Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
CES Medicina Veterinaria y Zootecnia
versão On-line ISSN 1900-9607
Ces. Med. Vet. Zootec. vol.8 no.1 Medellín jan./jun. 2013
Strangles: the most prevalent infectious respiratory disease in horses worldwide¤
Adenitis: la enfermedad respiratoria infecciosa de mayor prevalencia en caballos en el mundo
Adenite: a doença respiratória infeciosa de maior prevalência em cavalos ao redor do mundo.
María Patricia Arias Gutiérrez1*, MV, PhD
*Autor para correspondencia: María Patricia Arias. Facultad de Medicina Veterinaria, Universidad CES, Calle 10 A No 22-04, Barrio el Poblado, Medellín, Colombia. E-mail: marias@ces.edu.co.
1*Grupo INCA-CES, línea de investigación en fisiopatología equina, Facultad de Medicina Veterinaria, Universidad CES, Calle 10 No 43 A 50, Barrio el Poblado, Medellín, Colombia. E-mail: marias@ces.edu.co.
(Recibido: 20 de abril, 2013; aceptado: 15 de junio, 2013)
¤Para citar este artículo: Arias MP. Strangles: the most prevalent infectious respiratory disease in horses worldwide . Rev CES Med Zootec. 2013; Vol 8 (1): 143-159.
Abstract
Streptococcus equi subsp. equi is the causative agent of Strangles in horses, the most common respiratory infectious disease worldwide. Typical signs of the disease are fever, nasal, discharge, abscessation of retropharyngeal lymph nodes and inappetence; complications as Bastard Strangles and Purpura haemorrhagica may occur. Outbreaks may last for months or even years in farms and stables causing a great economical impact due to the large periods of convalescence, quarantine and treatments. Some horses suffer guttural pouch empyema, harbouring the bacteria in pus or chondroids, becoming carriers, which are a major problem for the prevention. Currently, many research groups all over the world are focus on the production of a safe and effective vaccine against this highly contagious disease. The control of the disease is difficult without a highly effectiveness vaccine. In Colombia, there is not well known the prevalence of Adenitis neither the microorganisms involved in this disease; moreover, in some countries of Latin America the carrier prevalence is markedly underestimated or unknown and it is necessary to focus more resources and effort to epidemiology studies in this disease.
Key words: Equine respiratory disease, guttural pouches, lymphadenophaty, quarantine, Streptococcus equi.
Resumen
Streptococcus equi sbsp. equi es el agente causal de la Adenitis en caballos, la enfermedad respiratoria infecciosa más común en todo el mundo. Los signos típicos de la enfermedad son fiebre, descarga nasal, abscesos en los nódulos linfáticos retro-faríngeos e inapetencia. Pueden ocurrir complicaciones como Adenitis bastarda y Purpura hemorrágica. Los brotes pueden durar meses y hasta años en fincas y establos, causando un gran impacto económico debido a los largos periodos de convalecencia, cuarentena y tratamientos. Algunos caballos sufren empiema de las bolsas guturales, alojando la bacteria en pus o en los condroides, convirtiéndose en portadores, los cuales son el principal problema para la prevención. Actualmente, muchos grupos de investigación en el mundo están enfocados en producir una vacuna segura y efectiva contra esta contagiosa enfermedad. El control de la Adenitis equina es difícil sin una vacuna altamente efectiva. En Colombia no se conoce bien la prevalencia de la adenitis ni los microorganismos involucrados en esta enfermedad; más aun, en muchos países de América Latina la prevalencia de los portadores es subestimada o se desconoce, y es necesario dedicar más recursos y esfuerzo a la investigación en la epidemiología de esta enfermedad.
Palabras clave: Bolsas guturales, cuarentena, enfermedad respiratoria equina, linfadenopatía, Streptococcus equi subsp. equi.
Resumo
Streptococcus equi subsp. equi é o agente causador da Adenite equina, a doença respiratória infeciosa mais comum em cavalos ao redor do mundo. Os sinais clínicos da doença são febre, secreção nasal, abscedação dos nódulos linfáticos retro-faríngeos além de inapetência. Podem ocorrer complicações como adenite bastarda e púrpura hemorrágica. As manifestações clínicas podem durar desde meses e ate anos nas fazendas e estábulos, causando um grande impacto econômico devido aos longos períodos de convalescência, quarentena e tratamentos que eles requerem. Alguns cavalos sofrem de empiema das bolsas guturais, alojando a bactéria em pus ou nos condroides, chegando a serem portadores, o qual é o principal problema para a prevenção. Atualmente, muitos grupos de pesquisa no mundo estão enfocados em produzir uma vacina segura e efetiva contra essa doença altamente contagiosa. O controle da Adenite equina é difícil sem uma vacina altamente efetiva. Na Colômbia não se conhece bem a prevalência da adenite nem os microrganismos involucrados nesta doença; ainda mais, em muitos países de América Latina a prevalência dos portadores é subestimada ou se desconhece, e é necessário dedicar mais recursos e esforços a pesquisa na epidemiologia desta doença.
Palavras chave: bolsas guturais, doença respiratória equina, linfadenopatia, quarentena, Streptococcus equi subsp. equi.
Introduction
Strangles, also known as Equine Distemper, caused by Streptococcus equi subsp. equi (S. equi equi), is the most commonly diagnosed respiratory infectious diseases of equids world-wide1,2. S. equi equi is a highly contagious and fast spreading microorganism, and mobile horse populations favor its widespread distribution3. Infected animals show classically pyrexia, severe inflammation of the oropharynx and nasopharynge mucosa, serous to nasal discharge that soon becomes purulent, swelling and often rupture of the head and neck lymph nodes, which produce large amounts of pus. Different presentations of the disease, from a mild nasal discharge to Bastard Strangles may be seen in infected horses4,5,6. Purpura haemorrhagica, meningoencephamyelitis and brain abscesses are sequels of the disease7,8. Established outbreaks may last for months or even years, particularly in large populations1,2. Due to long periods of convalescence and quarantine of infected animals, this disease causes great economical consequences for stables and riding schools9,10, and continues to wreak havoc despite decades of research. Control and prevention are difficult despite there are several commercially vaccines: Equilis StrepE has been approved for sale in Europe and Pinnacle IN is used in the USA and some other countries of the World. Timoney (2007)11 report adverse reactions to Equilis StrepE, although, it occurs when the vaccine is used inappropriately; Waller and Jolley (2007)2 states that the vaccine protect 50% and reduce clinical signs in a further 25% of horses; Guss et al., (2009)12, demonstrated the efficacy of a new subunit vaccine in horses; Florindo et al (2009)13 are working on the quantification of systemic and mucosal immune responses to a nanoparticle vaccine in mice. Currently, efforts of some research groups are focus on some immunogenic surface proteins of the bacterium to develop and produce a protective vaccine against this disease. Hopefully soon the equine veterinary practitioners may have a vaccine that helps to control this antique disease.
Etiology
S. equi equi is a gram-positive, beta-hemolytic streptococcus of Lancefield group C. It is highly host adapted to horses, donkeys and mules14. On blood agar the bacteria forms β-hemolytic colonies, and classically, it is highly capsulated giving it a "honey dew" appearance, which differentiate it from its parent Streptococcu subsp. zooepidemicus (S. equi zooepidemicus)15. Biochemically, it can be recognized because S. equi equi ferments salicin and sucrose but not sorbitol, lactose, trehalose, glycerine, or mannitol16. S. equi equi is a clone or biovar of S. equi zooepidemicus; it seems that the bacterium has evolved from this ancestral strain based on a high sequence homology17,18; their DNA are almost identical, and these pathogens share approximately 80% genome sequence identity with the important human pathogen Streptococcus pyogenes17. S. equi equi strains are known to be highly homogeneous and show limited genetic diversity. There is variation in some virulence factors, such as the hyaluronic acid capsule, the M-like proteins SeM and SzPSe, Streptolysin S, and Pyrogenic superantigenic exotoxins19. S. equi equi can survive for several weeks in water troughs, but dies quickly in soil or pasture10.
