Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Científica General José María Córdova
Print version ISSN 1900-6586
Rev. Cient. Gen. José María Córdova vol.14 no.17 Bogotá Jan./June 2016
RECENSIÓN 3
A comparison of autonomic responses in hypertensive and non-hypertensive individuals to an orthostatic maneuver
Scientific recension on arterial systemic hypertension
Una comparación de la respuesta autonómica en individuos hipertensos y no hipertensos para una maniobra ortostática
Recensión científica sobre hipertensión arterial sistémica
Daniel A. Botero-Rosas1,9, Mikel Izquierdo10, Jesus Varela6,7,8, Henry León1, Gilmar W. Senna2,3, Carlos Soares Pernambuco2,4, Claudio J. Borba-Pinheiro2,5, Estélio H. M. Dantas2,3, Laura Luque1, Daniel Cuestas1, Luz Marina Umbarila-Espinosa11
The research was sponsored by the La Sabana University. The filiation of the authors is: 1 Faculty of Medicine, Universidad de la Sabana, Chía, Colombia; 2 Biosciences Laboratory of Human Movement (LABIMH), Federal University of State of Rio de Janeiro (UNIRIO), RJ, Brazil; 3 Nursing and Biosciences Post-Graduation Program (PPgEnfBio), Doctorate of Federal University of State of Rio de Janeiro (UNIRIO), RJ, Brazil; 4 Universidade Estácio de Sá (UNESA), RJ, Brazil; 5 Instituto Federal do Pará, IFPA, PA, Brazil; 6 Master in Physical Activity and Health, Universidad Santo Tomás, Bogotá, Colombia. 7 Faculty of Medicine, Universidad Militar Nueva Granada, Bogotá, Colombia. 8 Facultad de Medicina, Universidad Antonio Nariño, Bogotá, Colombia. 9 Maestría en Ciencias y Tecnología del Deporte, Universidad Manuela Beltrán, Bogotá, Colombia. 10 Deparment of Health Sciences, Public University of Navarra, Navarra, Spain. 11 Facultad de Educación Física Militar, Escuela Militar de Cadetes General José María Cordova. Bogotá, Colombia
The Corresponding Author: Daniel Botero-Rosas, mail. daniel.botero@unisabana.edu.co.
Abstract
The aim of this paper is to analyze the autonomic behavior mediated by the baroreceptor response to an orthostatic maneuver (OM) in hypertensive and non-hypertensive subjects. The heart rate (HR) was obtained in 65 subjects (32 hypertensive and 33 non-hypertensive) using a frequency meter polar watch, before and after an OM (sudden standing). The R-R intervals were transformed into a heart rate, temporal series, and interpolation was applied due to a lack of heart rate periodicity. Sampling at 10Hz was performed, and a band-pass filter was applied followed by 1 Hz subsampling. The results showed an increase in sympathetic activity in the hypertensive group that did not occur in the non-hypertensive group after the OM (∆%=59.84%, P<0.01). For the parasympathetic results, decreases were found only in the hypertensive group and not in the non-hypertensive group after the orthostatic intervention (∆%= -43.4%, P<0.001). Increases in both groups were observed for the sympathovagal balance (hypertensive: ∆%= 45.63%, p=0.004; non-hypertensive: ∆%= 67.08%, p=0.013). The results showed increased sympathetic activity in contrast to a decreased parasympathetic response for hypertensive individuals after an OM.
Key-words: Autonomic nervous system, Arterial systemic hypertension, Orthostasism, Baroreceptors.
