Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Ingeniería y Ciencia
versión impresa ISSN 1794-9165
ing.cienc. vol.9 no.18 Medellín jul./dic. 2013
ARTÍCULO ORIGINAL
Physicochemical and Microbiological Characterization of Apis mellifera sp. Honey from Southwest of Antioquia in Colombia
Caracterización físico-química y microbiológica de la miel de Apis mellifera sp. del Suroeste de Antioquia, Colombia
A. M. Velásquez Giraldo1, L. M. Vélez Acosta2 and R. Zuluaga Gallego3
1 Ing. Agroindustrial, avelasquez@pubpol.umass.edu, Estudiante University of Massachusetts, Amherst, Estados Unidos
2 Mg. en Desarrollo, lina.velez@upb.edu.co, Universidad Pontificia Bolivariana, Medellín, Colombia.
3 PhD. en Ingeniería, robin.zuluaga@upb.edu.co, Universidad Pontificia Bolivariana, Medellín, Colombia.
Received: 23-03-2013, Acepted: 17-06-2013 Available online: 05-11-2013
Abstract
Characterizations of Apis mellifera honey produced in Southwest of Antioquia, an important coffee region of Colombia, have not been published in recent years. In the present work, seven samples of honey collected in the mentioned region, were physically (refractive index, specific rotation, density), chemically (moisture content, water activity, pH, free acidity, carbohydrates) and microbiologically (Clostridium, fungi and yeast) analyzed. The results show that the analyzed honeys meet both national (Resolución 1057 of 2010) and International (Codex-Stan 12 of 1981) standards for moisture content, free acidity, sucrose content and microbiological parameters, indicating their good quality. Fructose/glucose ratio, pH and specific rotation values indicate that the samples are blossom honeys.
Key words: honey; honey bee; apis mellifera; physicochemical parameters; microbiology analysis; quality standards.
Highlights
• Southwest of Antioquia honeys have different water activity behavior than honeys from other Colombian regions. • Absence of microorganisms reveals the application of good manufacturing practices. Fructose/ glucose ratio, pH and specific rotation values indicate that the samples are blossom honeys. • Southwest of Antioquia honeys meet current Colombian and International regulation.
Resumen
Caracterizaciones de miel de Apis mellifera producida en el Suroeste de Antioquia, una región cafetera de Colombia, no se han publicado recientemente. En el presente trabajo, siete muestras de miel recolectadas en la región mencionada, fueron físicamente (índice de refracción, rotación específica, densidad), químicamente (contenido de humedad, actividad acuosa, pH, acidez libre, carbohidratos), y microbiológicamente (Clostridium, hongos y levaduras) analizadas. Los resultados muestran que las mieles analizadas cumplen tanto los estándares nacionales (Resolución 1057 de 2010) como internacionales (Codex-Stan 12 de 1981) para contenido de humedad, acidez libre, contenido de sacarosa y los parámetros microbiológicos, indicando su buena calidad. Los valores de relación fructosa/glucosa, pH y rotación específica indican que las muestras son mieles florales.
Palabras clave: miel de abejas; abeja melífera; apis mellifera; parámetros físico-químicos; análisis microbiológico; estándares de calidad.
1 Introduction
The Codex Alimentarius regulation for honey, Codex-Stan 12 of 1981 [2], defines it as the natural sweet substance produced by Apis mellifera bees from the nectar of plants or extrafloral secretions that bees transform and store. Due to this nectar origin, honey is composed mainly of sugars, specially fructose and glucose, followed by sucrose and other saccharides. Honey also contains water, traces of organic acids, enzymes, amino acids, pigment, traces of pollen and wax [3], and volatile substances [4]. Depending on the origin of these components, honey exhibits specific properties that can be analyzed in order to determine its botanical origin, geographical origin or quality. Honeys of all over the world have been chemical, physical and microbiologically characterized in order to found differential aspects among them.
Besides the intrinsic characteristics, honey usually contains microorganisms like yeasts and spore-forming bacteria from the environment. Contamination with microbial organisms may come from primary sources (pollen, the digestive tracts of honey bees, dust, air, dirt and nectar), or from secondary sources (contamination due to human manipulation and transformation processes). Primary sources are very difficult to control but secondary sources of contamination can be controlled applying good manufacturing practices [5].
