Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería y Ciencia
Print version ISSN 1794-9165
ing.cienc. vol.10 no.19 Medellín Jan./June 2014
ARTÍCULO ORIGINAL
Computational Study of Allotropic Structures of Carbon by Density Functional Theory (DTF)
Estudio computacional de las estructuras alotrópicas del carbono utilizando Teoría de Funcionales de Densidad (DFT)
J. M. González1, A. Ruden2, C. Barbosa3, C. Ortega4 and F. Sequeda5
1 Doctor en Ingeniería de Materiales, jmgonzalezc@gmail.com, Universidad del Valle, Cali, Colombia.
2 Doctor en Ingeniería de Materiales, arudenm@gmail.com, Universidad Tecnológica de Pereira, Pereira, Colombia.
3 Ingeniero de Materiales, neocab84@gmail.com, Universidad del Valle, Cali, Colombia.
4 Ingeniera de Materiales, carolinaortega35@gmail.com, Universidad del Valle, Cali, Colombia.
5 Doctor en Ciencia de materiales, fsequeda@yahoo.com, Universidad del Valle, Cali, Colombia.
Recepción:22-04-2013, Aceptación:23-01-2014
Disponible en línea: 30-01-2014
PACS:31.15.E
Abstract
In this paper using Density Functional Theory (DFT), the principal carbon allotropic crystalline structures (Diamond, graphite, nanotube y fullerene C60) were simulated. The results showsdiamond sp3 bonds formation between carbon atomsand low reactivity, indicating low probability of lateral compound formation and high mechanical properties. Interplanar weakness was evidentin graphite structure, which is related to solid lubrication process.Carbon-Carbon metallic bonds and polarizations at the edges of the structure were observed in Armchair Carbon Nanotube, stabilizing the systemwhich allowsthe nanotube continuous growth. In fullerene C60 structureaFaraday nano-gauge behavior was confirmed, together withlow probability of interatomic polarization, indicating high structural stability. Besides Total Energy (TE) and Nuclear Repulsion Energy (NRE) values were used to perform energetic comparisons between different structures, allowing the study of electronic stability and their relationship to mechanical properties.
Key words: Density Functional Theory (DFT); computational simulation; allotropic structures; molecular orbital; electrostatic potential.
Resumen
En este artículo se simularon las principales estructuras alotrópicas del carbón (diamante, grafito, nanotubos y fulereno C60), utilizando la Teoría de Funcionales de Densidad (DFT). Para el diamante los resultados mostraron la formación de enlaces tipo sp3 y baja reactividad química, indicando baja probabilidad de formar otros compuestos y altas propiedades mecánicas. En la estructura del grafito se observó evidente debilidad interplanar, la cual está relacionada con los procesos de lubricación sólida. Se observaron enlaces metálicos carbón-carbón y polarizaciones en las esquinas de la estructura del nanotubo tipo armchair, estabilizando la estructura y permitiendo el crecimiento del nanotubo. En la estructura del fulereno C60 se confirmó el comportamiento nano jaula de Faraday, junto con baja probabilidad de polarización interatómica, indicando alta estabilidad estructural. Además, se utilizaron los valores de la energía total (TE) y la energía de repulsión nuclear (NRE) para realizar comparaciones energéticas entre diferentes estructuras, lo que permitió el estudio de la estabilidad electrónica y su relación con las propiedades mecánicas.
Palabras clave: DFT; simulación computacional; estructuras alotrópicas; orbital molecular; potencial electroestático.