As the environment of the host provides significant challenges to bacterial survival, this bacterium, as all the pathogenic species, has evolved mechanisms that enable its persistence in this environment16. S. equi equi expresses several virulence factors that mediate interactions with host cells, and explain the virulence and host adaption of this bacterium in comparison with its ancestor zooepidemicus20. Surface virulence components are grouped according its function: some of them promote bacterial adherence to host cells, others contribute to immune evasion allowing bacterial resistance to innate and adaptive host defences21, and several are involved in nutrient acquisition through degradative enzymes that may result in damage to the host or spread of the organism22. Surface exposed and secreted proteins of S. equi equi are involved in attachment, penetration, subversion of tonsilar innate immune defenses, evasion of phagocytosis and stimulation of immune responses23.
The virulence factors of S. equi equi
S. equi equi binds to receptors and colonize mucosal cells in a highly selective manner through their virulence factors which interact with a great variety of surface components, such as extracellular matrix molecules on host tissues and body fluid proteins (x). The adhesion of S. equi equi to host soluble components and tissues is an important step in this infection process24. The ability to interact with different substrates is likely to increase S. equi equi chances of surviving25. S. equi equi have a great variety of virulence factors that interact with host tissues and components, and favour bacteria colonization and growth19.
Hyaluronic acid capsula
A high molecular weight polimer formed by residues of N-acetylglucosamine and glucuronic acid forms the hyaluronic acid capsula of S. equi equi20. The antiphagocitic capsule reduces the contact of the bacteria with neutrophils and subsequently, phagocytosis26,27. In addition, the capsula increase the negative charge and hydrophobicity of the bacteria surface, generating a reducing environment that protects the oxygen labile proteases and toxins28,29. Therefore, without the capsula, the surface proteins may aggregate, losing their configuration and functionality10.
Collagen binding protein (Cne)
The adhesion of the bacteria to Fibronectin, a dimeric cell-adhesive extracellular matrix glycoprotein secreted by mesenchymal cells and assembled into insoluble matrices is mediated by Fibronectin-collagen binding protein30. The main function of Fibronectin is to mediate substrate adhesion of eucaryotic cells, involving the binding of Fibronectin-binding proteins to certain domains of the Fibronectin molecule31. Binding between bacterial cell surface Fibronectin binding proteins and immobilized fibronectin promotes internalization of Streptococci to epithelial cells32. Some streptococcal Fibronectin-binding proteins display additional enzymatic activities, such as serine protease activity, and trigger intracellular signaling pathways12. Two Fibronectin-binding proteins have been identified in S. equi equi, which harbor genes encoding FNE and SFS32.
Collagen-like proteins
SclC found in S. equi equi is a member of a seven collagen-like proteins family found in Gram-positive bacteria called SclC-SclI. Using PCR, the sclC gene was detected in strains of S. equi equi and also in S. equi subsp. Zooepidemicus33. In sera from horses previously diagnosed with Strangles, antibodies against SclC were detected suggesting that these proteins are expressed during the infection, and may play a key role on immunogenic response34.
Fibrinogen binding proteins
Some S. equi equi strains can bind human and equine fibrinogen through different proteins35; some of them interact with fibrinogen, specifically, by attachment to the C-terminal serine protease domain of equine fibrinogen to form active plasmin which hydrolyses fibrin, facilitating the spreading and the dispersion of the bacteria on host tissues36. Others functions of plasmin are activation of complement and production of low molecular nitrogen substances for promotion of bacteria growth37. M-like proteins also bind fibrinogen avidly through residues located at the extreme N-terminus of the molecule38.
M-like proteins
S. equi equi produces two M-like proteins: SeM, which is unique to S. equi and SzPSe, which is homologue of the M-like protein SzP produced by S. equi zooepidemicus39. Both proteins have shown a strong binding to equine fibrinogen and also antiphagocytic activity40. SeM is the major virulence factor and protective antigen of S. equi equi for its ability to provide phagocytosis resistance to the bacteria41. The antiphagocytic activity of M-like proteins are associated with their ability to inhibit deposition of the complement component C3b on the bacterial surface and also to bind fibrinogen to the N-terminal portion of the protein, as described above, which consequently inhibits phagocytosis26,28.
S. equi equi with truncated SeM has been isolated form outwardly healthy horses, therefore, this protein play an important role on bacteria virulence and seems to be related with the carrier state42. It is no casual that SeM extract has been used to produce some commercial vaccines.
Se18.9 protein. Se18.9 is an H factor binding protein secreted by S. equi equi that reduces the bactericidal activity of equine neutrophils and also deposition of C3 on the bacterial surface. Se18.9 is being studying for its potential to be used in immunodiagnosis and also to understand the mucosal antibody response to S. equi equi infection43.
Streptolysin S
This protein is a 36 aa oligopeptide with bactericin like cytotoxin activity responsible for the β-hemolysis44. To carry out its biological activity, this protein requires stabilization by association with a carrier protein, like albumin. The binding of the Streptolysin-albumin complex to erytrocytes causes the formation of transmembrane pores and consequently, lysis of red blood cells45.
Pirogenic mitogens
All the pirogenic mitogens have high immunemodulating capacity; they are able of binding to MHC Class-II molecules on antigen presenting cells and also to the variable region of β-chain of the T-cell receptor molecules causing a stimulation of large numbers of T cells and misdirection of the immune response. The result is a non specific T-cell proliferation and proinflammatory cytokines release such as IL1, IL2, TNF-β and IL6, responsible for triggering the acute phase of Strangles with fever, neutrophilia and fibrinogenemia19. Supernatant preparations of clinical isolates of S. equi equi elicited potent mitogenic responses from peripheral blood mononuclear cells. Serum from a horse experimentally infected with the S. equi equi strain CF32 abolished the mitogenic response neutrilizing the acute effects caused by the mitogenic factors46. At least four phage associated bacterial superantigens, SeeH, SeeI, SeeL and SeeM, are known to be expressed by S. equi equi, but SeeH is inactive47.
Lipoteichoic acid (LTA)
S. equi equi produces a Hyaluronan-associated protein (HAP) that is nearly identical to S. equisimilis HAP, an extracellular threonine kinase with autophosphorylation activity48. Whether the HAP of S. equi equi possesses protein kinase activity and plays a role in cell wall shedding as HAP of S. equisimilis remains to be established, but the identification of two surface-expressed phosphatase activities in S. equi equi suggests that the organism has surface-located enzymes that may regulate a protein kinase activity, as occurs with S. equisimilis HAP which is, sensitive to endogenous phosphatases39. Therefore, there is a possibility that an extracellular kinase could regulate the degree of encapsulation of S. equi equi, in response to the level of ATP in the host environment48.
VicK
Two-component regulatory system VicK important to S. equi equi growth and virulence, resist to phagocytosis by polymorphoneuclear leukocytes, VicK is being considering as a potential live Strangles vaccine49.
Factors associated with nutrient acquisition. S. equi equi has a group of factors that facilitate nutrient acquisition and metabolism50, conformed by Acid Phosphatases, ATP-binding cassette (ABC) importer systems and some degradative enzymes51.
Currently, no one really knows which factor(s) exactly mediates immunity of S. equi equi infection and this certainly a clue aspect to have a better comprehension of the immune response against strangles19.