Until mid-2010, the only local existing regulation for honey was NTC1273 Colombian technical standard, issued in 1997 by the Instituto Colombiano de Normas Técnicas y Certificación(Icontec) (private entity). Since March 2010 came into effect Resolución 1057 by which the Ministry of Social Protection of the national government establishes the technical regulations on the health requirements to be met by honey for human consumption. Unlike the NTC 1273, Resolución 1057 is more lax in some parameters such as moisture content and hydroxymethylfurfural (HMF), perhaps in response to new proposals of the scientific community (e.g.the increase in the accepted HMF limit, suggested by [6]). Although some parameters are more flexible, the community of beekeepers should be prepared to meet these requirements, because the governmental character of the norm strengthens its application.
In Colombia, honey is mainly produced through the utilization of Africanized bees, Apis mellifera sp. By 2008, the Southwestern sub-region held the 65% of the beekeeping activity of Antioquia [7], making it necessary to concentrate efforts on the study of bee products from this specific area. Southwest of Antioquia is a mountainous, coffee production zone with a great potential for beekeeping due its geographic, climatic and botanic resources. Furthermore, since 2000 it has appeared some rural associations dedicated to this activity in the area, and they need support in the technical area to access to new markets, taking into account their product quality.
Preliminary efforts to characterize honey from the coffee zone of Southwestern Antioquia were reported by [8], who found reducing sugars content, moisture content, free acidity, diastase activity, HMF content and ashes for 18 samples from the coffee zone of Southwest of Antioquia. Even though this represents a first approach, these results must be updated in order to know the current honey conditions and the actual compliance to the regulations.
For all the reasons previously exposed, in this paper we analyze physical (refractive index, specific rotation, density), chemical (moisture content, water activity, pH, free acidity, carbohydrates) and microbiological (Clostridium, fungi and yeast) properties of Sothwest Antioquia honey, intending to provide the academic and industrial community with input information for their research projects and processes, respectively. These aspects are fundamental to understand the composition of local honey in order to exploit its potential in the subsequent transformation processes. The characterization also aims to determine the compliance to the existing parameters of Colombian [1] and International [2] regulations.
2 Materials and methods
2.1 Honey Samples
Analyses were carried out for seven samples of honey (n = 7) collected during the first semester of 2011, in Southwest of Antioquia. Samples were provided by local producers. They indicated that the samples had not been heated or pasteurized, and were less than 6 months old. At the laboratory, samples were stored at room temperature.
2.2 Physicochemical characteristics
All physicochemical analyses were carried out in triplicate to comply with standards suggested by [6] and the typical honey analysis found in the literature. The decision of the kind of analysis to conduct was based in the parameters requested by the existent regulations and the equipment available to the researchers.
2.2.1 Specific rotation (Polarimetry) The specific rotation analysis was applied to measure the chirality of the honey samples. It was conducted in a Jina Polarimeter, under the code 781 USP 33 of USP methods.
2.2.2 Density Honey samples were loaded into a Blaubrand Pycnometer (Reference 43420), with a 24:7023 cm3 volume. The weight of the sample was found by the difference between the weight before and after loading the pycnometer. Temperature of analysis was 23:4 °C ± 1:4. We report the temperature because in general, at a constant pressure, the density of a solution decreases as the temperature rises. Literature did not provide a mathematical correction for density in relation with temperature, and this concern should be address to those interested in using honey density data for further analysis.
2.2.3 Moisture content Water or moisture content was indirectly measured by refractometry using a B&C 32400 digital Abbe Refractometer, and correlating according to Chataway Charts [9]. All measurements were performed at 21:5 °C ± 2:3 and data was corrected to 25 °C as indicated by [10].
2.2.4 Water activity Water activity data was measured with a Rotronic Higropalm device, using the Awquick mode and a stabilization time of 15 min per sample. The temperature of analysis was 24 °C ± 1:2.
2.2.5 pH and free acidity A solution of 10 g of honey in 75 ml of distilled water was prepared. Initial pH value was measured, and then the solution was titrated with 0:1 M NaOH until the pH reached 8:5. The pH was measured with a Schott Instruments pH Meter (Lab 850 series) with a pH 14 BlueLine electrode.
2.2.6 Carbohydrate analysis High Resolution Liquid Chromatography (HPLC) analysis was conducted using a Prominence series Shimadzu device, equipped with an autoinjector (SIL 20A) and a refractive index detector (RID 10A).
The resolution of the sucrose, glucose and fructose was achieved using a Waters IC-Pack column Ion Exclusion of 150 mm x 7:8 mm, with 0:01 N sulfuric acid as mobile phase at a temperature of 30 °C and a flow of 0:6 ml=min. Fructose + glucose values are reported as % of total solids (dry weight).