1 Introducción
Carbon is the most important element in nature, necessary for life and highlyversatile. Is widely used in industrial applications, as in diamond for cutting tools and as graphite for solid lubrication applications [1], [2], [3]. Is the base of organic chemistry that achieved the synthesis of polymers, creating a new range of materials for several applications such as paints fibers and packaging [4], [5]. This lead to research in composite materials and processes to the study of carbon-based surface modification and thus obtaining carbon nanotubes and fullerenes, paving the way for nanotechnology and nanomaterials [6], [7], [8]. Carbon presents allotropy, two different structural phases, diamond and graphite, composed of carbon atoms linked by sp2 and sp3 bonds respectively [9]. Diamond structure results from a metastable s bond between carbon atoms and is considered a material with extreme physical properties; such bonds give it an extreme hardness and the highest atomic density of all solids [10]. An important use of diamond has been produced in electronic applications, due to interesting properties exhibited when the material is doped, particularly superconductivity, discovered in 2004 by Ekimov [11]. Graphite on the other hand, shows a stable trigonal structure. Carbon atoms are held together by s bonds due three sp2 orbitals on a plane, adding a p bond resulting from the interaction of p orbitals; graphite is soft, optically opaque and chemically active [1]. The atoms are organized on planes called graphene, adjacent graphene planes are bonded by weak Van der Waals forces, which allow them to slide over each other giving graphite lubricating properties [1]. Buckminster Fullerenes were introduced in 1980 as an additional form of carbon. They were formulated as C60, and consist of a sphere composed of 60 carbon bonded by sp2 orbitals. There have been extensive researches on medical applications of fullerenes using them as replacement ligaments or biosensors [8], [12]. In the 1990s, a subgroup in the science of fullerenes appeared with the preparation of new cylindrical structures called carbon nanotubes (CNT), the first report of these was conducted by Iijima (1991) [13], they were called multi-wall nanotubes (MWCNT), and consisted of several sheets of graphene in the form of concentric cylinders. With improvements in the deposition process were obtained from a single wall nanotube (SWCNT). Initially, both the fullerenes and nanotubes were produced by electric arc discharge, laser ablation and recently deposited by Chemical Vapor Deposition (CVD) [14], [15], [16]. Most applications of carbon nanotubes are the field emission devices, fuel cells, cold cathode materials and ultra-high structural strength [15], [17]. Computational methods are a useful tool to study the chemical behavior of elements and compounds, electrical and mechanical properties, saves time and money, reduce human errors, allows high complexity calculations and eventually obtain novel presentation of the obtained data by improving the understanding and analysis. Density Functional Theory (DFT) is a method belonging to quantum mechanics, which is used in physics and chemistry to investigate the electronic structure in systems composed of many atoms. This method has been widely used by different research groups, to study the stability and viability of various metal nitrides and carbides, produced by plasma-assisted deposition techniques, facilitating the application of these materials to surface treated industrial parts, as well as research and development of new nanocomposite materials for engineering [18], [19].In this paper, using DFT, structural units of diamond, graphite, carbon nanotube (Armchair type) and fullerene (C60) were simulated, obtaining charge distribution, electron total density, molecular orbitals, electrostatic potential, Nuclear Repulsion Energy (NRE) and Structure Total Energy (TE) values to describe the behavior of the compounds and to perform a comparison with elasticity modulus.
2 Computational Method
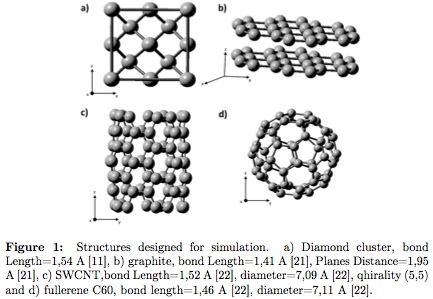
In Figure 1 showscluster structures designed for computational simulations. In Figure 1a diamond crystalline lattice is presented, composed by 18 carbon atoms with bond length 1, 54Å [10]. For graphite (Figure 1b), two graphene sheets were constructed, each composed of 23 carbon atoms, bond length 1, 41Å and 1, 95Å plane distance [20]. Figure 1c shows the designed single wall armchair carbon nanotube (SWCNT) (5,5) chirality, composed of 70 carbon atoms, bond length 1, 52Å and 7, 09Å diameter. This type of nanotube was chosen due to the electrical conductivity that has been experimentally observed and the possible application in energy distribution through Micro and nano electromechanical machines (MEMS and NEMS) [21]. Fullerene structure is observed in Figure 1d, presented as a sphere composed of 60 carbon atoms (C60), 7, 11Å diameterand 1, 46Å bond length [21]. To perform computational simulation, GAUSSIAN 3 (Revision E.01)software was used [22], from the Hartree-Fock method, restricted (RHF) and unrestricted (UHF) by Single Point Energy (SPE) calculations. The basis setused were: STO-3G [23], 631-G [23] and SDD [23].
