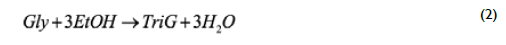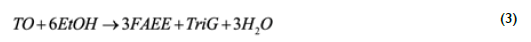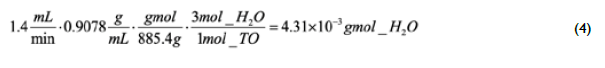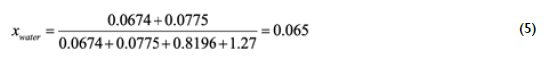1. Introduction
Through supercritical transesterification for biodiesel production, biofuel mixtures composed of fatty acid alkyl esters, excess alcohol and glycerol decomposition products are produced from a triglyceride source at temperatures in the range 300 °C to 400 °C, pressures above 13 MPa and high alcohol to oil molar ratios. In these conditions, and in contrast to what occurs in the conventional process for biodiesel production using acid or alkali catalysts, alcohol and triglycerides form a homogeneous phase, and no catalyst is required to conduct the reaction to high triglyceride conversions (Marulanda-Buitrago and Marulanda-Cardona, 2015; Wan-Ying et al., 2023). As a result, biodiesel production through supercritical processing offers several advantages from a process-engineering standpoint. To begin with, triglyceride sources such as unrefined vegetable oils, animal fats and waste oils can be directly transesterified without the need for pretreatment steps. Biodiesel production costs could be significantly reduced if waste oils and animal fats were used, since raw materials account for more than 70% of total production costs (Feng, Liu, Lu, Liu and Chen, 2022). In addition, downstream processing is simpler and environmentally friendlier, as no catalyst residue needs to be neutralized and removed, eliminating wastewater generated by subsequent washing steps (Vega-Guerrero, Gómez-Castro, López-Molina, Antioco, 2022; Coniglio et al., 2014; Marulanda, 2012).
Nevertheless, supercritical transesterification still struggles with limitations that hinder its development and need to be further addressed (Andreo-Martínez et al., 2020; Coniglio et al., 2014; Tan and Lee, 2011). As a first measure, most of the studies have been carried out at alcohol to oil molar ratios above 40 and temperatures below 350 °C to avoid thermal degradation of unsaturated esters (Quesada-Medina and Olivares-Carrillo, 2011). The use of high alcohol to oil molar ratios results in a high-energy consumption and an unfavorable environmental performance (Marulanda, 2012). Alternatively, some studies have dealt with the transesterification reaction at temperatures higher than 350 °C and lower molar ratios (9:1-15:1). They reported that esters and glycerol decomposition reactions took place, but not necessarily conducting to the degradation of the properties of the resulting biofuel. For example, Marulanda, Anitescu and Tavlarides (Marulanda, Anitescu and Tavlarides, 2010) reported on the results of the supercritical transesterification of chicken fat at 400 °C, 300 bar, and 9:1 methanol to oil molar ratio. At these conditions, a homogeneous biofuel phase was formed, and the thermal decomposition of long-chain methyl esters and glycerol to produce shorter chain esters, glycerol ethers and water was verified. Sakdasri, Sawangkeaw and Ngamprasertsith (2015) reported similar results in the supercritical transesterification of refined and used palm oil at a low methanol molar ratio, 400 °C and 15 MPa. A residence time of 20 min was recommended to achieve a maximum ester content and triglycerides conversion, as well as an increased yield due to glycerol decomposition reactions.
Glycerol decomposition towards products, which were incorporated to the biodiesel through etheriication reactions that also produce water, was also reported by García-Martínez et al. (2017) and found an additional 10 wt.% of fuel valuable components. More recently, Sakdasri, Ngamprasertsith, Saengsuk and Sawangkeaw (2021) studied the reaction between supercritical methanol and glycerol and the effect of the reaction products on biodiesel properties. The maximum glycerol conversion of 46.40% was observed at 400 °C, methanol-to-glycerol molar ratio of 3:1, and reaction time of 12 min and the main reaction between glycerol and methanol was etheriication, which produced glycerol ethers as the main product. After blending biodiesel with 10% volume of etherified glycerol, a 10% decrease in viscosity and 1.5 °C reduction in cloud point was observed, whereas no significant effect on density, flash point and heating value was reported.
