [ Links ]
Vogt, N.B., 1990. Multivariate ecotoxicological mapping of the relationships between sediment chemical composition and fauna diversity. The Sci. Total Environ. 90, 149-161. [ Links ]
Zwolsman, J. J., van Eck, G. T. M. and Burger, G. 1996. Spatial and temporal distribution of trace metals in sediments from the Scheldt Estuary, South-west Netherlands. Estuarine, Coastal and Shelf Science 43, 55 - 79. [ Links ]