Resumen
El propósito de este trabajo es analizar el comportamiento autonómico mediado por la respuesta barorreceptora a una maniobra ortostática (MO) en sujetos hipertensos y no hipertensos. La frecuencia cardiaca (FC) fue obtenida en 65 sujetos (32 hipertensos y 33 no hipertensos) usando un monitor polar de frecuencia cardíaca, antes y después de una MO (al ponerse de pie súbitamente). Los intervalos R-R fueron transformados en series temporales (ST) de frecuencia cardíaca. Se aplicó interpolación debido a una falta de periodicidad en la ST de FC. Un muestreo de 10 Hz fue realizado, adicionalmente un filtro pasa banda seguida de un sub muestreo a 1 Hz. Los resultados mostraron un incremento en la actividad simpática del grupo hipertenso, lo cual no ocurrió en el grupo no hipertenso luego de la MO (Δ%=59.84%, P<0.01). En cuanto a los resultados parasimpáticos se observaron disminuciones solamente en el grupo hipertenso y no en el grupo no hipertenso luego de la intervención ortostática (Δ%= -43.4%, P<0.001). Se observaron incrementos en ambos grupos para el balance simpático vagal (hipertensos: Δ%= 45.63%, p= 0.004; no hipertensos: Δ%= 67.08%, p= 0.013). Los resultados mostraron un incremento en la actividad simpática en contraste con una disminución de la respuesta parasimpática en individuos hipertensos luego de una MO.
Palabras clave: sistema nervioso autónomo, hipertensión arterial sistémica, ortostatismo, barorreceptores.
Introduction
Arterial systemic hypertension (ASH) is considered one of the main risk factors for coronary ischemic disease, stroke, renal dysfunction and cerebrovascular disease(WHO/ISH.2003). Several theories have been suggested to explain the genesis of ASH, although none have achieved this goal. Currently, dysfunction of short and long term arterial pressure control mechanisms are thought to be responsible for the genesis of ASH (Campagnaro et al., 2012).
Arterial baroreceptors are considered the main short term arterial pressure regulators (Grassi et.al., 1998; Tsekouras et al., 2011). The Autonomic Nervous System (ANS) and more specifically baroreceptor dysfunction have gained importance in recent investigations because it is thought that they might play an important role in the genesis of ASH (Davrath et al.,2003; Radaelli et al.,1994). However, their specific contributions to the mechanism of this pathology are still controversial(Schroeder et al., 2003).
Several factors can alter baroreceptor responses by causing thickening of the intima of the vessels, including age, atherosclerosis, obesity, dyslipidemia, smoking and autonomic dysfunction (Tsekouras et al., 2011; Kougias et al., 2010; Thrasher, 2005). This reduced arterial distensibility results in decreased deformation of the baroreceptors in response to pressure changes, leading to reduced afferent signaling and increased sympathetic outflow (Schroeder et al., 2003; Kougias et al.,2010; De Simone et al.,2006; Panza, 2001). However, the results of studies that have examined the role of arterial stiffness in baroreceptor dysfunction are still inconclusive.
Several studies have focused on investigating the ANS and orthostatic stress (Barantke et al., 2008; Gulli et al., 2007) in distinct populations with different geographic conditions (such as highlanders), genders and ages. Nevertheless, after rigorous examination, little evidence has been found regarding the response of the ANS to orthostatic maneuvers (OM) in hypertensive patients. Akselrod et al. (1997) examined the autonomic changes after an OM in mild hypertensive and normal individuals. In this study, the authors noted an increase in the sympathetic response in mild hypertensive compared to healthy individuals derived from sudden changes in body position. However, no difference was observed in the parasympathetic response during the OM.
The measurement of the Heart Rate Variability (HRV) has gained importance in clinical practice because modifications in sympathetic and parasympathetic modulation may increase the risk of acute cardiovascular events (Mourot et al., 2004; Seiler et al., 2007). Analysis of HRV in both the time and frequency domains has been successfully used to determine autonomic activity. Moreover, the frequency domain has recently been used to provide information that cannot be extracted from the time domain. The Power Spectral Density (PSD) allows analysis in the frequency domain (Wu et al.,2008) using a low frequency component (LF: 0.04-0.15Hz),which is under sympathetic control with parasympathetic modulation through the vagus nerve, and the parasympathetic activity, which is the main contributor to the high frequency component (HF: 0.15-0.40Hz). Furthermore, the LF/ HF ratio is an index of sympathovagal balance (Wu et al., 2008; Vinik & Ziegler, 2007).
The lack of studies that examine the responses of the ANS to sudden differences in body positions in patients with chronic ASH suggests that this subject should be further examined. Therefore, the aim of this study is to evaluate the response of the ANS to baroreflex stimuli through an orthostatic maneuver using analysis of the HRV in the frequency domain in hypertensive and non-hypertensive individuals.