Epidemiology
Strangles infection has been named by Schutz in 1888, but since ancient Rome this disease has been described by veterinarians52; nowadays, the disease prevail worldwide40. Equines of any age may contract the disease, but elderly and younger equines are more susceptible, except foals less than four months who are protected by colostrums derived passive immunity53. Elderly equines may have a weaker immune system and then, are the most severity affected with a longer duration of the disease54, however, it has been reported by several authors that older animals frequently present a mild form of the disease, possibly due to acquired immunity after natural infection1,6,42.
Although the majority of infected animals become subsequently immune after natural infection, some may contract the disease once again55. The severity of the clinical presentation depends on many factors, such as age, previous infections, vaccination and stabling conditions. Approximately 10% of the recovered horses fail to clear S. equi equi and continue bacteria shedding through nasal secretions for up to 39 months after strangles outbreaks, and they can be the cause of other outbreaks56. Horses recovered from the clinical disease may have persistent infection of S. equi equi in the guttural pouches and are source of infection57.
Guttural pouches are not only an important site of S. equi colonization, but also an integral to the S. equi equi carrier state9,1. The rupture of abscesses formed in retropharyngeal lymph nodes drains pus into the guttural pouches and these horses become persistently infected carriers58, therefore, horses with subclinical disease, such as some cases of guttural pouch empyema, may shed the organism for years56. These carriers transmit the organism to naïve horses and play an important role in disease spreading42. Persistent carriers (healthy animals) of S. equi equi harbour the bacteria in chondroids formatted in the guttural pouches and continue to spread the infection for prolonged periods of time59,60.
In susceptible populations, the morbility is 85% to 100%, although, mortality is low, from 4% to 8%3. Stressors factors as weaning, travelling, sudden climate changes and improper nutrition facilitate the transmission of Strangles23; in addition, communal drinking sources, high population density, and mobility favor the infection spreading61. Strangles is highly contagious, oral and nasal routes are the main way of transmission1; infected secretions can be transmitted by direct contact or through fomites10. Infected animals shed the bacteria for 2 to 3 days after the onset of fever, or 4 to 7 days after infection, and continue shedding 4 weeks after resolution of clinical signs58. A Strangles outbreak can last 4 to 6 months on a farm of a susceptible population with an inadequate isolation protocol62.
Pathogenesis
Nasopharingeal infection with S. equi equi generally follows contact with a shedding carrier horse or with an acutely infected animal16. S. equi equi enters via nose or mouth and attaches to the crypt cells of the palatine and lingual tonsils, oro and nasopharinx and lymphoid nodules. The organism multiplies in the lymph nodes1. The organism has been detected in tonsillar crypts, subepithelial follicular tissue and lymph nodes 3 hours after inoculation53. Migration of neutrophils into the lymph nodes causes swelling and abscessation 4 to 7 days after infection begins nasal shedding63. Clumps of S. equi equi are visible in the lamina propia 48 hours after infection57. At the onset of fever, tonsillar tissues and lymph nodes are infiltrated by neutrophils and also long chains of the bacteria26. The capsule facilitates the adherence process, and the expression of SeM by the bacteria, avoid phagocytosis20. This latter mechanism is an infectious advantage of S. equi equi in comparison Pathogenesis with its ancestral parent S. equi zooepidemicus, being the former more virulent18. The visual evidence of intracellular and extracellular multiplication of S. equi equi in tonsillar lymphoid tissues and lymph nodes indicates involvement of potent antiphagocytic activity and failure to innate immune defences16.
The hyaluronic acid capsule of S. equi equi facilitate bacteria colonization29, and the bacteria release some toxins and enzymes which damage the surrounding host cells, beginning the process of inflammation; at this point, the horse show fever, nasal discharge and pharyngitis64. At this early stage, the mucosal surface is hyperaemic but not ulcerated, and histological examination reveals neutrophilic exocytosis across the ephitelium, with accumulation of lymphocytes, plasma cells and neutrophils in the subjacent lamina propria62.
The SeM inhibition of C3 complement deposition on the bacterium avoids opsonisation, decreasing the neutrophil chemotaxis and microorganism clearance, therefore, the bacteria spread to the submandibular and retropharyngeal lymph nodes in few hours65. Despite the decrease of neutrophils chemotaxix, a lot of them are recruited into the lymph nodes; however, they are not able to phagocyte the bacteria due mainly to the antiphagocitic properties of SeM and the hialuronic acid capsule, and bacteria multiplication proceeds despite the polymorphonuclear leucocyte infiltration66. Gross pathology at this stage shows intranodal abscessation66. Histologically, lymphoid necrosis and massive neutrophils infiltration can be observed, and by Gram staining streptococci can be seen in necrosis areas, most of them are not phagocytosed by neutrophils, although the acute inflammation62.
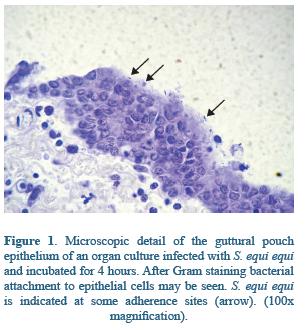
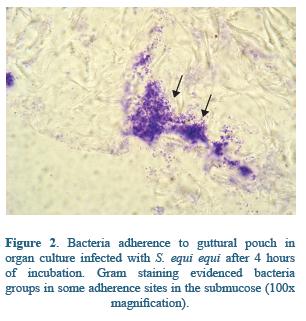
In the respiratory tract, glycosaminoglycanes of the mucosae have been shown to be ligands for streptococci bacteria25,67. In guttural pouches many proteins mediate the attachment of S. equi equi to mucosal surface. In the ephitelium the wide distribution of glucosaminoglycanes seems to be determinant of S. equi equi adhesion, and may facilitate S. equi equi colonization through SeM or other surface binding proteins68,69 (Figure 1). Indeed, the preference of S. equi equi for guttural pouches may be explain by the presence of a great variety of glycosaminoglycanes such as chondroitinsulphate B, heparin and heparin sulphate in the guttural pouches secretions, playing a key role on the pathogenesis of Strangles infection68. S. equi equi attaches also to the submucosal cells, mainly through Fibronectin Binding proteins FNE and SFS38, and extracellular matrix molecules70; these interactions have been well documented in naso and orofaringe14 (Figure 2). Although alterations caused by Strangles infection are well documented, some key points of the early pathogenesis, especially in relation to bacteria colonization, remain understood23.
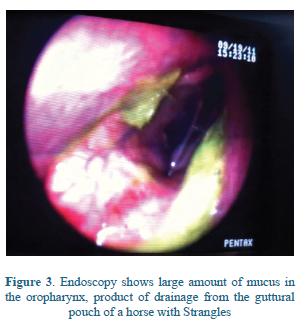
The bacteria occasionally spreads through lymphatic or hematogenous circulation during the early febrile stage and abscesses are formed in lymph nodes and body organs distant from the head and neck in a severe condition known as 'Bastard Strangles'4. Most of these abscesses are located on the abdomen and thorax, rarely, abscesses on other locations can be observed5. Guttural pouch empyema can occur as a consequence of rupture of retropharyngeal abscesses into the pouches and also from local spread of the infection from the pharynx through the pharyngeal openings of guttural pouches71 (Figure 3).