2.3 Microbiological parameters
2.3.1 Sulfite-reducing Clostridia This analysis was carried out according to the methodology proposed by [4] for the isolation of vegetative cells of sulfite-reducing Clostridia. Anaerobic bacteria were isolated on Sulfite Polymyxin Sulfadiazine (SPS) agar which contains per liter: peptone 15 g; yeast extract, 10 g; sodium sulfite, 0:5 g; ferric citrate, 0:5 g; Polymyxin B sulfate, 0:01 g; sulfadiazine sodium salt, 0:12 g, and agar, 13:5 g. First, 20 g of honey was suspended in 150 ml of peptone water, homogenized, and centrifuged at 8500 g and 4 °C during 60 min. The sediment was re-suspended in 7 ml of peptone water. Then, a series of dilutions (1 : 10) were cultured in Miller?Pricket tubes containing SPS medium. The tubes were sealed with Vas-Par and incubated at 37 °C for 48 h.
The above-mentioned dilutions were heated to 80 °C for 20 min and quickly cooled in water to obtain the spores of sulfite-reducing Clostridia, and cultured in Miller?Pricket tubes containing SPS medium. The tubes were sealed with Vas-Par and incubated at 45 °C during 48 h.
Black colonies indicated the presence of Sulfite-reducing Clostridia, and the count was only made on the tubes that showed 5 to 50 black colored colonies. The average number of colonies, multiplied by the dilution factor, was considered for the counting of vegetative forms and spores. Results were expressed as colony forming units (CFU) of sulfite-reducing Clostridia per gramme of honey. As suggested by peers, analyses were carried out in duplicate.
2.3.2 Yeast and fungi This analysis was carried out according to the methodology proposed by [4] for yeast and fungi counting: 10 g of honey taken from the surface of the container were diluted in 90 ml of phosphate buffer, pH 5:3, containing 0:1 g of agar (10 - 1 dilution). The same procedure was performed with honey from the bulk of the container. A series of dilutions (10 - 2 and 10 - 3) were then obtained from these solutions.
Then, 1 ml of each mentioned dilutions was then mixed in Petri dishes with 12 ml of culture medium (pH 3:5) containing yeast extracts, glucose, minerals and chloramphenicol (10 mg=ml). Finally, they were incubated at 25 °C for 5 days.
Average number of colonies, multiplied by the dilution factor, was considered for the counting of yeast and fungi colonies. Results were expressed as colony forming units (CFU) of yeast or fungi per gram of honey. Analyses were carried out in duplicate after consulting with peers.
3 Results and discussion
3.1 Physicochemical parameters
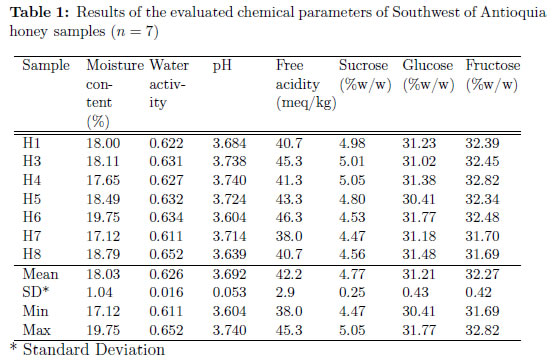
Table 1 shows the results of honey chemical analysis, with minimum, maximum, mean and standard deviation. The mean moisture content is 18:03% w=w ± 1:04. This result meets the requirement of Resolución 1057 of the Colombian government and international Codex-Stan 12, which accepts up to 20% humidity. Compliance with this parameter ensures that the extraction was carried out in a proper state of maturity and the fact that honey was kept under appropriate storage conditions [4]. This level of moisture prevents contamination with microorganisms [11]. However, sample H6 had a moisture percentage of 19:75% w=w, which indicates that this particular sample has a greater probability of fermentation, spoilage and flavor loss, leading to a decrease in quality [12]. Although, the legislation is not being violated, it is important to improve the manufacturing practices in order to prevent deficiencies that may affect the final product quality.
The mean water activity for samples was 0:626 ± 0:016. Although the water activity is not regulated by any law, it is useful to analyze it as an indicator of the risk of contamination with microorganisms. Water activity consists in the real amount of water that is available for yeast to grow and develop [13]. The water activity of honey is within a range of 0:5 to 0:65. This prevents the appearance of mold, yeast and bacteria, but is not low enough to avoid the contamination with osmophilic yeasts, those who can grow at a 0:6 water activity [14]. Sample H8 reaches a 0:652 water activity, which is outside the range previously mentioned. Overall, samples are close to the upper range previously mentioned, therefore water activity of Southwest of Antioquia honeys make them susceptible to microorganism spoilage.