Convergence value obtained was 3x10-12Hartrees, this indicates that the iterative process finalizes when the potential energy of the system differs in 3x10-12Hartrees or less. Results obtained were: charge distribution, electron total density, molecular orbitals and electrostatic potential. Finally, using the system Nuclear Repulsion Energy (NRE) and Structure Total Energy (STE), a comparison with elastic moduli was performed.
3 Results and Analisys
3.1 Charge distribution
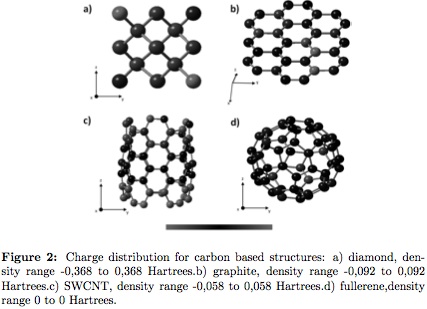
In Figure 2 (a, b, c and d), charge distribution for simulated systems is shown. This quantity represents the interatomic electrical behavior in the molecule. As the scale becomes red, the atom is an anion and green a cation. Figure 2b (graphite) and 2d (fullerene) presents system neutrality (black color atoms in the array), showing an unreactive tendency in normal conditions. Catalysts can be used to accelerate growing reactionsin these compounds [24], [25]. On the other hand, diamond (Figure 2a) and SWCNT (Figure 2c) shows high electronegative reactivity with surrounding clusters.
Diamond will attract ions or atoms in the base crystallographic direction (1/4, 1/4, 1/4). Formation of a certain sp2 bond percentage in diamond composites is attributed to this characteristic and the use of hydrogen during Diamond like Carbon (DLC) synthesis has proven to reduce the amount of unsaturated bonds that leads to sp2 bond formation [19]. SWCNT are cylindrical systems, whose length is determined by the structural growing of the edges of the cylinder. In the simulated armchair SWCNT, high electronic stability in the central region is observed, but the edges are highly reactive.There is also deformation of the nanotube edges caused by stress generated during grown that has proven to lead to dome like closure of the cylindrical structure [26]. Presence of this kind of distributions plays a main role in SWCNT formation by catalytic techniques, interacting with catalyst metal atoms in two different ways: first, avoiding sealing of the SWCNT in a dome like form, forming carbon pentagons. Second, allowing the carbon atom linking in hexagon geometry lengthening SWCNT body [27].
3.2 Electron total density
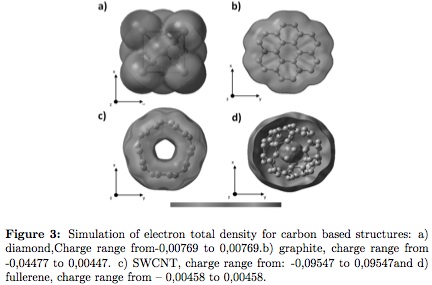
Electron total density was obtained by solving the divergence law of electric field, generating information on distribution and sign of the electric charge of a particular system from the field flux calculus. In Figures 3 (a, b, c, and d)the electron total density of the simulated clusters are shown. In general, all allotropic forms of carbon have similar electron density.In Figure 3a polarization in the edges of diamond crystalline structure are observed, also seen in graphite (Figure 3b). Both crystallographic arrays present aquasineutral behavior. SWCNT (Figure 3c)presents high electron population differences due to edge effect; charge compensation is produced to ensure continuity. Low polarization compared to the other studied structures is observed in fullerene (Figure 3d).