A complex of purpura haemorrhagica can be development in horses during or after Strangles infection64. Affected horses characteristically have particularly high levels of anti-M protein antibodies and it is thought that these generate M-protein immune complexes responsible for this condition72,73. The S. equi equi M-protein may trigger the immune complex disease purpura haemorrhagica inducing the formation of complexes that bind to the β2-integrins of host neutrophils, leading to their activation and subsequent realise of heparin binding protein, an inflammatory mediator that induces generalised vasculitis, oedema and toxic shock which is frequently fatal74. It is quiet intriguing the fact that purpura haemorrhagica is also associated with some strangles vaccines63.
Clinical findings and complications
The signs appear after an incubation period of 3 to 8 days approximately, and they generally last for 3 or 4 weeks9. The disease develops suddenly with complete anorexia, depression, fever, and serous nasal discharge which rapidly becomes copious and mucopurulent6 (Figure 3).
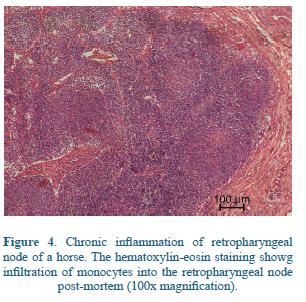
Anorexia, loss of condition and depression increase with the disease progress61. Sometimes, a moist cough may be present75. Retropharyngeal lymph node enlargement may cause obstruction of the oro- and nasopharynx with subsequent dyspnea54, submandibular lymph nodes become enlarged, firm and painful, and usually also causes dysphagia40 (Figure 4).
The swelling of the lymph nodes may, in severe cases, restrict the airway and it is this clinical feature that gave ‘Strangles’ its name63; death by asphyxiation may occur at this time in severe cases, although is not common1. In the acute phase of the disease, fibrinogen plasma concentration and leukocyte counts increase62.Abscessed lymph nodes burst 7 to 10 days after the clinical signs presentation discharging high infectious pus, and if complications do not occur, they recover 1 or 2 weeks thereafter76.
An atypical form of the disease can occur in older animals with residual immunity to S. equi equi, which is characterized by a short transient fever, slight nasal discharge, and anorexia, sometimes accompanied by lymphadenopathy77. Some authors have been associated this mild presentation of the disease with certain low virulence S. equi equi strains, rather than age or immunity42,73.
The most common complication is the rupture of abscesses in the retro-pharyngeal lymph nodes on the roof of the nasopharynx that become inflamed and drain into overlyng guttural pouch, causing infection with empyema77. Marked inflammation of the lymph nodes that drain the pharyngeal area (the primary site of infection) cause the classical equine “septic sore throat”, and is manifested by grossly enlarged submandibular lymph nodes that frequently drain outside54.
Purpura haemorrhagica is an immune complex that may be trigger by S. equi equi and other streptococcal infections in horses, associated with leucocytoclastic vasculitis, edema in the head and limbs, petechial hemorrhages in mucosae, musculature and viscera, and sometimes glomerulonephritis72. Anatomical changes as infarction of the skeletal musculature, skin, gastrointestinal tract, pancreas, and lungs have been found at necropsy, also leukocytoclastic vasculitis in numerous tissues and acute coagulative necrosis. Neutrophilia with a left shift and toxic changes, hyperproteinemia, hypoalbuminemia, and high serum creatine kinase and aspartate transferase activities are frequent hematologic and serum biochemical abnormalities78. Immune complexes containing IgA and S. equi equi specific antigens are found after Strangles infection, as consequence of autoimmune vasculitis72. Purpura haemorrhica is frequently fatal55.
Metastatic infection, also known as "Bastard Strangles", results in the formation of abscesses in any organ or body site, but most commonly in the lungs, mesenteric lymph nodes, liver, spleen, kidneys and brain5. Metastatic brain abscesses positive for S. equi equi and other soft tissue lesions has been confirmed by magnetic resonance imaging in horses with Bastard Strangles7. Deeply located abscesses usually do not produce any well defined clinical syndrome and are often undiagnosed. Because of the large size of many Strangles abscesses (30 cm diameter or more), penetration of antibiotics is inadequate14. Horses with a history of recent Strangles infection or exposed to strangles cases, that are suffering from colic, weight loss, respiratory signs, fever and present raised white cell count and acute phase proteins should be suspected of Bastard Strangles72. Also horses with neurological signs after Strangles outbreaks should be examined by magnetic resonance imaging78. Some authors associate this condition with the use of the intranasal vaccine14.
Less common complications have been observed. In foals with neurologic signs that had a history of Strangles or were exposure to infected horses, meningoencephalomyelitis caused by S. equi equi was reported8. The development of suppurative necrotic bronchopneumonia secondary to the aspiration of pus from ruptured abscesses or metastatic infection of the lungs is another reported complication78.
Authors agree about the fact that these different presentations of the disease indicate a change in virulence of certain strains of S. equi equi4,42,31.
Immunity and vaccination
Protective immunity is mediated by a combination of serum opsonic and nasopharyngeal mucosal humoral responses19. Convalescent horses exhibit a protective immune response, mainly the local production of antibodies against SeM protein, an antiphagocytic and opsonogenic S. equi M-like protein, known as the major protective antigen against strangles. Although SeM is highly antigenic, other bacterial proteins can stimulate antibodies production involved on the immune response. Nasopharingeal mucosal IgA and IgG also confers protection11. After natural infection, nasopharingeal mucosal IgA and IgG levels are high and immunity is enough to protect against another infection for 4 years after natural infection in most of the animals, however, 1/4 of animals do not develop a good immune response, becoming susceptible to a new infection 6 months later79.
Foals that receive adequate high quality colostrum from exposed or vaccinated mares have serum IgG and nasopharyngeal mucosal IgA that provide resistance to S. equi equi infection80; calostral antibodies against SeM cover the oropharyngeal mucosa during suckling and IgA is absorbed from the gastrointestinal tract during the three first days of birth. Foals remain protected for up to three months because of acquired IgG on serum and IgA on the mucosa64.
As protective immune responses have little impact on resolution of abscesses, therefore, an ideal practice would be to stimulate an immune response that function early into the tonsilar complex62. Acquired immunity after natural infection appears to block tonsillar entry of the bacteria, as shown by absence of serum antibody responses to its immunogenic surface proteins64. The problem is that the immune mechanism involved in the protective response remains understood, and this fact difficult the development of an effective vaccine79.
Current vaccines against Strangles rely on immunisation with inactivated bacteria, S. equi bacterin, or SeM extracts. Two commercially available vaccines contain purified bacterial extracts of S. equi equi M-like protein SeM81,82. In animals which are at increased risk of contracting the disease, vaccination protocols with SeM has been used, and although this vaccination elicit an increase reactive antibody level, its use is still controversial since it leads limited protection and untoward effects83. These vaccines may reduce the clinical attack rate by 50%, a level of protection much lower than that produced during recovery from Strangles63. In addition, some of these vaccines should be administered intramuscularly, causing frequently swelling and pain at the injection site. Injection into the pectoral muscles is preferred since injection on the neck muscles may cause the horse to be unable to lower its head for several days. Another side effect is that some vaccinated horses produce high SeM antibody titers, and purpura hemorrhagica has been associated with administration of this vaccines63. A recombinant vaccine with S. equi hyaluronate associated protein is available83, but neither this one have showed significant protection79.
A live non-specifically attenuated S. equi vaccine made of live attenuated strain, which lacks the hyaluronic acid capsule and for intranasal inoculation has been used in USA64. This vaccine was developed by chemical mutagenesis on the bacteria genome, and random mutations may prone to back and thus, to reverse to full virulence, therefore, this vaccine has not been licensed for sale in Europe because of safety concerns84,29,82. Care should be taken to avoid contamination of injections elsewhere in the horse, since concurrent injection of other vaccines has resulted in S. equi equi abscesses at these sites, presumably through inadvertent contamination. Various adverse effects, including pharyngeal lymphadenopathy, limb edema, and bastard strangles abscesses were reported after the use of this vaccine63. Occasional reports of lack of efficacy may reflect failure of the vaccine to block antibodies or to reach the tonsil79.