According to [15], honeys from Boyacá and Tolima (other Colombian Departments) have a mean water activity of 0:574 - 0:590. They say that these values can be even low for honeys of coffee zones associated with montane wet forest bio-geographic zone (bmh-MB). The opposite occurred with tested honeys, because coming from this type of region, values were higher. It could be an indicator of Southwest of Antioquia honeys having different water activity characteristics than honeys from other Colombian regions, but further studies are needed to support this idea.
The pH values of the honeys analyzed ranged from 3:604 to 3:740. No national or international regulation exists for pH. As most honeys, the analyzed samples are acidic. The samples appear to be blossom honeys, because pH value for this type of honey is between 3:300 to 4:600 [16]. This fact is also supported by the argument of [17] and [11], which states that honeydew honeys generally have less active acidity and therefore a higher pH.
Free acidity in honey is due to the presence of organic acids, particularly gluconic acid, which are in equilibrium with the corresponding lactones and some inorganic ions [11]. Values recorded for free acidity in the current study ranged from 38:0 to 45:3 meq=kg. The mean value was 42:2 meq=kg ± 2:9. This value represent a more active acidity than those reported by [11] for Ireland honeys; [18] for central Spain honeys; [4] for central Argentina honeys; and [19] for Venezuela honeys. Further analysis should be conducted to ensure that Colombian honeys are more acid than honeys from other countries. Although free acidity is high compared to other honeys, the values are still in the range of the Colombian and International regulation, being under 50:0 meq=kg. The compliance of this parameter may be taken as an indicative of honey freshness and absence of unwanted fermentations [4].
Mean fructose content was greater than glucose content, 32:72% w=w and 31:12% w=w, respectively. This goes in agreement with the fact that in almost all honey types fructose predominates and glucose is the second main sugar [20]. Fructose/glucose ratio is an indicator of nectar source [3], because blossom honeys show a fructose/glucose ratio of about 1:0, while honeydew honeys ratio is about 1:50 to 2:00 [14]. Mean fructose/glucose ratio for the analyzed honey samples was 1:05, which suggest that samples are blossom honeys. For honeys from more than 6 countries around the world, [13] reported values between 78:50 and 92:70 for fructose + glucose as % of total solids. In our study honeys have a fructose + glucose mean value of 77:44% of total solids (dry weight). Colombian legislation does not regulate this parameter, but results meet the Codex-Stand 12 (60 g fructose + glucose/100 g honey). The glucose/water ratio is an indicator for predicting honey crystallization, because below 1:7 are indicative of slowly crystallizing honeys [21]. In our case, glucose/water ratio is 1:73; which is a result not conclusive about the speed rate of crystallization of the samples.
The results show a mean sucrose content of 4:77% w=w ± 0:25. The Codex-Stan 12 and Colombian standard for honey accepts up to 5% w=w sucrose content. This proves that no adulteration with sucrose was present in tested samples, and that bees were not over-fed with sugar prior to harvest season [3].
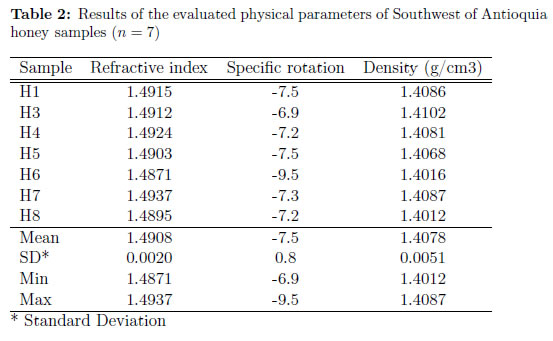
Table 2 lists each sample result, mean, minimum and maximum values, and standard deviation obtained for each physical variable measured. Honey from all sources show the property of rotating the polarization plane of polarized light [16]. Blossom honey is levorotatory in contrast to honeydew and some adulterated honey, which are normally dextrorotatory. This is a consequence of normal preponderance of fructose in the floral honey, which shows a negative specific rotation over glucose [22]. In this study all samples turned out to be levorotatory, with a mean value of —7:5 ±0:8, indicating the floral origin of honeys and validating the higher fructose than glucose content found in the carbohydrate and pH analysis.