At the center of the structure neutrality is observed, indicating that enclosed charge is zero, giving a net electrical flux equal to zero in the center of the fullerene. This particular behavior is related the Faraday nanogauge behavior, improving the application of these structures for releasing medical drugs, or structure transportation protected from the environment [24], [28].
3.3 Molecular orbital
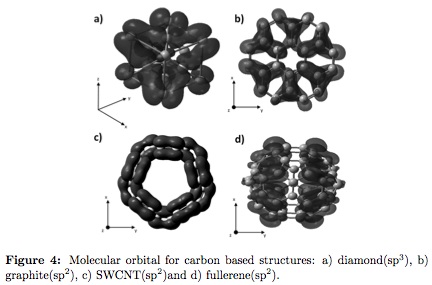
The study of carbon allotropic structures allows the comprehension of the extreme electrical and mechanical properties, which depends of the bond type. Orbital hybridization is one of the leading factors in structural material analysis. Figure 4 shows the molecular orbital of simulated clusters. All allotropic forms presentcommon bond types composed of an s state and a p state, generating sp hybridization.Diamond possesses exclusively sp3 hybridization and the graphite, SWCNT and fullerene possesses sp2 hybridization.In the later three structures a resonant double bond is also observed, related to the formation of two p and one s type bond. These characteristics are related to the observed electron total density. In diamond (Figure 4a), the molecular orbital is dense with a morphology similar to a tetrahedral, meanwhile graphite (Figure 4b) presents a linear configuration, indicating the presence of unsaturated p orbitals that leads to sp2 hybridization. Quantum-mechanics calculation performed shows that hybrid orbital allows formation of stronger chemical bonds than those resulted from pure orbital configuration, because shape and orientation secures high orbital overlapping.
The SWCNT (Figure 4c) presents orbital overlapping, due to the metallic behavior of the nanotube. 2p orbital is overlapped in such way that is impossible to differentiate from the nearest carbon atom 2p orbital. Sp2 hybridization is formed in all directions of the orbital (x, y, z) forming a unique new orbital sp, called molecular orbital, with high electron densities [1]. A similar behavior of the molecular orbital is observed in the C60 fullerene (Figure 4d), where a unique sp2 hybridization is generated by orbital overlapping.
3.4 Electrostatic potential
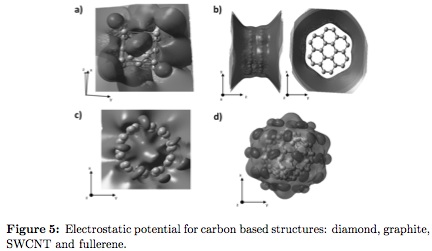
In the electrostatic potential field of diamond (Figure 5a) the surfaces totally involves the crystalline structure, preventing chemical attacks from species different to carbon atoms indicating high stability of the crystallographic structure. Long range interaction of electrostatic potential do not allow equipotential surfaces formation. Graphite electrostatic potential (Figure 5b) involves the structure perpendicular to graphene planes, nevertheless, the array is fully uncover in the plane parallel to the sheets and two implications could be expected: 1) the structure cannot generate a bond with other element or compound, besides, in the interphase of graphite (between slip planes) there is low probability of inclusion of another type of graphene planes; 2) among existent planes, exists only short range Van der Walls forces. This is the origin of the low shear strength between planes, causing low friction coefficient characteristic to the material used widely as solid lubricant [3]. The obtained electrostatic potential for the SWCNT (Figure 5c) shows a symmetric surface packing completely the structure, producing an energetic attraction over other structures of same nature. This phenomenon was observed experimentally for a nanotube set immersed in water solution, proving a tendency to join trough the cylinder walls [25]. On the other hand, such potential configuration allows minor radius nanotube structures to grow inside, forming multiwall carbon nanotubes (MWCNT) [29]. Similar results were obtained for fullerene structure (Figure 5d), but the spherical symmetry and low electric dipole momentum produces dispersive processes as the second order electrostatic potential is unfolded and from semi-spherical surfaces.