A live-attenuated deletion mutant, strain TW928, was constructed and evaluated. Despite the sub-mucosal vaccinations in the inner side of the upper lip caused small transient swellings, these resolved completely within two weeks. In comparison with the side effects of the SeM protein vaccines, the sub-mucosal vaccination appeared to be safe in foals, but not efficacious, since mucosal immune response were not observed in vaccinated horses82.
Different live attenuated S. equi equi mutants administered by the intranasal route are also commercially available. The vaccines were reported to be not enough protective2. Furthermore, some complications such as guttural pouch empyema, purpura hemorrhagica and focal vasculitis in the upper airways associated with this vaccine were reported11.
Two endopeptidases of S. equi equi and S. equi zooepidemicus, IdeE2 and IdeZ2, have been studied as potential vaccine components in a mouse infection model85. In this vaccination and challenge study, both enzymes induced protection against S. equi infection79. This group is still studying the use of these components for the development of a Strangles vaccine.
Florindo et al., are focus on the development of a new intranasal vaccine S. equi equi antigens encapsulated in poly(lactide-co-glycolide) nanospheres. This vaccine is based on the use of purified recombinant SeM and S. equi protein extract-entrapped nanospheres as potential carriers for the delivery of S. equi equi antigens, in order to induce a mixed Th1 and Th2 response86,79.
The Strangles research group of The Animal health Trust directed by Andrew Waller is working on the identification of potential vaccione components based on the genomic sequences of S. equi equi and S. equi zooepidemicus. They are focus on seven recombinant S. equi equi immunogenic proteins (five surface localized proteins and two IgG endopeptidases) that may stimulate mucosal response, serum opsonin and neutralizing antibody to the non pyrogenic exotoxins.
A safe and efficient vaccine which confers broad protection to horses throughout the world is desired by all veterinary practitioners; hopefully this vaccine will be soon available.
In Colombia, there are not commercial vaccines for Strangles; some vaccines against influenza are available but there are specific for orthomyxovirus, and these do not prevent Streptococcus infection.
Diagnosis
Additionally to the history and clinical signs, diagnostic should be confirmed by culture of nasal swabs, washes, or lymph nodes aspirates and PCR for the identification the bacterium3. The encapsulated bacteria forms a honey colored mucoid colony with a zone of beta-hemolysis on blood agar, and does not ferment lactose, trehalose or sorbitol, these characteristics differentiate S. equi equi from S. equi zooepidemicus18, although, it is important to confirm the subsp. by PCR63. Unfortunately, culture may fail to detect the organism during the incubation period, in early clinical phases, and in the guttural pouch carriage in apparently normal horses following recovery from strangles63.
Persistent carriers of S. equi equi may be identified by culture and PCR testing of lavage fluid from the nasopharynx or guttural pouches and nasopharynx swabs after recovery from acute disease and at postmortem examination87. PCR increases the carrier detection rate because it is three times more sensitive than cultures and detects the DNA sequence of S. equi SeM gene and also Sod A gene, but this test does not differentiate between dead and live bacteria, therefore, a positive test cannot correlate with an active infection24.
Serology is not very useful in the detection of S. equi equi infections. Serum levels of SeM antibody can be measured by ELISA88; titres peak 4 to 5 weeks after natural exposure and remain high for 6 to 8 months, but false negative titters may occur if exposure has occurred within the previous 7 days, because of insufficient time to induce seroconversion47. ELISA does not predict carrier status after a strangles outbreak18. A complete blood count and plasma fibrinogen concentration are useful to support the diagnosis and may also differentiate horses with acute S. equi equi infection from horses with S. equi zooepidemicus infection88 acute viral processes40. Hyperfibrinogenemia is characteristic of both the acute and chronic disease. Leukocytosis with neutrophilia and hyperproteinemia attributable to a polyclonal gammaglobulinemia is characteristic of metastatic and chronic abscessation58.
In conclusion, a guttural-pouch and upper airways endoscopy with subsequent culture and PCR testing to detect S. equi equi remains the most accurate method available for the identification of persistent carriers15,18. Other diagnostic as pharyngeal radiography and lymph node ultrasonography may help to have an accurate diagnosis54.
Strangles should be differentiated clinically from other upper respiratory tract diseases of horses3. Chronic weight loss due to metastatic infection should be differentiated from equine infectious anemia, parasitism, inadequate nutrition, and neoplasia76.
Treatment
Whether or not to treat an animal with Strangles with antibiotics is still controversial among veterinarians53. Treatment of an animal in the early stages of the disease is usually effective and is not associated with side effects. Some authors have described that antimicrobials impairs the development of acquired immunity after natural infection by decreasing the antigenic exposure, allowing hematogenous bacteria spreading; however, it has not been established a correlation between antimicrobial use and S. equi equi bacteremia36. Some Treatment veterinarian practitioners believe that treatment predisposes the horse to prolonged infection and even to suffer bastard strangles, but there is no evidence of this fact40.
The use of antimicrobials is also controversial. S. equi equi is highly susceptible to penicillin G, which is recommended at 22,000 IU/kg in early stages63. The application of penicillin after abscesses draining speed the recovery and prevents complications78. The organism is also susceptible to ampicilin, ceftiofur, erythromycin, rifampicin, tetracycline; all of them are effective in treating field cases. Trimethoprim / sulphonamides have been use also but are less effective than beta-lactam antimicrobials40.
If the disease is advanced, most veterinarians do not use antibiotics but rather recommend nursing care and allow growing the abscesses to drain those56. Non-steroid anti-inflammatory drugs to reduce fever and pain are also useful, as resting in a warm setting and soft-moist feeding40.
In animals with abscessed lymph nodes, antibiotic treatment is usually ineffective once lymphadenopathy is detected48. Antimicrobials may slow the progression of the disease and delay the abscess maturation, and may not clear the infection, maybe because to local inhibitors factors, such as fibrosis, that prevents adequate reaching of the organism by antimicrobials in abscessed lymph nodes75. Premature discontinuation of antimicrobials may result in the recurrence, for this reason it is recommended to avoid their use in uncomplicated cases.
Asymptomatic guttural pouch carriers should be tested for Strangles by PCR, and also by endoscopy. In the cases of guttural pouch empyema, these should be lavaged daily with 1 to 2 liters of isotonic saline or polyionic fluids for 2 to 3 days following evidence of resolution1.
In horses with lymph nodes inflammation of the head and neck or with internal abscesses, high doses of beta-lactam antimicrobials and drainage of abscesses are recommended. In severe cases of Strangles, airflow may be impeded by retrophayngeal lymphadenopathy, and these animals may require tracheotomy7. In animals with dysphagia, intravenous fluids and nasogastric feeding may be necessary62.
Control and Prevention
Dealing with outbreaks is not easy, then, it is absolutely necessary to implement in each farm or stable prophylactic management practices to prevent introduction and spreading of the bacteria. During an outbreak of Strangles, not only ill animals should be isolated, but also those that have been exposed and do not show clinical signs of the disease. Areas occupied by infected horses should be disinfected appropriately. Personnel should wear separate clothing and footwear and infected horses should not be handled before healthy horses. Separate tacks, feed buckets, grooming and water supplies should be implemented to decrease transmission by this way59. Clothing, shoes and hands of personnel should be gently washed before and after handling an affected horse, and boots must be disinfected with disinfectant-impregnated mats placed outside each stall40. The organic material, especially food, should be store in an area that is not use for horses, in addition, stall walls, feeders, floors and waterers should be washed to remove organic material before applying a disinfectant. Stalls and padocks should not be use after being disinfected59.