Methods
Subjects
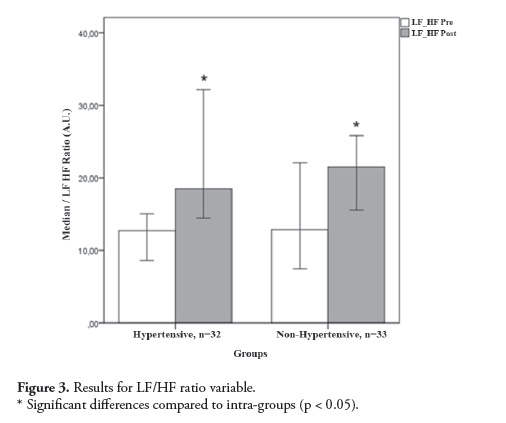
All subjects were examined at the Clinical Research Center at La Sabana Faculty of Medicine (Bogotá, Col). Sixty-five subjects aged 18-65 years were divided into two groups according to blood pressure conditions (hypertensive or non-hypertensive subjects). The hypertensive group included 32volunteers with chronic ASH, and the non-hypertensive group included 33 healthy volunteers. The descriptive characteristics of the subjects are presented in Table 1. The exclusion criteria consisted of secondary hypertension, metabolic syndrome, renal dysfunction, intake of caffeine or alcoholic beverages on data collection day and the presence of any medical condition that could influence the experimental procedures. Each subject was informed of the experiment procedures before the study and gave their written informed consent. The ethics committee of the Universidad de La Sabana approved the study protocols.
Analysis of heart rate variability
The R-R intervals obtained with the frequency meter were downloaded onto a computer through an infrared device and processed with MatlabSoftware®. The R-R intervals were then transformed into a heart rate temporal series, revealing the measurement of the HR value for each heartbeat. The lack of periodicity between each heart beat made it necessary to use the Spline Cubic Interpolation technique to obtain a 10Hz sampling rate. Next, the linear tendency was removed from each tachogram, and a band-pass filter (Butterworth 8th order, frequency cutoff between 0.04-0.4Hz, phase zero) was applied. Finally, subsampling at 1Hz was implemented to obtain the HR signal for each second. To extract the PSD of the temporal series, a Fast Fourier Transform (FFT) was applied to the series obtained during the 5 minutes at rest and the 5 minutes after the OM. Then, the values of the power at the LF (0.04-0.15Hz), HF (0.16-0.4Hz) and the sum of both frequencies were obtained for each subject through a trapezoidal numeric integration method applied to the spectrum. Finally, the values of the power at LF and HF and the LF/HF ratio were calculated.
Experimental procedures
A frequency meter polar watch (Polar RS800)was used to obtain the temporal series of the R-R interval in the supine position during 5 minutes at rest and 5 minutes after an OM(ESC/NASPE,1996; Quintana ET AL.,2012). All volunteers were submitted to an active OM that is a variant of a passive postural maneuver(tilt table test), which is recognized as the most effective response to evaluate cardiac sympathetic and vagal changes because it induces the baroreceptor reflex and involves contraction of the muscles of the lower limbs(Smit et al.,1999). The OMhas been described previously (Smit et al.,1999). The subjects changed their body position in response to a verbal command, without any help or verbal stimulation. No attempt was made to control the movement velocity during the OM. All tests were conducted at the same time of day under controlled conditions (23 to 26°C and 50-60% humidity). Briefly, to minimize errors, standard instructions concerning the testing procedures were given to the participants before the test.
Statistical Analysis
A value ofp≤0.05 was considered statistically significant for all study variables. A descriptive analysis of the variables of the groups with measures of central tendency and dispersion was initially performed. Then, the Shapiro-Wilk test (normal distribution) and Levene's test (variance) were applied, which allowed the application of the Kruskal-Wallis test followed by the Student Newman Keul post hoc test for multiple comparisons. The percentage difference was determined by the formula:
Δ% = ((post-test -test) *100/test).
SPSS 20.0 for Windows® was used for all data analysis.
