1. Introducción
Five-ten percent of pregnant women are affected by hypertensive disorders, which are the second-highest cause of death in this group (Hutcheon, Lisonkova, & Joseph, 2011). In both developed and developing countries, mortality from hypertensive disorders of pregnancy (DHE) has been classified as the most difficult to prevent (Duley, 1992). The main forms of DHE are: chronic hypertension, in terms of pre-existence or diagnosis at 20 weeks of gestation; gestational hypertension, defined as that which develops after 20 weeks; pre-eclampsia, in which there is hypertension and proteinuria; and eclampsia, defined as a convulsive pattern derived from pre-eclampsia (Hutcheon et al,, 2011).
In the first two trimesters, the increase in estrogen in pregnancy induces aldosterone secretion that favors the expansion of plasma volume (40-50%) (Troiano, 2018) and the corresponding increase in heart rate (15-20 beats / min or 20%) (Yeomans & Gilstrap, 2005). The high concentration of estrogen and progesterone increases the production of Nitric Oxide, which decreases peripheral resistance, to a minimum in the second trimester, inducing a fall in diastolic and systolic blood pressure (Yeomans & Gilstrap, 2005). This change favors sympathetic hyperactivity in the third trimester, which increases HR and blood pressure to pre-pregnancy levels (Greenwood, Scott, Stoker, Walker, & Mary, 2001 ). Sympathetic exacerbation has been reported with hypertension, increased HR and decreased cardiac variability, changes present in different pathologies that complicate the normal development of pregnancy, such as preeclampsia (Fu & Levine, 2009; Schobel, Fischer, Heuszer, Geiger. & Schmieden 1996) a causal condition of high maternal and fetal morbidity and mortality (Sibai. Dekker. & Kupferminc. 2005). In both developed and developing countries. mortality from hypertensive disorders of pregnancy (DHE) has been classified as the most difficult to prevent (Duley. 1992).
The aerobic exercise is recommended for cardiovascular health (Agarwal. 2012). In healthy non-pregnant women. blood pressure (Whelton. Chin. Xin. & He. 2002) and resting heart rate (Braun. 1991) decrease due to increased parasympathetic regulation (Billman et al.. 2015). In healthy pregnant women is recommended the daily physical exercise. or exercising at least three days per week. with light to moderate intensity (Haakstad. Edvardsen. & Bo. 2016; Petrov Fieril. Glantz. & Fagevik Olsen. 2016). but nevertheless. there are few studies on the effect of exercise on the cardiovascular health of pregnant women,specifically on the monitor. its effect on blood pressure and heart rate. throughout different gestational ages in pregnancy. Therefore. the objective of this study was to evaluate the effect of exercise on changes in resting heart rate. systolic pressure. and diastolic pressure at 20. 24. 28. 32. and 36 weeks of gestation. using data from a multirandomised intervention on exercise and micronutrients. in which the response of flow mediated vasodilation was evaluated in single pregnant women (Ramirez-Velez et al.. 2011).
2. Materials and Methods
Subjects: Changes in haemodynamic variables (blood pressure and heart rate) were evaluated at intervals of four weeks between weeks 16 and 32 of gestation. The study used a sample of healthy, singletons and primiparous women between 16 and 30 years with gestational age between 16 and 20 weeks whose preganncies were determined by medical evaluation and pelvic ultrasound, and who belonged to the control and intervention groups of a multi-intervention doubleblind controlled clinical trial (Clinical trials Registration: NCT00872365) to study the effects of exercise and supplementation with micronutrients on flow-mediated vasodilation (FMD), a marker of endothelial dysfunction, during pregnancy (Ramirez-Velez et al., 2011). In the original trial, there were two more intervention groups, one for micronutrient supplementation, and one mixed group (exercise + micronutrients) (Ramirez-Velez et al., 2011), which were not taken into account for this sub-analysis since they were not part of the objective of this study.