The traffic through areas where there are infected horses should be limited to trained personnel, and the movement of animals through these areas should be avoided. It is recommended to isolate affected horses at least for one month after resolution of clinical signs48. In farms with susceptible animals, new horses should be quarantined for 3 to 4 weeks and monitored during this period.
A clue aspect for the control of this disease is the early detection and its prevention by efficient methods for detection of the carrier state89. Carriers are usually horses that recover from clinical disease but remain with persistent infection (empyema or chondroids) in the guttural pouches90. These carriers should be detected by culture and PCR, this latest been more sensitive3. PCR tests are expensive but necessary to prevent new cases of the disease. Detection, together with isolation and treatment of carriers should be used to eradicate the bacterium31. Despite vaccination with a live vaccine decrease the incidence and severity of the disease, it may interfere with the detection and eradication approach to control65.
For the detection of carriers, a series of three nasopharyngeal swabs spaced over two or three weeks enable the detection of carrier state3. Identification of carriers should be done before a new animal is introduced into a stable or herd, or 30 days following recovery of a horse from strangles. Three consecutive negative cultures and PCR reactions must be obtained to declare the absences of carriers in a stable15. An endoscopic examination of guttural pouches should be carried out in order to identify the presence of pus or chondroids, and eliminate them by swabs or surgery77.
If an animal is positive to nasopharyngeal swabs culture or PCR, it is recommended to evaluate the guttural pouch by endoscopy, in order to treat guttural pouches flushing and infusion of penicillin G or to remove chondroids if are present50. In addition, these horses should be isolated and then retested with the 3 consecutive series of nasopharyngeal swabs and culture. PCR is not usually recommended in these animals because it may be confusing due to identification of dead bacteria giving a "positive" reaction15,87. Animals that remain positive should go through a repeat treatment with beta-lactam antimicrobials48.
Farms and stables should be continuously monitored to look for evidence of Strangles. Although these recommendations to control the disease are expensive, the financial costs of Strangles outbreaks are higher than prevention strategies in terms of disruption of training activities, long periods of convalescence and quarantine, immobilization of animals, cost of veterinary care and treatments. As there is no doubt that carriers are the major problem to prevent Strangles, and may cause new outbreaks, the identification and treatment of these horses is essential to control this highly contagious disease. The potential risk of transmission by carriers should be properly recognized in all countries and resources must be invested on prevention, rather that treatment.
The prevention in Colombia is particularly difficult because it is not known if S. equi or S. zooepidemicus is the cause of Adenitis in equine population. To date, we do not know if PCR isolation has been done in this country, and it makes more difficult to control a disease without known the presence of specific microorganism involved in Strangles infection. It is necessary to pay more attention and focus resources on Strangles research. Although Strangles is not a mandatory notifiable disease to the veterinary authorities in this country, there are many case report of human infection by equine transmission91.
Conclusion
Although research about Strangles has been conducted for decades, this disease continues to have the higher prevalence worldwide among respiratory infections in horses. There are different presentations of this disease: horses may have mild signs of respiratory infection, others can show the classic signs of the typical adenitis, and frequently, several horses develop Bastard Strangles; in addition, sequels, such as vasculitis and Purpura Haemorrhagica worse the situation. The biggest control problem is the asymptomatic carrier, an animal who is persistently infected after suffering empyema and harbor S. equi equi in pus or chondroids, for this reason, it is important to take strict preventive measures to avoid its spreading, and if it is possible, to check always the guttural pouches of new introduced animals into a stable or before their mobilization.
Ackowledgements
This work is part of the doctoral thesis 'Interactions between five strains of S. equi with different Sem protein sequences and glycosaminoglycanes of the equine guttural pouches mucosa in an air-interface organ culture model', carried out in the University of Camerino, Italy.
References
1. McGorum Di Bruce C, Dixon Padraic M, Robinson N. Equine Respiratory Medicine and Surgery. Saunders Elsevier. 2007. [ Links ]
2. Waller AS, Jolley KA. Getting a grip on strangles: recent progress towards improved diagnostics and vaccines. Vet. J. 2007a May;173(3):492-501. [ Links ]
3. Laus F, Preziuso S, Spaterna A, Beribè F, Tesei B, Cuteri V. Clinical and epidemiological investigation of chronic upper respiratory diseases caused by betahaemolytic Streptococci in horses. Comp. Immunol. Microbiol. Infect. Dis. 2007 Jul;30(4):247-260. [ Links ]
4. Sweeney CR, Whitlock RH, Meirs DA, Whitehead SC, Barningham SO. Complications associated with Streptococcus equi infection on a horse farm. J. Am. Vet. Med. Assoc. 1987 Dic 1;191(11):1446-1448. [ Links ]
5. Slater JD. Strangles, bastard strangles, vives and glanders: archaeological relics in a genomic age. Equine Vet. J. 2003 Mar;35(2):118-120. [ Links ]
6. Robinson N. Current therapy in equine medicine. 6° ed. St. Louis Mo.: Saunders Elsevier; 2009. P. 288-292. [ Links ]
7. Spoormakers TJP, Ensink JM, Goehring LS, Koeman JP, Ter Braake F, van der Vlugt-Meijer RH, et al. Brain abscesses as a metastatic manifestation of strangles: symptomatology and the use of magnetic resonance imaging as a diagnostic aid. Equine Vet. J. 2003 Mar;35(2):146-151. [ Links ]
8. Finno C, Pusterla N, Aleman M, Mohr FC, Price T, George J, et al. Streptococcus equi meningoencephalomyelitis in a foal. J. Am. Vet. Med. Assoc. 2006 Sep 1;229(5):721-724. [ Links ]
9. Timoney JF. Strangles. Journal of Equine Veterinary Science. 2000;20(9):572. [ Links ]
10. Harrington DJ, Sutcliffe IC, Chanter N. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 2002 Abr;4(4):501-510. [ Links ]
11. Timoney JF. Strangles vaccines in trouble again. Equine Vet. J. 2007 May;39(3):196. [ Links ]