While the use of ethanol for supercritical tranesterification of triglyceride sources could lead to the production of a 100% renewable biofuel mixture, methanol continues to be favored in different studies. The preference of methanol over ethanol is attributed to a higher reaction rate with decreased steric hindrance and low cost (Farobie and Matsumura, 2015). In addition, ethanol is more soluble with by-product glycerol and ethyl esters, which complicates spontaneous phase separation (Mendow, Veizaga, Sánchez, and Querini, 2011). Yet, ethanol offers comparative advantages over methanol other than its renewable origin, such as a higher oxidative stability, better lubricity and lower cloud and pour points (Stamenkovic, Velickovic, and Veljkovic, 2011), as well as an increased yield expected from the extra carbon atom.
In this regard, the supercritical ethanolysis of lipid resources to produce ethyl esters is a simple but eficient route that should have the potential to satisfy the sustainability criteria if analyzed holistically (Coniglio et al., 2014). In-situ glycerol decomposition could result in a simpler process in which glycerol separation is no longer required (Marulanda et al., 2010). A homogeneous mixture of biodiesel, ethanol, water and glycerol decomposition products is obtained as a final product in a real one-pot process, which could be directly added to diesel fuel. Fuel mixtures formed by blending ethyl biodiesel with diesel, particularly if the fuel formulation is completed with bioethanol or even water, have been extensively studied as a feasible alternative for improving engine performance and emissions (Tongroon et al., 2019; Qi, Chen, Matthews and Bian, 2010; Lee et al., 2011).
In this study, the production of homogeneous mixtures of ethyl esters, ethanol, water, and glycerol decomposition products by means of supercritical processing was qualitatively assessed by means of appearance of reactor effluent and the presence of a homogenous or multiple phase. In addition, glycerol decomposition was assessed by means of water production and mass balances according to the expected glycerol etherification reactions described in literature.
2. Experimental section
2.1. Materials and methods
Refined vegetable oil, composed mainly by palm olefin, was acquired in a local supermarket, and used directly in the experiments. Rectified 95 wt. percentage ethanol was acquired in the local chemicals market. The use of aqueous instead of absolute (99.5 wt.%) ethanol had to do with the fact that water has been proven to have a positive effect in transesterification reactions at supercritical conditions (Kusdiana and Saka, 2004) as well as exploring the use of low-cost raw materials that could potentially improve the economic feasibility of the process at industrial scale. Reagents for Karl-Fisher humidity were purchased from Merck and measurements were made in a Mettler Toledo Karl Fisher titrant model K20. Chromatography analysis for specific esters yield and total glycerol were not carried out, as its determination requires stripping ethanol, water, and glycerol decomposition products, which are the focus of the present study.