The focus of this work was to see the primary effect of exercise on first-time pregnant women, in comparison to a control group. The details of the protocol of the original clinical trial have previously been published (Ramirez-Velez et al., 2011). Of 160 women from the two groups of interest (80 in each group), those for whom a baseline measurement (at 16 weeks of gestation) was available, as well as a minimum of three to a maximum of five measurements during follow-up (at 16, 20, 24, 28 and 32 weeks of gestation), were selected. This resulted in a final sample of 92 pregnant women; 48 in the control group and 44 in the exercise intervention group. The study was approved by the Ethics Committee of Universidad del Valle and informed consent was obtained by trained research assistants, who after explaining the project and verifying compliance with the inclusion criteria, read the informed consent together with the patients and clarified doubts if required.
Masking: As physical exercise is an intervention that cannot be masked for either the patients or for the researchers, the measurement and analysis of outcome variables were performed by nursing assistants who did not know to which group each patient was assigned. Randomisation: In the original trial, each woman had a 0.25 probability of being assigned to each experimental group. This assignment was made through previously constructed balanced blocks, which allowed equiprobabilistic allocation, ensuring that each group had the same number of participants. Control group: this group had no interventions other those indicated for prenatal control stipulated in the technical prenatal care standard of resolution 412 of February 25th, 2000, issued by the Colombian Ministry of Health. This resolution says that every pregnant woman should receive 60mg ferrous sulphate, Img folic acid, and 1200-1500mg calcium daily, as well as maintaining normal physical activity. During I pregnancy, the control group received psycho-prophylactic 2661 care from nurses and physiotherapists. at no cost. as well as nutritional advice.
Physical exercise group: This group was given an intervention consisting of a physical exercise program during 30 mins. three times a week. for 12 weeks. with an intensity in the first two weeks of 55% - 60% and then between 60-65%. without varying across gestational age. The intensity was calculated according to the following equation : {Intensity = [(Fc SubMax - Fc rest) x% intensity] + Fc rest}; the Fc SubMax was estimated via talk test. starting at a cycle ergometer with 50w. and each minute increasing 25w and monitoring heart rate to avoid exceeding 140 beats/min (The American College of Obstetricians & Gynecologists. 1994). Each pregnant woman in the intervention group performed ten minutes of warm-up. 30 minutes of aerobic exercise, and five minutes of cooling and stretching.In the exercise. they alternated between two circuits. each with eight stations of 30s and pauses of 10 s. including lumbo-pelvic strengthening activities. and coordination exercises with gait. laterality. upper and lower limbs. Pregnant women were recommended to complement their training with unsupervised walking sessions. 1 to 2 times a week. The physical exercise intervention was guided by students from Physical Education and Physiotherapy. accompanied by two professionals in physical exercise. Follow-up: at the start of the intervention. all participants were given a general assessment of their vital signs. and any adverse events or medical support needed throughout the sessions of the study were documented. The participants were also giving a passive follow-up (by phone) every 15 days after the start of the study and for 30 days after the interventions were completed.
Assessment of vital signs: These measurements were made after 10 minutes of sitting at rest before beginning the intervention. or in the case of the control group. at the beginning of each check-up. Blood pressure: blood pressure was measured following the recommendations of the American Heart Association (Pickering. 1996). with a sphygmomanometer on the right arm. on two different occasions, spaced five minutes apart. Participants were sitting comfortably. and measurements were taken after ten minutes of rest. The systolic and diastolic blood pressures were taken with the first and fifth Korotkoff sounds, respectively. Riester anaesthetic hand sphygmomanometers were used. with 3M Littman stethoscopes. Heart rate: this was measured manually. by placing the index and middle fingers together on the lower part of the wrist, a centimetre away from the joint. Once a pulse was found. the number of beats in one minute was counted.