12. Guss B, Flock M, Frykberg L, Waller AS, Robinson C, Smith KC, et al. Getting to grips with strangles: an effective multi-component recombinant vaccine for the protection of horses from Streptococcus equi infection. PLoS Pathog. 2009 Sep;5(9):e1000584. [ Links ]
13. Florindo HF, Pandit S, Gonçalves LMD, Alpar HO, Almeida AJ. New approach on the development of a mucosal vaccine against strangles: Systemic and mucosal immune responses in a mouse model. Vaccine. 2009a Feb 18;27(8):1230-1241 [ Links ]
14. Timoney JF. Strangles. Vet. Clin. North Am. Equine Pract. 1993 Ago;9(2):365-374. [ Links ]
15. Flanagan J, Collin N, Timoney J, Mitchell T, Mumford JA, Chanter N. Characterization of the haemolytic activity of Streptococcus equi. Microb. Pathog. 1998 Abr;24(4):211-221. [ Links ]
16. Timoney JF. The pathogenic equine streptococci. Vet. Res. 2004 Ago;35(4):397-409. [ Links ]
17. Mathew T.G. et al. Evidence for the Evolution of Streptococcus equi: Host Restriction, Increased Virulence, and Genetic Exchange with Human Pathogens. PLoS Pathol. 2009 Mar; 5 (3). [ Links ]
18. Lanka S, Borst LB, Patterson SK, Maddox CW. A multiphasic typing approach to subtype Streptococcus equi subspecies equi. J. Vet. Diagn. Invest. 2010 Nov;22(6):928-936. [ Links ]
19. Paillot R, Darby AC, Robinson C, Wright NL, Steward KF, Anderson E, et al. Identification of three novel superantigen-encoding genes in Streptococcus equi subsp. zooepidemicus, szeF, szeN, and szeP. Infect. Immun. 2010b Nov;78(11):4817-4827. [ Links ]
20. Anzai T, Timoney JF, Kuwamoto Y, Fujita Y, Wada R, Inoue T. In vivo pathogenicity and resistance to phagocytosis of Streptococcus equi strains with different levels of capsule expression. Vet. Microbiol. 1999a Jul 1;67(4):277-286. [ Links ]
21. Kline KA, Fälker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe. 2009 Jun 18;5(6):580-592. [ Links ]
22. Gentry-Weeks C, Coburn PS, Gilmore MS. Phages and other mobile virulence elements in grampositive pathogens. Curr. Top. Microbiol. Immunol. 2002;264(2):79-94. [ Links ]
23. Timoney JF, Kumar P. Early pathogenesis of equine Streptococcus equi infection (strangles). Equine Vet. J. 2008 Nov;40(7):637-642. [ Links ]
24. Paillot R, Robinson C, Steward K, Wright N, Jourdan T, Butcher N, et al. Contribution of each of four Superantigens to Streptococcus equi-induced mitogenicity, gamma interferon synthesis, and immunity. Infect. Immun. 2010a Abr;78(4):1728-1739. [ Links ]
25. Votava M. Handbook of bacterial adhesion: Principles, methods and applications Y.H. Humana Press Inc., Totowa; 200. [ Links ]
26. Anzai T, Sheoran AS, Kuwamoto Y, Kondo T, Wada R, Inoue T, et al. Streptococcus equi but not Streptococcus zooepidemicus produces potent mitogenic responses from equine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 1999b Feb 22;67(3):235-246. [ Links ]
27. Kyossev Z, Weigel PH. An enzyme capture assay for analysis of active hyaluronan synthases. Anal. Biochem. 2007 Dic 1;371(1):62-70. [ Links ]
28. Cole JN, Pence MA, von Köckritz-Blickwede M, Hollands A, Gallo RL, Walker MJ, et al. M Protein and Hyaluronic Acid Capsule Are Essential for In Vivo Selection of covRS Mutations Characteristic of Invasive Serotype M1T1 Group A Streptococcus. MBio [Internet[. 2010; 1(4). Available from: http://www.ncbi.nlm.nih.gov/pubmed/20827373. [ Links ]
29. Chanter N, Ward CL, Talbot NC, Flanagan JA, Binns M, Houghton SB, et al. Recombinant hyaluronate associated protein as a protective immunogen against Streptococcus equi and Streptococcus zooepidemicus challenge in mice. Microb. Pathog. 1999 Sep;27(3):133-143. [ Links ]
30. Lindmark H, Guss B. SFS, a novel fibronectin-binding protein from Streptococcus equi, inhibits the binding between fibronectin and collagen. Infect. Immun. 1999a May;67(5):2383-2388. [ Links ]
31. Guss B. Fibronectin-collagen binding protein in virulence. In: Getting to grips with Strangles. Edinburgh: 2008 May. p. 18-19. [ Links ]
32. Lindmark H, Jonsson P, Engvall E, Guss B. Pulsed-field gel electrophoresis and distribution of the genes zag and fnz in isolates of Streptococcus equi. Res. Vet. Sci. 1999b Abr;66(2):93-99. [ Links ]
33. Åsa Karlströma, Karin Jacobssona, Margareta Flockb, Jan-Ingmar Flockb and Bengt. Identification of a novel collagen-like protein, SclC, in Streptococcus equi using signal sequence phage display. Veterinary Microbiology. 104(3-4). December 2004. Pages 179-188. 45. [ Links ]
34. Åsa Karlström, Karin Jacobsson and Bengt Guss. SclC is a member of a novel family of collagen-like proteins in Streptococcus equi subspecies equi that are recognised by antibodies against SclC. Veterinary Microbiology. Volume 114, Issues 1-2, 16 April 2006, Pages 72-81. [ Links ]
35. Boschwitz JS, Timoney JF. Characterization of the antiphagocytic activity of equine fibrinogen for Streptococcus equi subsp. equi. Microb. Pathog. 1994 Ago;17(2):121-129. [ Links ]
36. Davidson A, Traub-Dargatz JL, Magnuson R, Hill A, Irwin V, Newton R, et al. Lack of correlation between antibody titers to fibrinogen-binding protein of Streptococcus equi and persistent carriers of strangles. J. Vet. Diagn. Invest. 2008 Jul;20(4):457-462. [ Links ]
37. Meehan M, Lynagh Y, Woods C, Owen P. The fibrinogen-binding protein (FgBP) of Streptococcus equi subsp. equi additionally binds IgG and contributes to virulence in a mouse model. Microbiology (Reading, Engl.). 2001 Dic;147(Pt 12):3311-3322. [ Links ]
38. Meehan M, Lewis MJ, Byrne C, O'Hare D, Woof JM, Owen P. Localization of the equine IgG-binding domain in the fibrinogen-binding protein (FgBP) of Streptococcus equi subsp. equi. Microbiology (Reading, Engl.). 2009 Ago;155(Pt 8):2583-2592. [ Links ]
39. Timoney JF, Artiushin SC, Boschwitz JS. Comparison of the sequences and functions of Streptococcus equi M-like proteins SeM and SzPSe. Infect. Immun. 1997 Sep;65(9):3600-3605. [ Links ]
40. Timoney JF, Mukhtar MM. The protective M proteins of the equine group C streptococci. Vet. Microbiol. 1993 Nov;37(3-4):389-395. [ Links ]
41. Anzai T, Kuwamoto Y, Wada R, Sugita S, Kakuda T, Takai S, et al. Variation in the N-terminal region of an M-like protein of Streptococcus equi and evaluation of its potential as a tool in epidemiologic studies. Am. J. Vet. Res. 2005 Dic;66(12):2167-2171. [ Links ]
42. Chanter N, Talbot NC, Newton JR, Hewson D, Verheyen K. Streptococcus equi with truncated M-proteins isolated from outwardly healthy horses. Microbiology. 2000 Jun;146 ( Pt 6):1361-1369. [ Links ]
43. Tiwari R, Qin A, Artiushin S, Timoney JF. Se18.9, an anti-phagocytic factor H binding protein of Streptococcus equi. Vet. Microbiol. 2007 Mar 31;121(1-2):105-115. [ Links ]
44. Hashikawa S, Iinuma Y, Furushita M, Ohkura T, Nada T, Torii K, et al. Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J. Clin. Microbiol. 2004 Ene;42(1):186-192. [ Links ]
45. Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 2009 Sep;73(3):407-450. [ Links ]