Results
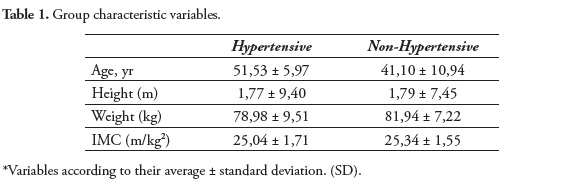
Figure 1 shows the results for the Sympathetic (LF) variable. The Kruskal-Wallis test demonstrated a significant difference (H = 21.54; p= 0.0001)between the pre- and post-test values. However, analysis with the Student Newman Keuls post hoc test for multiple comparisons showed an important significant difference only for the comparison between the pre- and post-test values for the Hypertensive group(pre-test OM= 172.22 and post-test OM= 275.28, ∆% = 59.84%, p= 0.005).
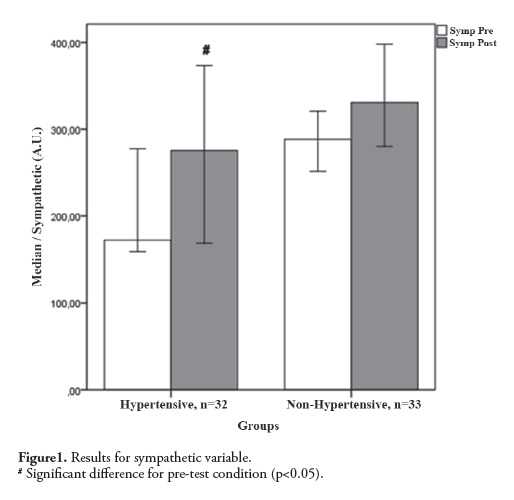
Figure 2 demonstrates the results for the parasympathetic (HF) variable. The Kruskal-Wallis test showed a significant difference (H = 14.98; p = 0.0018) between the pre-and post-test values only for intra-group comparisons, with high post-test values for the Hypertensive group (pre -test OM = 21.12 and post-test OM = 12. 0, ∆% = -43.4%; p = 0.0007).
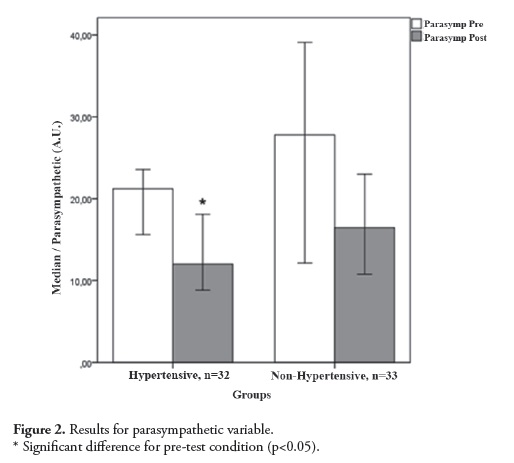
Figure 3 shows the sympathovagal (LF/ HF ratio) variable results. The Kruskal-Wallis test following the Student Newman Keuls post hoc test showed a significant difference (H = 14.69, p= 0.002) between the pre- and post-test values for both groups. The analysis of multiple comparisons showed a significant difference in intra-group differences for the Hypertensive group (pretest OM= 12.69and post-test OM= 18. 48; Δ% = 45.63%;p= 0. 004)and for the Non-Hypertensive group (pre-test OM= 12.85 and post-test OM= 21.47; Δ% = 67.08%; p= 0.013). However, no difference was observed for the comparison between groups.
Discussion
One of the main results of our study was that hypertensive individuals had significant sympathetic elevations after the OM compared to baseline values, which did not occur in non-hypertensive subjects. In addition, a significant decrease in parasympathetic activity after the OM was observed in the hypertensive group but not in the non-hypertensive group. Another key finding of this experiment was that elevations of the sympathovagal value compared the baseline value (supine position) (HF/LF ratio) differed significantly (p < 0.05) between the hypertensive and non-hypertensive groups after the OM. The increased activity of the sympathetic nervous system reduces parasympathetic modulation and the HF/LF ratio in hypertensive patients after an OM, which suggests dysfunctional baroreceptors as the cause of ASH, which occurs even in controlled chronic hypertensive individuals. This result, suggest that at some key times, such as in the morning (Boudreau et al.,2012), OM may potentiate the risk factors for ventricular tachycardia, ventricular fibrillation, and sudden cardiac death in patients with coronary heart disease (Carney et al., 2001). Our results contribute to the understanding of this increased cardiovascular vulnerability after waking.