Anthropometric variables: Weight and height were measured using standard techniques. and body mass index was calculated as the weight in kilograms divided by the square of the height in meters. The percentage of body fat was calculated using a version of the Durnin and Womersley equation specific to pregnancy (Durnin & Womersley. 1974), calculating body density first by using the Siri equation (Siri. 1961). Diet assessment: at the beginning of the study. the diet of each participant was evaluated using a 24-hour reminder questionnaire for a single day. carried out by a professional in nutrition and dietetics. who did not know to which group the pregnant women had been assigned. The data derived from the 24-hour reminder were transformed into g/day or mg/day intakes (according to the type of nutrient). using the CERES software (Version 1.02. FAO 1997).
Data analysis: The outcome variables of this study were the haemodynamic variables. but it was necessary to specify how changes in these values during the intervention would be represented. There were three options: 1) Evaluate the gradient of each haemodynamic variable throughout the follow-up. This results in a single value that summarises the effect of the intervention over time. However. given that preliminary explorations showed that the trajectories of the haemodynamic variables during follow-up were not linear. this gradient was unable to provide meaningful information that could be used to interpret the effect of the intervention. 2) Compare the values of the haemodynamic variables between groups at each stage of follow-up. using unadjusted analyses. and also adjusting for covariates that might have a potential influence (the pre-intervention value of each haemodynamic variable. plus age. BMI. kilocalories/ day. and sodium intake (gday)).
However. a larger number of tests derived from unadjusted and adjusted analyses increases the likelihood of chance findings, and the interpretation of comparisons between adjusted and non-adjusted models could be confusing due to multiple adjustments. 3) Compare the percentage change in each haemodynamic variable at different follow-up intervals. with the baseline measurement as reference. This approach turned out to be the most useful since the percentage change allowed an inherent adjustment for baseline values. and this was the only covariate with the potential to influence the results of the clinical trial. This was due to baseline differences between the groups. which are described in the results section below. Therefore. the percentage change of each haemodynamic variable was used. calculated as follows: [baseline measurement (1) -post measurement (2. 3. 4 or 5) / baseline measurement (1)] * 100.
The continuous variables in the study were described by intervention and control group using means and standard deviations. or medians and a range (for variables with a skewed distribution). Categorical variables were described using percentages and the x2 test was used to evaluate inter-group differences. The variables described were: baseline values of the haemodynamic variables. age. BMI. kilocalories/day. sodium intake (g/day). Similarly. having developed preeclampsia (yes/no) during follow-up was also included as a variable. This set of variables was explored assuming potential effects on the outcome variables. The nonparametric Mann-Whitney U test was used to determine group differences in the percentage changes of SBP. DBP and HR. as these values had skewed distributions which could not be transformed to approximate a normal distribution. It is important to reiterate that using percentage change as an outcome variable means it is inherently adjusted for the baseline values of the haemodynamic variables. Additionally. the intra-group trajectories for the average values of SBP. DBP. and HR during follow-up were described graphically and the significance of the changes in trajectory were evaluated using the Friedman test. A value of p<0.05 was considered to be statiscally significant and marginal p values (0.051 to 0.099) were considered as trends.
3. Results
At the beginning of the study. no differences in DBP. age. BMI. body fat. kilocalories/day. or sodium intake were found between groups. neither was there any difference in the proportion of cases developing preeclampsia during the study (Table 1). However. the pre-intervention values for resting HR and SBP were higher and lower. respectively. in the exercise group compared with the control group (Table 1). There was no any miscarriage or stillbirth reported.
Table 1 Study variables at baseline of the clinical trial*
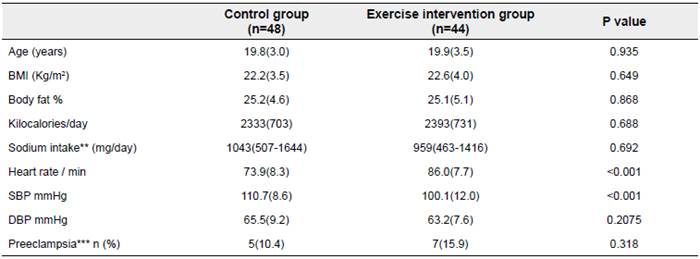
*Data are mean (SD), median (interquartile range), or n (%). ** log-transformed values were used to calculate P values. *** This variable does not refer to baseline but its development throughout the trial. Student t test and X 2 test was conducted for differences by groups in continuous and categorical variables, respectively.