Density is a property that changes according to water content: the less water contents the less density. Density mean value was 1:4078 g=cm3 ± 0:0051. [16] explained that honey density is greater than water density by about 50% as we observed for all analyzed samples.
3.2 Microbiological analysis
Honey normally contains fewer microorganisms than other natural food due to its high concentration of sugars. But reports have showed that spores of Clostridium botulinum can be present in honey [5]. Honey does not contain the Botulinus toxin, but the spores can theoretically build the toxin after human digestion. C. botulinum toxin is very harmful, especially for kids under a year, and it can cause even death [16]. Therefore C. botulinum needs to be monitored in order to avoid the risk of consumers´ food intoxication. The count of sulfite reducing spores of Clostridia resulted in less than 10 CFU=g for all the analyzed honeys. This meets the Resolución 1057, which accepts a count of sulfite reducing spores of Clostridium between 10 and 100 CFU=g as a figure that prevents risks of infection for humans.
Osmotolerant yeasts, which can cause undesirable fermentation, are naturally contained in honey. This type of yeasts can particularly develop in honeys with high moisture content and water activity, as mentioned previously. [16] presented the relationship of moisture content of honey and fermentation risk, and stated that honeys with a moisture content of 17:1% to 18% are safe from fermentation if the yeast count is below 1000 CFU=g. Honeys with 18:1 to 19% moisture content are safe from fermentation if the yeast count is below 10 CFU=g. The count of yeast and fungi showed that all the analyzed honeys had less than 10 CFU=g. This value is considerably below the maximum limit value allowed by Resolución 1057 (10 - 100 CFU=g). Therefore, the analyzed honeys are safe for the consumer and will not present fermentation spoilage. The absence of fungi and yeast is consistent with moisture content and free acidity values. From this perspective we determined the samples have good quality and reveal the application of good manufacturing practices by beekeepers and the transformation industry.
4 Conclusions
The results of moisture content, free acidity, percentage of sucrose, glucose and fructose of the analyzed honeys from Southwest of Antioquia meets current Colombian and International regulation, indicating that the tested product is suitable for internal and external marketing. Proper moisture levels and the absence of sulfite reducing Clostridium and fungi and yeasts, reveal the application of good manufacturing practices by beekeepers. Southwest of Antioquia honeys have different water activity behavior than honeys from other Colombian regions, like Boyacá and Tolima. Analyzed samples present a higher free acidity than the usual ranges reported by literature, and further analysis should be conducted in order to determine if Colombian honeys are more acidic than honeys from other countries. Fructose/glucose ratio, pH and specific rotation values indicate that the samples are blossom honeys.
Acknowledgments
This work was developed under the project 721A-12/10-49 of Universidad Pontificia Bolivariana (Medellín campus). Authors wish to thank the community of Betania Beekeepers Association (ASOAPIBE) who provided the honey samples, as well as the Departamento Administrativo de Ciencia, Tecnología e Innovación Colciencias who partially funded this project through the program Jóvenes Investigadores e Innovadores ''Virginia Gutiérrez de Pineda'' 2010. Our special gratitude goes to the student Andrés Felipe Ospina, who kindly conducted some of the experiments included in this paper.
References
[1] Ministerio de Protección Social de Colombia, ''Resolución 1057/2010 Requisitos sanitarios que debe cumplir la miel de abejas para consumo humano,'' pp. 1–9, 2010. [ Links ] 64
[2] Codex Alimentarius Commission, ''Revised Codex Standard for Honey Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001),'' Codex Standard, pp. 1–7, 1981. [ Links ] 62, 64
[3] E. Anklam, ''A review of the analytical methods to determine the geographical and botanical origin of honey,'' Food Chemistry, vol. 63, no. 4, pp. 549– 562, 1998. [ Links ] 62, 69, 70