In a similar study, after an increase in orthostatism, Akselrod et al. (1997) observed similar patterns of sympathetic responses and vagal withdrawal in mild-hypertensive and healthy individuals. In addition, Akselrod et al., (1997) observed differences in the HR fluctuations, demonstrating the lack of a parasympathetical tone mediated by the alteration of baroreflex control in mild hypertension. Akselrod et al. (1997) examined the autonomic changes that occur after an OM by measuring the HRV and AP variability in mild hypertensive on normal individuals. Our results show changes in sympathetic and parasympathetic activity in hypertensive individuals after the OM but not in non-hypertensive subjects, which is not consistent with the results of Akselrod et al. (1997) because we did observe a non-significant sympathetic increase in non-hypertensive individuals. Other aspects that differentiated the two studies included a reduced parasympathetic response that occurred only in hypertensive subjects in our experiment. Our results suggest that this alteration in hypertensive individuals may have occurred due to modifications in baroreflex control.
Certain factors could explain the discrepancy between the results obtained in our study and those obtained by Akselrod et al. (1997); first, our patients had chronic hypertension and were receiving anti-hypertensive medication that did not directly alter the ANS, while Akselrod's patients had mild hypertension and were un medicated. Additionally, the low oxygen pressure at the site of our experimental protocol (Bogotá-Colombia, at 2600 meters above sea level) could have potentiated the sympathetic response due to an interaction between baroreceptors and chemoreceptors, increasing the effect of baroreceptor dysfunction on heart rate regulation(Halliwill & Minson ,2002) and other factors, including the hematocrit, plasma volume, and blood volume, and may have contributed totheobserved discrepancies.
Several studies have investigated the ANS and orthostatic stress (Barantke et al., 2008; Gulli et al., 2007). Specifically, Barantke et al. (2007) examined the effects of aging on the ANS reflex response induced by an OM. Ninety-five healthy volunteers (10 - 70 years of age) underwent noninvasive continuous measurements of heart rate, blood pressure, and baroreflex sensitivity to assess the influence of aging on the responses to orthostatic. It was observed that the pressure reflex in response to body positioning, in contrast to the baroreflex sensitivity response, was not significantly affected by aging. Moreover, the baroreflex sensitivity response was influenced by the blood pressure reflex, as both are interrelated. These observations provided important information about the complex interactions between the autonomic reflexes and physiological orthostasis associated with healthy aging. Additionally, Barantke et al. (2008) examined the effects of sex and aging on the autonomic response to an OM. A total of 362 volunteers (206 women),all between 10 and 88 years of age, underwent continuous noninvasive monitoring of the heart rate and blood pressure in supine and upright body positions. A continuous decline in baroreflex sensitivity and the LF and HF components were observed with increasing age in both males and females regardless of body posture. A comparison of genders values showed significantly higher LF (sympathetic) components in men than in women. The HF (parasympathetic) components did not show differences between genders. Thus, Barantke et al. (2008) concluded that men and women exhibited significant differences in cardiac autonomic modulation according to the posture, and different degrees of this autonomic response to an OM may be associated with variations in age. The results of studies by Barantke et al. (2007, 2008) determined whether sex and age are related to differences in orthostatic tolerance.
As an addition to the growing body of knowledge of orthostatic tolerance and autonomic responses, our study was partially consistent with the studies of Barantke et al. (2007, 2008).Our results showed significant differences between ANS responses to an OM in hypertensive and non-hypertensive patients. This increase in the activity of the sympathetic nervous system in hypertensive patients after an OM suggests baroreceptor dysfunction as the primary cause of AHS. These observations suggest the existence of an error in reception and/or transduction of the information during short term PA changes. According to our results, we hypothesize that the short term sympathetic and parasympathetic responses observed in hypertensive individuals may not be caused by baroreceptor dysfunction.