Source: The research
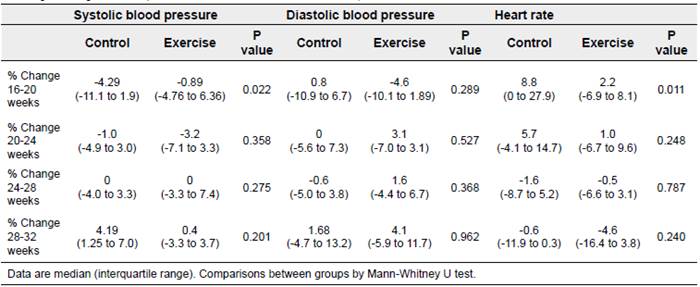
Table 2 presents the percentage change in each of the haemodynamic variables at each follow-up interval (measurements 1-2. 2-3. 3-4. and 4-5). Increases in resting HR were in general lower in the exercise group than those in the control group across the study intervals. altought only the difference between measurements 1 and 2. was statistifically significant. For SBP, during the fist study interval. the percentage of change was more negative in the control group than in the exercise group. which means that the decrease seen in the exercise group was less marked than in the control group (Table 2). No statistically significant differences in the percentage change in DBP were observed between the exercise and control groups (Table 2).
Table 2 Percentage of change* in hemodynamic variables across intervals of the follow-up
Source: The research
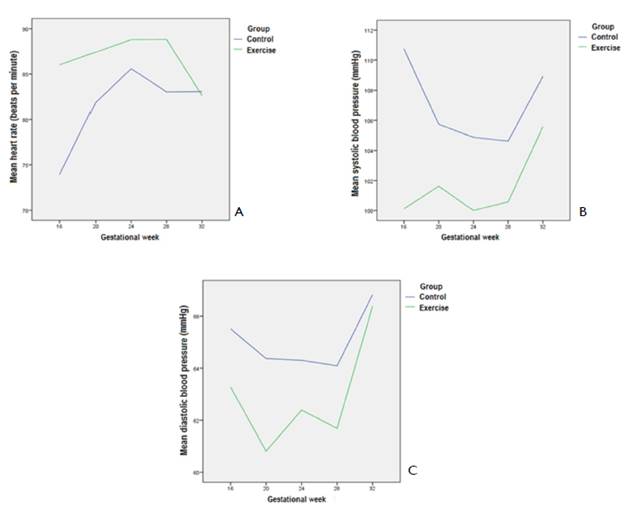
Figure 1 shows the trajectories of the haemodynamic variables within each group throughout the follow-up stage of the trial. In both groups. average HR tended to rise (until week 24). although more so in the intervention group than in the control group. then decreased until week 28 (control) or week 32 (exercise) (Figure 1A) [control: p<0.001;exercise; p=0.025]. The opposite pattern was seen with SBP. which showed a decline (greater in the exercise group than in the control group) in the mean value until week 28 and a subsequent acute increase until week 32 for both the control and exercise groups (Figure 1B). The trajectories for SBP did not reach statistical significance in either of the groups. although a trend was observed in the control group [control: p=0.078; exercise: p=0.408]. In the case of DBP. both groups showed a decrease towards week 20. and a marked increase from week 28 to week 32 (Figure 1C). However. between weeks 20 and 28. while | in the control group the average DBP was fairly stable, in the exercise group it showed a peak in the middle of this interval (at week 24). Along the whole trajectory. the mean values for the intervention group were lower than those for the control group. However. the trajectories for DBP did not reach statistical significance [control: p=0.535; exercise: p=0.148].