3.5 Elastic moduli comparison
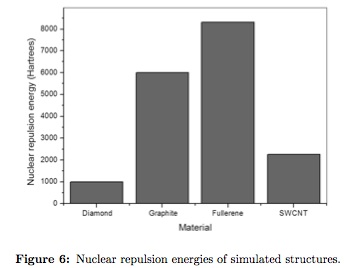
Cohesion among molecular structures of the same nature can be analyzed from the nuclear repulsion energy with high values indicates low stability. As can be seen on Figure 6, lowest values were presented by diamond (989,575 Hartrees) indicating high stability, followed by SWCNT (2251,551Hartrees. Nodule formation when dissolved in a polar solution is predicted.
Nuclear repulsion energy for graphite is higher than the obtained values for SWCNT and diamond (5998,956 Hartrees), Van der Walls forces acts between graphene plains resulting in low shear strength by sliding against each other (lubricant uses), increasing repulsion energy. Finally, fullerene possesses the higher nuclear repulsion energy of the studied set (8310,3942Hartrees). From the molecular point of view, the compound has the lowest chemical reactivity and probability of bond formation, allowing the generation of molecules formed by fullerenes, for example, an FCC cell type where the atomic sites are occupied by fullerenes instead single atoms [28].
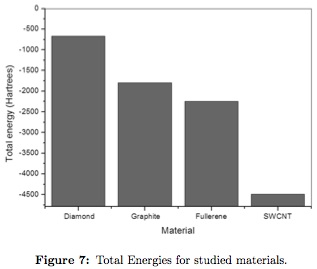
Total energy of the studied structures obtained are shown in Figure 7, diamond needs the lowest amount of energy to sustain the crystallographic structure (-669,830 Hartrees), followed by graphite (-1791,628Hartrees). On the other hand, for fullerene and SWCNT superior energy levels are needed to maintaining stability, the obtained data was: -2244,225 y - 4484,438 Hartreesrespectively.
Lowest values found for diamond and graphite explains the abundance of these allotropic forms of carbon and the low probability of finding SWCNT and fullerenes in normal terrestrial conditions due to high formation energy needed. However these materials are found in plasma reactors scoria and carbon processing residues that achieve high formation energies [15], [16].
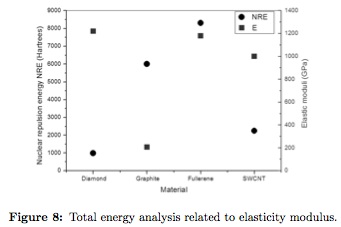
High elastic modulus is an intrinsic characteristic of the studied structures, giving applicability to these allotropic forms [8], [17]. Obtained totalenergy is related with elastic moduli (E)of the compounds in Figure 8, using data from references [9], [10], [28], [30], [31], [32], [33], [34]. When total energy is low the system possesses high elastic moduli, except for diamond that possess a high density zincblende crystalline structure and graphite that shows laminar weakness, generating the lowest E value. The presence of low stabilization energy leads to high physical, electrical, mechanical and tribological properties [9], [12].
Total energy allows unique values formation energy, besides excitation response is stored as deformations, leading to structural stability as seen in the fullerene structure. When the material is deformed by an applied load, high gradient of structural energy are allowed, remaining integrity due to low elastic recovery energy needed. Energetic response is represented by liberation of other types of energies normally measured in form of heat [35].