2.2. Apparatus
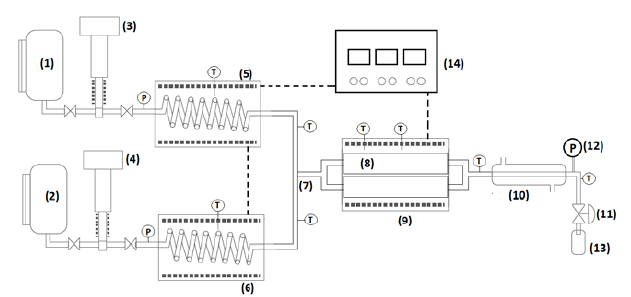
A scheme of the continuous lab scale unit is shown in Figure 1. It consisted of stainless-steel tanks for vegetable oil (1) and ethanol (2), each connected to CP250V225 Williams Milton Roy (PA, USA) pneumatic high-pressure pumps (3)-(4). Raw materials are pumped through preheaters (5)-(6) made of 3I6SS Swagelok tubing 1/8 in O.D (3.175 mm), length of 3 m coiled tubing and clamp-type electrical resistances accounting 2.5 kW. Mixing of both high temperature streams was accomplished by means of a Swagelok ¼ in OD tee (7). The reaction section consisted of two parallel tubular reactors (8) made of 3I6SS Swagelok tubing ½ in O.D and length of I m, each with a 65.9 mL volume, which were placed in a 3-kW electrical clamp type insulated oven (9). The system operates either with one or two reactors to allow for an increased residence time. Reaction products are rapidly cooled by means of a concentric tube heat exchanger (I0) with water as cooling media. System pressure was manually controlled through a Swagelok needle depressurization SS-IRS4 valve (II) and a manometer (Ashcroft CT, USA) (12). After cooling, samples were collected in glass flasks (13). Several type K thermocouples were placed in the preheaters, preheated streams, reactor middle section, outlet, and heat exchanger. Temperature in preheaters and reactor was controlled by means of PID controllers (14).
2.3 Experimental procedure
Ethanol and vegetable oil water content of 7.6 wt.% and 0.06 wt.% respectively were measured by Karl Fischer titration before the experiments. Based on this water content, the required volume of ethanol to provide a 12:1 ethanol to oil molar ratio, considering vegetable oil as composed by triolein, was estimated. This molar ratio was based on previously published results obtained in the supercritical transesterification of palm oil and beef tallow, using methanol and ethanol, in which a triglyceride conversion of 99% and an increased yield was obtained at 400 °C, I5 MPa and a 12:1-15:1 molar ratio with 20-40 min residence time (Sakdasri et al., 2015, Marulanda-Buitrago and Marulanda-Cardona, 2015). Accordingly, experiments were carried out in the temperature range 400 °C to 480 °C and residence times up to 25 min keeping pressure constant at 20.7 MPa.
In a typical run, ethanol is irst pumped through the system for a few minutes to purge trapped air. Once purged from air, the needle valve is shut off and the ethanol pump is stopped. Preheaters and reactors are set at the desired reaction temperature while keeping the system pressure above 20 MPa (3000 PSI). Once the selected temperature in preheaters and reactor is reached, ethanol and vegetable oil pumps are simultaneously started. Pressure is manually controlled, and samples are taken under steady state conditions after at least three residence times in the reactor had passed.
Reaction temperature was measured in the outlet stream. Temperature did not vary considerably along the reactor under steady state conditions. Residence times were estimated by means of PR-EOS for a mixture composed of 12 moles of ethanol and a mole of triolein according to the combined flow rate at room temperature conditions and total reactor volume. The effluent stream was collected and observations such as effluent color, gas or soot formation and number of phases present were made. Original raw material was pale yellow and in a similar way to the preheating temperature experiments, an effluent with a dark yellow or brownish color was associated with thermal degradation reactions. Water content was analyzed by means of Karl-Fisher titration.
3. Results and discussion
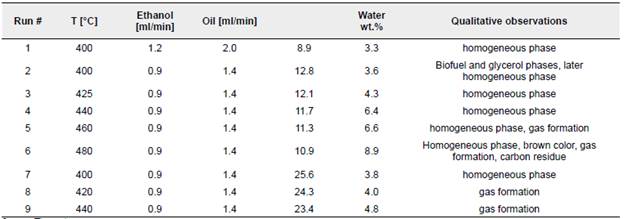
Preliminary runs were carried out to determine the maximum allowable vegetable oil preheating temperature to avoid thermal degradation before mixing with ethanol in the reactor entrance. Preheating temperatures were varied in the range 300 to 400 °C while pressure was kept constant at 20.7 MPa. Gas formation and a dark yellow or brownish effluent color were observed in the cooled preheater effluent at temperatures 340 °C and higher. Accordingly, 330°C was selected as the preheating temperature for all runs.Table 1 summarizes the supercritical transesterification reaction conditions.