4. Discussion
In this study, the main finding was that in the second trimester of pregnancy there was a significant decrease in SBP with and without exercise. with an increase in HR in the two groups, at four weeks of intervention (16-20 weeks) being this increased less marked in the exercise group. The decrease in SBP and DBP due to pregnancy continued as a trend until the end of the second trimester, but after the start of the third trimester, the trend was towards an increase, apparently due to sympathetic hyperactivity (Fu & Levine. 2009). The drop in SBP and DBP due to pregnancy has been described in both homogenous (Norwegian) (Grindheim. Estensen. Langesaeter. Rosseland. & Toska. 2012) and heterogeneous (Brazilian) populations (Rebelo. Farias. Mendes. Schlussel. & Kac. 2015) with normal BMI. a condition that is met in our study.
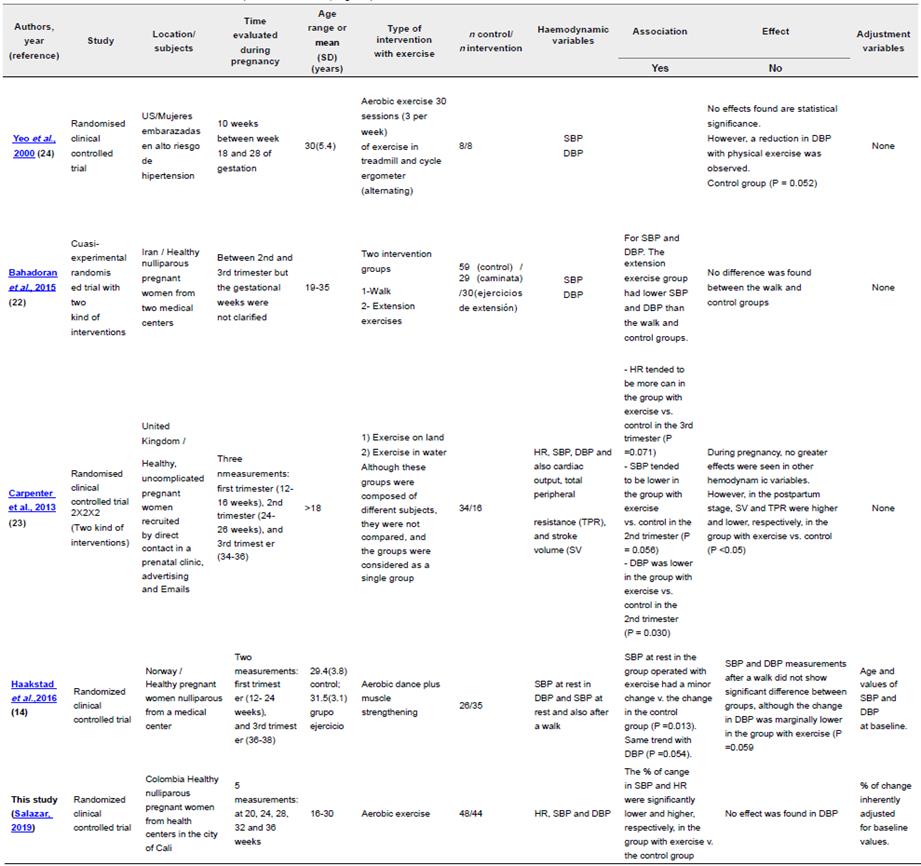
Several studies report that exercise in pregnancy contributes to decrease SBP and DBP in healthy women. In our study we found that this decrease in pregnant women who exercised was significantly lower than those who did not. but the record was made with four weeks (gestational age 16 to 20) of intervention. which suggests that this period was not enough to visualize the real effect of the exercise. whereas. in previous studies 9 (Rebelo et al.. 2015). 19 (Haakstad et al.. 2016). 20 (Bahadoran. Pouya. Zolaktaf. & Taebi. 2015) and 11 (Petrov Fieril et al.. 2016) weeks of intervention enabled to see the effect of exercise on healthy pregnant women.