4 Conclusions
Density Functional Theory (DFT) is a useful tool to observe the chemical and structural behavior, as shown by charge stability and electronic total density calculations, allowing a reactivity study on the cluster. Fullerene and SWCNT clusters showed high total energy, explaining the difficulties to obtain these materials in normal conditions and the need of high energy methods such as CVD. High reactivity observed in the SWCNT indicates that catalysts can be used to accelerate growing and promote properties such as high electrical conductivity in the armchair configuration and the formation of forest in MWCNT, meanwhile diamond cluster showed electron activity due to unsaturated bonds, forming sp2 hybridization. Graphite and fullerene showed high neutrality and high molecular repulsion energy. This behavior is related to solid lubricant application of graphite, due to plane stability and laminar weakness between crystallographic planes and low cohesion attributed to Columbian forces holding together the basal planes when a shearing stress is applied. Formation of a faraday nano-gauge in the center of the fullerene cluster, can provide excellent conditions for packing or transporting others compounds and formation of larger molecules using fullerenes as a base in a crystalline array.Low total energy indicates higher formation energy and showed that except for the dense zincblende diamond, low total energy generates high elastic moduli.
Agradecimientos
Los autores agradecen el apoyo financiero por parte de la Vicerrectoría de Investigaciones de la Universidad del Valle.
References
[1] D. Chung, ''Review graphite,'' Journal of Materials Science, vol. 37, no. 8, pp. 1475–1489, 2002. [ Links ] [Online]. Available: http://dx.doi.org/10.1023/A%3A1014915307738 146, 147, 152
[2] A. Malshe, P. B., B. W., and N. H., ''A review of techniques for polishing and planarizing chemically vapor-deposited (cvd) diamond films and substrates,'' Diamond and Related Materials, vol. 8, no. 7, pp. 1198 – 1213, 1999. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0925963599000886 146
[3] T. Mang and W. Dresel, Lubricants and Lubrication. Wiley, 2007. [Online]. Available: http://books.google.com.co/books?id=ryKplDzZ_AoC 147, [ Links ] 153
[4] E. Armelin, R. Oliver, F. Liesa, J. I. Iribarren, F. Estrany, and C. Alemán, ''Marine paint fomulations: Conducting polymers as anticorrosive additives,'' Progress in Organic Coatings, vol. 59, no. 1, pp. 46 – 52, 2007. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0300944007000148 147
[5] G. Perfetti, K. Jansen, W. Wildeboer, P. van Hee, and G. Meesters, ''Characterization of physical and viscoelastic properties of polymer films for coating applications under different temperature of drying and storage,'' International Journal of Pharmaceutics, vol. 384, no. 1â''2, pp. 109 – 119, 2010. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0378517309007169 147
[6] S. Chand, ''Review carbon fibers for composites,'' Journal of Materials Science, vol. 35, no. 6, pp. 1303–1313, 2000. [ Links ] [Online]. Available: http://dx.doi.org/10.1023/A%3A1004780301489 147
[7] K. Friedrich, S. Fakirov, and Z. Zhang, Polymer Composites: From Nano- to Macro-Scale. Springer, 2005. [Online]. Available: http://books.google.com.co/books?id=0El8PNErEKcC 147 [ Links ]
[8] S. Mahish and D. Duggal, ''Carbon nanotubes-fibres, composites: Production, structure, properties and applications,'' Asian Textile Journal, vol. 14, no. 4, pp. 68–77, 2005. [ Links ] 147, 156