Kougias et al. (2010) found that any inhibition of baroreceptor function could generate an increase in sympathetic activity and therefore produce higher arterial pressure. Based on the previous mechanism, Kougias et al. (2010) targeted chronic sympathetic activity as the main cause of sustained hypertension. Atherosclerosis has gained prominence over the last decade as an explanation for baroreceptor dysfunction (Thrasher, 2005). Recently, is was suggested that individuals with carotid atherosclerosis showed histological changes of the vascular wall that could alter the elastic properties of the carotid sinus, resulting in reduced the vessel compliance and enhanced baroreceptor sensitivity (Tsekouras et al., 2011; Thrasher, 2005). To confirm this hypothesis, the vessels compliance in animals was experimentally reduced by applying specially designed plastic clamps around the internal and external carotid arteries (Crandall et al., 1957). The authors noted a change in the mean arterial pressure from 126 to 167mmHg after clamp placement. Tsekouras et al. (2011) stated that echogenic carotid plaques are associated with lower baroreflex sensitivity and that it is possible that patients with echogenic plaques have stiffer barosensitive regions that are not sensitive to transmural pressure changes. Additionally, an association between an altered chronotropic response and reduced baroreceptor sensitivity was described (Fukuma et al., 2004). We observed a defectivechronotropic response in hypertensive individuals thought to be generated by stiffer arterial walls, which is consistent with the idea that patients with atherosclerosis exhibit dysfunctional baroreceptors (Nasr et al., 2005; Chao et al., 2003; Gianaros et al., 2002).
Conclusion
A key finding of our study was that the experimental procedures allowed us to observe the behavior of the ANS, which plays an important role in maintaining homeostasis. We found significant differences between the responses of the ANS to an OM in hypertensive, but not in non-hypertensive, subjects. This increase in activity of the sympathetic nervous system and reduced parasympathetic modulation in hypertensive patients after an OM suggests dysfunctional baroreceptors as the cause of this pathology. Although our study supports the theory that dysfunctional baroreceptors area major cause of AHS, investigations with different populations, diseases and pharmaceutical statuses, as well as geographic conditions, should be conducted to properly explain the behavior of HRV in different situations as well as to identify the key mechanisms for this physiological self-regulation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We gratefully acknowledge the support of our universities, without which the present study could not have been completed.
References
1. WHO/ISH. 2003 World Health Organization(WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-92. [ Links ]
2. Campagnaro BP, Gava AL, Meyrelles SS, Vasquez EC. Cardiac-autonomic imbalance and baroreflex dysfunction in the renovascular Angiotensin-dependent hypertensive mouse. Int J Hypertens. 2012;968123. [ Links ]
3. Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31(1):68-72. [ Links ]
4. Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20(9):1675-88. [ Links ]
5. Tsekouras NS, Katsargyris A, Skrapari I, Bastounis EE, Georgopoulos S, Klonaris C, et al. The role of carotid plaque echogenicity in baroreflex sensitivity. J Vasc Surg. 2011;54(1):93-9. [ Links ]
6. Davrath LR, Goren Y, Pinhas I, Toledo E, Akselrod S. Early autonomic malfunction in normotensive individuals with a genetic predisposition to essential hypertension. Am J Physiol Heart Circ Physiol. 2003;285(4):H1697-704. [ Links ]
7. DeQuattro V, Feng M. The sympathetic nervous system: the muse of primary hypertension. J Hum Hypertens. 2002;16 Suppl 1:S64-9. [ Links ]
8. DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension. 2004;43(2):147-50. [ Links ]
9. Radaelli A, Bernardi L, Valle F, Leuzzi S, Salvucci F, Pedrotti L, et al. Cardiovascular autonomic modulation in essential hypertension. Effect of tilting. Hypertension. 1994;24(5):556-63. [ Links ]
10. Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42(6):1106-11. [ Links ]