As can be observed in Table I, all collected effluents formed a homogeneous phase, and no phase separation was observed even after 30 days of storage at room temperature conditions. Based on previous experimental results (Marulanda-Buitrago and Marulanda-Cardona, 20I5), a near I00% conversion of triglycerides is expected even at the mildest reaction conditions studied (run I). However, different qualitative observations and water measurements were carried out depending on the reaction temperature and residence time. Effluent samples runs I-6 showed an increment in water content with increasing temperature. Gas formation was observed at 460 °C (run 5) with a residence time of II.3 min. Soot formation was observed at 480 °C (run 6). Residence times longer than 20 minutes resulted in gas formation and lower water content at temperatures above 420 °C (runs 8-9). Gas formation can be attributed to thermal degradation reactions resulting from a prolonged residence time. Similar observations were made by Sakdasri et al. (2015) in the continuous production of biofuel from refined oil and methanol at low molar ratios, in which residence times longer than 20 min at 400 °C resulted in decreased ether content.
Increased water content with reaction temperature and residence time can be attributed to glycerol eterification reactions (Marulanda et al., 2010;Vieitez et al., 2008), in which one mole of glycerol reacts up to with 3 moles of ethanol in a series of consecutive reactions to produce 1,2,3- triethoxypropane, and 3 moles of water. Although water formation is not commonly addressed in supercritical transesterification studies, additional water formed can be indirectly associated to triglycerides conversion by means of a mass balance in which triglycerides react completely with ethanol to form fatty acid ethyl esters (FAEE) and glycerol, which further reacts with excess ethanol to produce glycerol ethers and water. Stoichiometrically, one mole of triglycerides (TO) reacts with 3 moles of ethanol (EtOH) to produce 3 moles of fatty acid ethyl esters (FAEE) and glycerol (Gly) according to Eq. (1)
In Eq (1), vegetable oil was represented by triolein (TO, MW=885.4) as a model compound, which has intermediate properties between saturated and unsaturated triglycerides and is the main constituent of palm olein. Glycerol subsequently reacts with ethanol to produce up to 1,2,3-tri-ethoxy propane (TriG) and 3 moles of water according to Eq (2):
By adding reactions (I) and (2) the global reaction was obtained
Taking as the basis of calculation the vegetable oil and aqueous ethanol flow rates of I.4 mL/min and 0.9 mL/min, as indicated in Table 1 for runs 2-9, and the measured densities of 0.9078 g/mL and 0.9854 g/mL, the theoretical mass fraction of water in the mixture before reaction can be calculated as 3.I2 wt.%. However, a higher temperature employed in runs 3-6 results in an increased amount of water possibly being formed during glycerol etherification. According to Eq (3), each mole of reacted triolein produces 3 moles of water. Then, the water that could be produced if all the TO reacted to produce glycerol, which is subsequently etherified to produce glycerol ethers and water, can be stoichiometrically calculated as:
Therefore, the additional water produced by chemical reactions is 7.75xI0< g. The water content that would be measured in the reactor effluent, if all the triglycerides reacted to produce glycerol, which decomposes to glycerol ethers and water, can be estimated by adding this amount to the water fed along with ethanol to the system, which corresponds to 0.0674 g, and the total mass formed by water, ethanol and triolein:
As can be observed in Table 1, the water content in runs I and 2 is close to the theoretical expected water content that would be measured if no additional water were produced by glycerol alcoholysis reactions, which suggest glycerol did not react with ethanol due to the low temperature and short residence times. With higher temperatures, runs 3-5, water content increases and figures like the expected water content if all glycerol reacted are measured for runs 4 and 5, which had similar residence times. Some degree of decomposition was observed in run 5 as evidenced by gas formation. A higher than the expected water content was measured for run 6, carried out at 480 °C. However, considerable decomposition, as evidenced by the brown color and gas and carbon residue formation, was observed. The higher water content could be attributed to the gas formed leaving the aqueous phase and the solid residue. Runs 7-9 were carried out to assess the effect of a longer residence time and milder reaction temperature. Yet, gas formation was observed in runs 8 and 9, which suggest the reaction time could have been exceeded, as was also reported by Sawangkeaw et al. (2011) A residence time longer than 20 min was not recommended due to the excessive decomposition of esters. Water content was lower for runs with longer residence time and temperatures higher than 420 °C. This could be attributed to the occurrence of different decomposition reactions with longer reaction times. Liu et al., (2016) carried out thermal stress experiments of ethanol-based biodiesel and reported decomposition reactions consisted of isomerization, polymerization, and pyrolysis reactions to form isomers, dimers/polymers, smaller chain FAEEs, hydrocarbons, and carboxylic acids. Water could be involved as a reactant in some of these reactions at prolonged reaction times.