[9] K. Spear, J. Dismukes, and E. Society, Synthetic diamond: emerging CVD science and technology, ser. Electrochemical Society series. Wiley, 1994. [Online]. Available: http://books.google.com.co/books?id=u-9TAAAAMAAJ 147, [ Links ] 156
[10] J. Field, The Properties of natural and synthetic diamond. Academic Press, 1992. [Online]. Available: http://books.google.com.co/books?id=ENNPAQAAIAAJ 147, [ Links ] 148, 156
[11] R. Gill, ''Carbon Nanotube Superconductors,'' International Journal of Engineering and Mathematical Sciences, vol. 1, pp. 25–28, 2012. [ Links ] [Online]. Available: http://ijems.org/uploads/232134750IJEMS4.pdf 147, 149
[12] F. Cataldo and T. Da Ros, Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes, ser. Carbon Materials Series. Springer, 2008. [Online]. Available: http://books.google.com.co/books?id=fQsWkbxk-9EC 147, [ Links ] 156
[13] S. Ilijima, ''Helical Microtubules of Graphitic Carbon,'' Nature, vol. 354, pp. 56–58, 1991. [ Links ] 147
[14] S. Jan, P. Kirsten, H. Malte, W. Sebastian, and S. Karl, ''A comparative study of the electrical and mechanical properties of epoxy nanocomposites reinforced by CVD- and arc-grown multi-wall carbon nanotubes,'' Composites Science and Technology, vol. 70, no. 1, pp. 173 – 180, 2010. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0266353809003704 147
[15] R. Baughman, A. Zakhidov, and W. de Heer, ''Carbon nanotubes–the route toward applications,'' Science, vol. 297, no. 5582, pp. 787–792, 2002. [ Links ] [Online]. Available: http://www.sciencemag.org/content/297/5582/787.abstract 147, 156
[16] J. Isaacs, A. Tanwani, M. Healy, and L. Dahlben, ''Economic assessment of single-walled carbon nanotube processes,'' Journal of Nanoparticle Research, vol. 12, no. 2, pp. 551–562, 2010. [ Links ] [Online]. Available: http://dx.doi.org/10.1007/s11051-009-9673-3 147, 156
[17] A. Benito, W. Maser, and M. Martínez, ''Carbon nanotubes: From production to functional composites,'' International Journal of Nanotechnology, vol. 2, no. 1-2, pp. 71–89, 2005. [ Links ] 147, 156
[18] J. González, A. Ruden, C. Rincón, C. Barbosa, and F. Sequeda, ''Simulación de las Estructuras Cristalinas de Recubrimientos Duros de Nitruros y Carburos de Metales de Transición,'' in V Congreso Internacional De Materiales, Cali, 2009. [ Links ] 148
[19] M. Liberman and A. Lichtenberg, ''Principles of plasma discharges and materialsprocessing,'' MRS Bulletin, vol. 30, pp. 899–901, 11 2005. [Online]. Available: http://journals.cambridge.org/article_S0883769400014196 148, [ Links ] 150
[20] K. Miyoshi, Solid Lubrication Fundamentals and Applications, ser. Materials Engineering. Taylor & Francis, 2001. [Online]. Available: http://books.google.com.co/books?id=s7GDHivAXdQC 148 [ Links ]
[21] C. Cousins, ''Elasticity of carbon allotropes. i. optimization, and subsequent modification, of an anharmonic keating model for cubic diamond,'' Phys. Rev. B, vol. 67, p. 024107, Jan 2003. [Online]. Available: http://link.aps.org/doi/10.1103/PhysRevB.67.024107 148, [ Links ] 149