11. Kougias P, Weakley SM, Yao Q, Lin PH, Chen C. Arterial baroreceptors in the management of systemic hypertension. Med Sci Monit. 2010;16(1):RA1-8. [ Links ]
12. Nasr N, Pavy-Le Traon A, Larrue V. Baroreflex sensitivity is impaired in bilateral carotid atherosclerosis. Stroke. 2005;35(9):1891-5. [ Links ]
13. Thrasher TN. Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R819-27. [ Links ]
14. De Simone G, Devereux RB, Chinali M, Roman MJ, Best LG, Welty TK, et al. Risk factors for arterial hypertension in adults with initial optimal blood pressure: the Strong Heart Study. Hypertension. 2006;47(2):162-7. [ Links ]
15. Lucini D, Mela GS, Malliani A, Pagani M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation. 2002;106(21):2673-9. [ Links ]
16. Panza JA. High-normal blood pressure--more "high" than "normal". N Engl J Med. 2001;345(18):1337-40. [ Links ]
17. Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, et al. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol. 2008;19(12):1296-303. [ Links ]
18. Barantke M, Ortak J, Lieb W, Wilke IK, Schunkert H, Bonnemeier H. Effects of aging on reflex autonomic nervous response induced by orthostatic maneuvers. Pacing Clin Electrophysiol. 2007;30 Suppl 1:S198-202. [ Links ]
19. Freitas J, Santos R, Azevedo E, Carvalho M, Boomsma F, Meiracker A, et al. Hemodynamic, autonomic and neurohormonal behaviour of familial amyloidotic polyneuropathy and neurally mediated syncope patients during supine and orthostatic stress. Int J Cardiol. 2007;116(2):242-8. [ Links ]
20. Gulli G, Claydon VE, Slessarev M, Zenebe G, Gebremedhin A, Rivera-Ch M, et al. Autonomic regulation during orthostatic stress in highlanders: comparison with sea-level residents. Exp Physiol. 2007;92(2):427-35. [ Links ]
21. Mourot L, Bouhaddi M, Tordi N, Rouillon JD, Regnard J. Short- and long-term effects of a single bout of exercise on heart rate variability: comparison between constant and interval training exercises. Eur J Appl Physiol. 2004;92(45):508-17. [ Links ]
22. Seiler S, Haugen O, Kuffel E. Autonomic recovery after exercise in trained athletes: intensity and duration effects. Med Sci Sports Exerc. 2007;39(8):1366-73. [ Links ]
23. Wu JS, Lu FH, Yang YC, Lin TS, Chen JJ, Wu CH, et al. Epidemiological study on the effect of pre-hypertension and family history of hypertension on cardiac autonomic function. J Am Coll Cardiol. 2008;51(19):1896-901. [ Links ]
24. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387-97. [ Links ]
25. ESC/NASPE. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354-81. [ Links ]
26. Quintana DS, Heathers JA, Kemp AH. On the validity of using the Polar RS800 heart rate monitor for heart rate variability research. Eur J Appl Physiol. 2012;112(12):4179-80. [ Links ]
27. Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519 Pt 1:1-10. [ Links ]
28. Boudreau P, Yeh WH, Dumont GA, Boivin DB. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int. 2012;29(6):757-68. [ Links ]
29. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104(17):2024-8. [ Links ]
30. Akselrod S, Oz O, Greenberg M, Keselbrener L. Autonomic response to change of posture among normal and mild-hypertensive adults: investigation by time-dependent spectral analysis. J Auton Nerv Syst. 1997;64(1):33-43. [ Links ]
31. Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol. 2002;93(3):857-64. [ Links ]
32. Crandall EE, Mc CH, Sukowski EJ, Wakerlin GE. Pathogenesis of experimental hypertension produced by carotid sinus area constriction in dogs. Circ Res. 1957;5(6):683-92. [ Links ]
33. Fukuma N, Oikawa K, Aisu N, Kato K, Kimura-Kato YK, Tuchida T, et al. Impaired baroreflex as a cause of chronotropic incompetence during exercise via autonomic mechanism in patients with heart disease. Int J Cardiol. 2004;97(3):503-8. [ Links ]
34. Chao AC, Chern CM, Kuo TB, Chou CH, Chuang YM, Wong WJ, et al. Noninvasive assessment of spontaneous baroreflex sensitivity and heart rate variability in patients with carotid stenosis. Cerebrovasc Dis. 2003;16(2):151-7. [ Links ]
35. Gianaros PJ, Jennings JR, Olafsson GB, Steptoe A, Sutton-Tyrrell K, Muldoon MF, et al. Greater intima-media thickness in the carotid bulb is associated with reduced baroreflex sensitivity. Am J Hypertens. 2002;15(6):486-91. [ Links ]