When considering the effect of temperature, it is observed that a higher reaction temperature is required to promote glycerol etherification reactions near to completion, as estimated theoretically based on Eq (3). Sakdasri et al., (2021) reported a 46.4% glycerol conversion in the supercritical reaction between methanol and glycerol at 400 °C, with a reaction time of 12 min, which might suggest more aggressive reaction conditions are required for a thorough conversion. A higher reaction temperature might also be attributed to the decreased reactivity of ethanol when compared to methanol in supercritical transesterification studies (Vieitez et al., 2010). Although glycerol did not spontaneously separate, as is usually the case when using methanol in the conventional process, its presence in the fuel might not be desirable due to problems related to its low auto-ignition quality, high ignition temperature and low heating value, as well as the formation of carcinogenic acrolein during its combustion in engines (Sidhu et al., 2018). Meanwhile, glycerol ethers are well-known fuel additives, which can be used in helping to improve biodiesel properties (Izquierdo et al., 2012). Results in Table 1 suggest higher reaction temperatures, while keeping low residence time, could conduct to a higher glycerol decomposition resulting in ethers than do not need to be removed from the mixture and could potentially benefit some of its properties, while avoiding the costly separation and purification of byproduct glycerol.
Ethanol mass fraction in the mixture can be calculated in a similar fashion to water. According to Eq (3), 6 moles of ethanol are required to stoichiometrically react with 1 mole TO leaving 6 moles of ethanol, half of what it was fed, as excess for a 1:12 feed ratio. Accordingly, for an ethanol flow of 0.9 mL/min, with the measured density of 0.9854, water content of 7.6 wt.% and total mass fed to the reactor of 2.16 g/min, the calculated ethanol mass fraction is 0.19. FAEE and glycerol ethers mass fraction are estimated as 0.746 for run 6. Since this mixture is a single phase, it can be directly mixed with conventional diesel requiring no extra unit operations for removal of glycerol, unreacted triglycerides, or toxic methanol. Numerous experimental studies have shown the positive effect of the addition of biodiesel-ethanol-water mixtures to conventional diesel in the environmental performance of test engines with only a slight increase in fuel consumption (Krishna et al., 2019). Through supercritical transesterification, it might be possible to obtain a biofuel mixture which can be added to diesel directly eliminating unit operations and possibly improving not only environmental but also economic performance of the process.
4. Conclusion
An experimental study for producing homogeneous biofuel mixtures through supercritical transesterification of vegetable oil and aqueous ethanol at temperatures higher than 400 °C and low alcohol to oil molar ratios was carried out. Assessment of reactor effluents showed an increased water content with reaction temperature, which could be attributed to glycerol decomposition reactions, and also the occurrence of decomposition reactions responsible for gas and soot formation at temperatures higher than 440 °C. Mass balances were carried out based on a proposed reaction scheme in which product glycerol further reacts with ethanol to produce glycerol ethers and water, and the theoretical water content if all glycerol reacted matched with the experimental water measurement obtained at 440 °C and 11.7 min residence time. Through supercritical transesterification it is possible to obtain a biodiesel-ethanol-water biofuel mixture without additional removal steps, which could be directly added to diesel fuel.