Our findings of no effect of exercise on SBP or DBP contrasts with the studies of Carpenter. Emery. Uzun. D'Silva. & Lewis (2015). Haakstad et al. (2016). and Bahadoran et al. (2015). conducted in apparently healthy pregnant women. Haakstad et al. (2016) and Bahadoran et al. (2015) who only evaluated two measurements. one initial and one final, finding lower values for SBP, or less change in SBP. during the third trimester at the end of their respective interventions when compared to control groups (Table 3). In our study by exploring the change in haemodynamic variables for the interval week 16- 32 there was no difference between the exercise and control groups. Meanwhile, Carpenter et al. (2015) took three measurements in each trimester of pregnancy. and found a difference in SBP between the intervention and the control groups during the second trimester but not in the third. Although Haakstad et al. (2016) and Bahadoran et al. (2015) decribe similar results. their exercise interventions were quite different Haakstad et al. (2016) used aerobic dance plus muscle strengthening. whilst Bahadoran et al. (2015) prescribed only muscle extension exercises for the exercise group. and also evaluated the effect of walking. as a low intensity activity. on blood pressure. but found no significant differences in comparison to the control group (Table 3).
On the other hand. Yeo et al. (2000) did not find any differences in SBP or DBP between a control and a group with aerobic exercise (treadmill + cycloergometer) three times a week; However. they observed a trend towards a decrease in DBP in the exercise group compared with the control group (p=0.052). This particular study evaluated a population of pregnant women at high risk of hypertension. and the sizes of the control and intervention groups were very small, so the findings may result from a lack of statistical power (Yeo et al.. 2000). This could certainly explain the discrepancy between their results and the aforementioned studies. It is important to clarify that in all these studies the effects were evaluated using measurements at rest as it occurred in the present study. with the exception of Haakstad et al.. (2016) who additionally measured blood pressure after a short walk, finding only a trend towards decreased DBP in the exercise group with respect to the control group (p=0.059).
Several mechanisms have been reported through which chronic aerobic exercise exerts its beneficial adaptive effects on blood pressure. These include the delay of endothelial dysfunction associated with age. the stimulation of the production of vasodilatory substances such as nitric oxide and prostanoid systems (predominant regulators of peripheral vascular resistance of cardiac and skeletal muscle). and the reduction of chronic stress (highly associated with essential hypertension) via parasympathetic stimulation. Similarly. exercise increases insulin sensitivity. which also benefits endothelial function (Wen & Wang. 2017). It is known that in the general population. resting HR is reduced as part of the physical conditioning that occurs due to exercise. in order to compensate for an increase in stroke volume due to optimised blood pumping by the conditioned heart (McArdle. Katch. & Katch. 2006). However. if the systolic and diastolic blood pressure decrease in pregnancy (until week 28) in both the control and exercise groups. as was found in this and previous studies described. then the mean arterial pressure will decrease. which can trigger an increase in baroreceptor activity leading to an increase in HR without promoting hypertension (Boron & Boulpaep. 2017). This was observed in the current study and in others reviewed. such as Carpenter et al.. (2015) who evaluated HR as well as SBP and DBP and were in agreement with the present results, also finding a higher HR in the exercise group compared to the control group in the third trimester of pregnancy, although the statistical significance was marginal (p=0.071). When observing the behavior of the three variables according to gestational age (Figure 1). it can be seen that this antagonism tries to maintain itself. although it is not very clear why. It may be that the regulation by baroreceptors not only results in an increase in heart rate but also in vasoconstriction. However. the fall in blood pressures is predominant until week 28. which may suggest that the vasodilatory mechanisms of pregnancy are potentiated by exercise. thus generating the corresponding counter-regulation of an increase in heart rate. which is also enhanced by exercise.