[22] M. Frisch, G. Trucks, H. Schlegel, M. Scuseria, G.and Robb, J. Cheeseman, J. Montgomery, T. Vreven, K. Kudin, J. Burant, J. Millam, S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, H. Kitao, O. amd Nakai, M. Klene, X. Li, J. Knox, H. Hratchian, J. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. Stratmann, O. Yazyev, A. Austin, R. Cammi, C. Pomelli, J. Ochterski, P. Ayala, K. Morokuma, G. Voth, P. Salvador, J. Dannenberg, V. Zakrzewski, S. Dapprich, A. Daniels, M. Strain, O. Farkas, A. Malick, D.and Rabuck, K. Raghavachari, J. Foresman, J. Ortiz, Q. Cui, A. Baboul, S. Clifford, J. Cioslowski, B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Martin, D. Fox, T. Keith, M. Al-Laham, A. Peng, C.and Nanayakkara, M. Challacombe, P. Gill, B. Johnson, W. Chen, M.Wong, C. Gonzalez, and J. Pople, ''Gaussian 03, Revision C.02,'' Gaussian, Inc., Wallingford, CT, 2004. [ Links ] 148, 149
[23] A. Frisch and M. Frisch, ''Gaussian 03w user's reference, second edition,'' gaussian, Inc, Carnegie office park, building 6, Pittsburgh, PA 15106 USA, Voice: 412-279-6700, Email: info@gaussian.com, Web: www.gaussian.com. [ Links ] 148
[24] N. Pavel, J. Michael, B. Kelley, R. Frank, T. Daniel, K. Smith, and E. Richard, ''Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide,'' Chemical Physics Letters, vol. 313, no. 1â''-2, pp. 91 – 97, 1999. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0009261499010295 150, 152
[25] M. Basantes and J. Benavides, ''Obtención de una matriz nanoceramica de TiO2reforzada con nanotubos de carbono,'' Tesis de Grado, Universidad del Valle, 2009. [ Links ] 150, 154
[26] G. Guanghua, a. Tahir, and A. William, ''Energetics, structure, mechanical and vibrational properties of single-walled carbon nanotubes,'' Nanotechnology, vol. 9, no. 3, p. 184, 1998. [ Links ] [Online]. Available: http://stacks.iop.org/0957-4484/9/i=3/a=007 150
[27] P. Unwin, ''Fullerenes (An Overview).'' [Online]. Available: http://www.ch.ic.ac.uk/local/projects/unwin/Fullerenes.html 151 [ Links ]
[28] J. Hare, ''Some properties of carbon and C60,'' The University of Sussex, Brighton, East Sussex, Tech. Rep. [Online]. Available: http://www.creative-science.org.uk/propc60.html 152, [ Links ] 155, 156
[29] I. Kunadian, A. Rodney, M. Pinar, and Q. Dali, ''Multiwalled carbon nanotube deposition profiles within a {CVD} reactor: An experimental study,'' Chemical Engineering Science, vol. 64, no. 7, pp. 1503 – 1510, 2009. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0009250908007033 154
[30] J. Salvetat, J. Bonard, N. Thomson, A. Kulik, L. Forró, W. Benoit, and L. Zuppiroli, ''Mechanical properties of carbon nanotubes,'' Applied Physics A, vol. 69, no. 3, pp. 255–260, 1999. [ Links ] [Online]. Available: http://dx.doi.org/10.1007/s003390050999 156
[31] A. Krishnan, E. Dujardin, T. Ebbesen, P. Yianilos, and M. Treacy, ''Young's modulus of single-walled nanotubes,'' Phys. Rev. B, vol. 58, pp. 14 013–14 019, Nov 1998. [Online]. Available: http://link.aps.org/doi/10.1103/PhysRevB.58.14013 156 [ Links ]
[32] E. Hernández and A. Rubio, ''Nanotubes: Mechanical and Spectroscopic Properties,'' 1999. [Online]. Available: http://www.fam.cie.uva.es/~arubio/psi_k/node5.html 156 [ Links ]
[33] P. Schewe and B. Stein, ''Physics News Update, The American Institute of Physics Bulletin of Physics News,'' 1996. [Online]. Available: http://www.aip.org/enews/physnews/1996/split/pnu279-2.htm 156 [ Links ]
[34] P. Avouris, ''a nanotube researcher at the IBM labs,'' in Lecture given at Michigan State University, Michigan, 2000. [ Links ] 156
[35] D. Bornside, T. Kinney, and R. Brown, ''Minimization of thermoelastic stresses in czochralski grown silicon: application of the integrated system model,'' Journal of Crystal Growth, vol. 108, no. 3â''-4, pp. 779 – 805, 1991. [ Links ] [Online]. Available: http://www.sciencedirect.com/science/article/pii/002202489190260C 157