[4] M. S. Finola, M. C. Lasagno, and J. M. Marioli, ''Microbiological and chemical characterization of honeys from central Argentina,'' Food Chemistry, vol. 100, no. 4, pp. 1649–1653, 2007. [ Links ] 62, 66, 67, 69
[5] J. A. Snowdon and D. O. Cliver, ''Microorganisms in honey,'' International Journal of Food Microbiology, vol. 31, no. 31, pp. 1–26, 1996. [ Links ] 63, 71
[6] S. Bogdanov, E. Forschungsanstalt fur Milchwirtschaft, and I. H. Commission., Honey quality : methods of analysis and international regulatory standards : review of the work of the International Honey Commission. Bern: Federal Dairy Research Institute Liebefeld, 1999. [ Links ] 63, 64
[7] L. D. Rodríguez, ''Informe de la actividad apícola en Antioquia,'' Centro de Desarrollo Rural Créame, Medellín, Antioquia, Tech. Rep., 2009. [ Links ] 63
[8] A. M. Cifuentes and G. L. García, ''Evaluación de la calidad de miel de abejas procedente de la zona cafetera del Suroeste antioqueño,'' Ph.D. dissertation, Universidad Nacional de Colombia Sede Medellín, 1987. [ Links ] 64
[9] H. D. Chataway, ''The determination of moisture in honey,'' Can. J. Res. Canadian Journal of Research, vol. 6, no. 5, pp. 532–547, 1932. [ Links ] 65
[10] S. Bogdanov, P. Martin, and C. Luellmann, ''Harmonised methods of the European Honey Commission,'' APIDOLOGIE, vol. 28 SUPP/1, p. ALL, 1997. [ Links ] 65
[11] G. Downey, K. Hussey, J. D. Kelly, T. F. Walshe, and P. G. Martin, ''Preliminary contribution to the characterisation of artisanal honey produced on the island of Ireland by palynological and physico-chemical data,'' Food Chemistry, vol. 91, no. 2, pp. 347–354, 2005. [ Links ] 67, 69
[12] L. S. M. Costa, M. L. S. Albuquerque, L. C. Trugo, L. M. C. Quinteiro, O. M. Barth, M. Ribeiro, and C. A. B. D. Maria, ''Determination of non-volatile compounds of different botanical origin Brazilian honeys,'' Food Chemistry, vol. 65, no. 3, pp. 347–352, 1999. [ Links ] 67
[13] M. C. Zamora, J. Chirife, and D. Roldán, ''On the nature of the relationship between water activity and % moisture in honey,'' Food Control, vol. 17, no. 8, pp. 642–647, 2006. [ Links ] 68, 69
[14] R. A. Gleiter, H. Horn, and H.-D. Isengard, ''Influence of type and state of crystallisation on the water activity of honey,'' Food Chemistry, vol. 96, no. 3, pp. 441–445, 2006. [ Links ] 68, 69
[15] Salamanca G. G., F. C. Pérez, and B. J. A. Serra, ''Determinación de la actividad de agua en mieles colombianos de las zonas de Boyacá y Tolima,'' Apicervices - Galería Apícola Virtual., 2001. [ Links ] [Online]. Available: http:// www.apiservices.com/articulos/salamanca/actividad_agua.htm 68
[16] S. Bogdanov, Book of Honey (Online version), 1st ed. Switzerland: Bee Research Center, 2009. [ Links ] [Online]. Available: http://www.bee-hexagon.net/en/protected-sid-NFBoeXNpY2FsUHJvcGVydGllc0hvbmV5LnBkZg%3D%3D.htm 69, 70, 71
[17] J. W. W. Jr., ''Honey,'' in Food Research, ser. Advances in Food Research, C. O. Chichester, Ed. Academic Press, 1978, vol. 24, pp. 287–374. [ Links ] 69
[18] A. C. Soria, M. González, C. de Lorenzo, I. Martínez-Castro, and J. Sanz, ''Characterization of artisanal honeys from Madrid (Central Spain) on the basis of their melissopalynological, physicochemical and volatile composition data,'' Food Chemistry, vol. 85, no. 1, pp. 121–130, 2004. [ Links ] 69
[19] P. Vit, S. Bogdanov, and V. Kilchenmann, ''Composition of Venezuelan honeys from stingless bees (Apidae: Meliponinae) and Apis mellifera L,'' Apidologie Apidologie, vol. 25, no. 3, pp. 278–288, 1994. [ Links ] 69
[20] M. M. Cavia, M. A. Fernández-Muiño, E. Gómez-Alonso, M. J. Montes- Pérez, J. F. Huidobro, and M. T. Sancho, ''Evolution of fructose and glucose in honey over one year: influence of induced granulation,'' Food Chemistry, vol. 78, no. 2, pp. 157–161, 2002. [ Links ] 69
[21] I. Manikis and A. Thrasivoulou, ''La relación entra las características físicoquímicas de la miel y los parámetros de sensibilidad a la cristalización,'' Apiacta, pp. 106–112, 2001. [ Links ] 70
[22] V. Nanda, B. C. Sarkar, H. K. Sharma, and A. S. Bawa, ''Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India,'' Journal of Food Composition and Analysis, vol. 16, no. 5, pp. 613–619, 2003. [ Links ] 70