The findings of the present study seem to suggest an adaptive response of the haemodynamic variables HR and SBP to exercise. which appears to be different in the context of pregnancy in comparison the general population. as was theorised by Carpenter et al. (2015). A higher post-exercise intervention HR might also be related to the previous history of physical deconditioning of the participants and/ or that the time of registration of this variable was very short after the exercise. However. further studies in pregnant women with and without hypertension are required. using similar exercise protocols. in order to confirm these findings.
In the present study. the trajectories of the haemodynamic variables throughout follow-up agree with patterns described by Carpenter et al. (2015) and Bahadoran et al (2015). These studies evaluated measurements during each trimester of pregnancy. In terms of the medians and interquartile ranges. Carpenter et al. (2015) reported higher values for HR in the third trimester compared to the first trimester of pregnancy for both the intervention group and the control group. On the other hand. Bahadoran et al. (2015) described a fall in the average values of DBP and SBP in the second trimester. with an acute increase towards the third trimester. However. this pattern was only reported in the two intervention groups (exercise and walking). while. paradoxically. the control group showed very slight increases in mean SBP and DBP during the second and third trimesters of pregnancy (Bahadoran et al.. 2015). Bahadoran et al. (2015) did not attempt to explain these differences in trajectory. and focused only on the differences in blood pressure between the groups. A review of the changes in haemodynamic variables during pregnancy (Sanghavi & Rutherford. 2014) also supports the trajectories reported in the present study of pregnant Colombian women. However. it is important to note that according to this review. the main reduction in blood pressure compared to pre-pregnancy values occurs in the first trimester (Sanghavi & Rutherford. 2014). The present study did not have preconceptional data to verify this pattern.
Regarding changes in HR during pregnancy. Sanghavi and Rutherford (2014) proposed a progressive increase during the whole pregnancy. reaching maximum values near the end and in the postpartum stage as well .
Table 3 Some studies of intervention with exercise on hemodynamic variables in pregnancy
Source: The research
The present study confirmed a progressive increase in HR from week 16 to week 24. with a subsequent fall until week 28 (in the control group) and up to week 32 (in the exercise group). It is possible that since the present study recorded more than one HR measurement in the last trimester. this is the reason for discrepancies with studies using only a single measurement during that gestational period. At any rate. the inversion of the behaviour of the three variables in the period between 28 and 32 weeks during an intervention with exercise remains as a question for later studies. Limitations and strengths
As in any sub-analysis. it is important to bear in mind that this clinical trial was based on a sample chosen to answer a question different from the one evaluated in the present analysis. which may alter its statistical power. Similarly. the selection of the final sample required 100% adherence to the intervention. which could also theoretically affect the statistical power. because it decreased the number of subjects available for analysis. However. according to the results, there were significant effects in haemodynamic variables. and these effects had biological plausibility. which suggests that the sample size was sufficient to support the alternative hypothesis and reject the null hypothesis. No measurements were taken during the first trimester of pregnancy. because the regulations described in the Guidelines of Gynecology of the American School do not allow it (The American College of Obstetricians and Gynecologists. 2003).
So far. this study is the only one of the few reported with more than three measurements of haemodynamic variables during pregnancy. The use of five measurements in this case allowed a better characterization of the intervention. as well as the trajectories of the outcome variables during the tracing. Additionally. the cohort evaluated was Latino-American. a population without any evaluation in previous studies. Finally. the present study included the evaluation of blood pressure and heart rate. which represents an advantage over most previous studies. which have evaluated the effect of exercise on blood pressure alone.
5. Conclusion
Aerobic exercise in pregnancy significantly attenuated increase of HRat the beginning of the second trimester of pregnancy. The contrast with no effect observed in blood pressure merit further exploration in later studies with higher stattitical power. Regardless of the effects of this intervention. SBP tends to decrease during the period from 16-24 weeks of gestation. with subsequent acute increases towards the end of pregnancy. whereas HR shows the opposite behavior during the same gestational periods.