46. Muhktar MM, Timoney JF. Chemotactic response of equine polymorphonuclear leucocytes to Streptococcus equi. Res. Vet. Sci. 1988 Sep;45(2):225-229. [ Links ]
47. Alber J, El-Sayed A, Estoepangestie S, Lämmler C, Zschöck M. Dissemination of the superantigen encoding genes seeL, seeM, szeL and szeM in Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus. Vet. Microbiol. 2005 Ago 10;109(1-2):135-141. [ Links ]
48. Srivastava SK, Barnum DA. The role of lipoteichoic acids on the adherence of Streptococcus equi to epithelial cells. Vet. Microbiol. 1983b Oct;8(5):485-492. [ Links ]
49. Liu M, McClure MJ, Zhu H, Xie G, Lei B. The Two-Component Regulatory System VicRK is Important to Virulence of Streptococcus equi Subspecies equi. Open Microbiol J. 2008;2:89-93. [ Links ]
50. Banu LD, Conrads G, Rehrauer H, Hussain H, Allan E, van der Ploeg JR. The Streptococcus mutans serine/threonine kinase, PknB, regulates competence development, bacteriocin production, and cell wall metabolism. Infect. Immun. 2010 May;78(5):2209-2220. [ Links ]
51. Hamilton A, Harrington D, Sutcliffe IC. Characterization of acid phosphatase activities in the equine pathogen Streptococcus equi. Syst. Appl. Microbiol. 2000 Oct;23(3):325-329. [ Links ]
52. Pelagonius. Ars Veterinaria. In: Fischer KD, ed. Pelagonii ars veterinaria. Leipzig, Germany: BG Teubner, 1980;13-16. [ Links ]
53. Srivastava SK, Barnum DA. Adherence of Streptococcus equi on tongue, cheek and nasal epithelial cells of ponies. Vet. Microbiol. 1983a Oct;8(5):493-504. [ Links ]
54. Hardy J, Léveilé R. Diseases of the guttural pouches. Vet. Clin. North Am. Equine Pract. 2003 Abr;19(1):123-158, VII. [ Links ]
55. Golland LC, Hodgson DR, Davis RE, Rawlinson RJ, Collins MB, McClintock SA, et al. Retropharyngeal lymph node infection in horses: 46 cases (1977-1992). Aust. Vet. J. 1995 May;72(5):161-164. [ Links ]
56. Newton JR, Wood JL, Dunn KA, DeBrauwere MN, Chanter N. Naturally occurring persistent and asymptomatic infection of the guttural pouches of horses with Streptococcus equi. Vet. Rec. 1997a Ene 25;140(4):84-90. [ Links ]
57. Hamlen HJ, Timoney JF, Bell RJ. Epidemiologic and immunologic characteristics of Streptococcus equi infection in foals. J. Am. Vet. Med. Assoc. 1994 Mar 1;204(5):768-775. [ Links ]
58. Newton JR., Wood JL., Chanter N. Strangles: Long term carriage of Streptococcus equi in horses. Equine Veterinary Education. 1997b;9(2):98-102. [ Links ]
59. George JL, Reif JS, Shideler RK, Small CJ, Ellis RP, Snyder SP, et al. Identification of carriers of Streptococcus equi in a naturally infected herd. J. Am. Vet. Med. Assoc. 1983 Jul 1;183(1):80-84. [ Links ]
60. Verheyen K, Newton JR, Talbot NC, de Brauwere MN, Chanter N. Elimination of guttural pouch infection and inflammation in asymptomatic carriers of Streptococcus equi. Equine Vet. J. 2000 Nov;32(6):527-532. [ Links ]
61. Holcombe SJ, Jackson C, Gerber V, Jefcoat A, Berney C, Eberhardt S, et al. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet. J. 2001 May;33(3):244-249. [ Links ]
62. Sweeney CR, Timoney JF, Newton JR, Hines MT. Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles. J. Vet. Intern. Med. 2005 Feb;19(1):123-134. [ Links ]
63. Taylor S., Wilson D. Streptococcus equi subsp. equi (Strangles) Infection. Clinical Techniques in Equine Practice. 2006;5(3):211-217. [ Links ]
64. Galan JE, Timoney JF. Mucosal nasopharyngeal immune responses of horses to protein antigens of Streptococcus equi. Infect. Immun. 1985 Mar;47(3):623-628. [ Links ]
65. Hinchkliff K, Kaneps A, Geor R. Equine Sports Medicine and Surgery. USA: Sounders; 2004. [ Links ]
66. Galán JE, Timoney JF. Immunologic and genetic comparison of Streptococcus equi isolates from the United States and Europe. J. Clin. Microbiol. 1988 Jun;26(6):1142-1146. [ Links ]
67. Frick I.M., Schmidtchen A., and Ulf S. European Journal of Biochemestry. Interactions between M proteins of Straptococcus pyogenes and glycosaminoglycans promote bacterial adehsion to host cells. 2003. 270: 2303-2311. [ Links ]
68. Parillo F, Arias MP, Supplizi AV. Glycoprofile of the different cell types present in the mucosa of the horse guttural pouches. Tissue Cell. 2009b Ago;41(4):257-265. [ Links ]
69. Arias M., Magi GE., Parillo F., Cutteri V., Renzoni G. Evaluation of a three-dimensional culture of equine guttural pouches to study interactions between Streptococcus equi and mucopolysaccharides: preliminary results. En: Book of Abstracts: ESPV 26th Annual Meeting. Zagreb, Croazia: 2008. [ Links ]
70. Patti JM, Allen BL, McGavin MJ, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 1994;48:585-617. [ Links ]
71. Piché CA. Clinical observations on an outbreak of strangles. Can. Vet. J. 1984 Ene;25(1):7-11. [ Links ]
72. Heath SE, Geor RJ, Tabel H, McIntosh K. Unusual patterns of serum antibodies to Streptococcus equi in two horses with purpura hemorrhagica. J. Vet. Intern. Med. 1991 Oct;5(5):263-267. [ Links ]
73. Pusterla N, Watson JL, Affolter VK, Magdesian KG, Wilson WD, Carlson GP. Purpura haemorrhagica in 53 horses. Vet. Rec. 2003 Jul 26;153(4):118-121. [ Links ]
74. Kaese HJ, Valberg SJ, Hayden DW, Wilson JH, Charlton P, Ames TR, et al. Infarctive purpura hemorrhagica in five horses. J. Am. Vet. Med. Assoc. 2005 Jun 1;226(11):1893-1898, 1845. [ Links ]
75. Reile LJ., Genetzky RM. Equine strangles: a brief overview. 1983. p. 16-19. [ Links ]
76. Smith K., Timoney JF. Getting to grips with strangles. Edinburgh, UK: 2008. p. 30-31. [ Links ]
77. Safia B. Handbook of Equine Respiratory Endoscope. 1º ed. UK: Saunders Ltd.; 2006. [ Links ]
78. Dixon PM. Strangles complications and clinical solutions. Edinburgh, UK: R and W Communications; 2008. p. 54. [ Links ]
79. Florindo HF, Pandit S, Gonçalves LMD, Videira M, Alpar O, Almeida AJ. Antibody and cytokine-associated immune responses to S. equi antigens entrapped in PLA nanospheres. Biomaterials. 2009b Oct;30(28):5161-5169. [ Links ]
80. Galan JE, Timoney JF, Lengemann FW. Passive transfer of mucosal antibody to Streptococcus equi in the foal. Infect. Immun. 1986 Oct;54(1):202-206. [ Links ]
81. Flock M, Frykberg L, Hirst TR, Franklin A, Guss B, et al. Recombinant Streptococcus equi proteins protect mice in challenge experiments and induce immune response in horses. Infect. Immun. 2004 Jun;72(6):3228-3236. [ Links ]
82. Waller A, Flock M, Smith K, Robinson C, Mitchell Z, Karlström A, et al. Vaccination of horses against strangles using recombinant antigens from Streptococcus equi. Vaccine. 2007b May 4;25(18):3629-3635. [ Links ]
83. Hoffman AM, Staempfli HR, Prescott JF, Viel L. Field evaluation of a commercial M-protein vaccine against Streptococcus equi infection in foals. Am. J. Vet. Res. 1991 Abr;52(4):589-592. [ Links ]
84. Galan JE, Timoney JF. Immune complexes in purpura hemorrhagica of the horse contain IgA and M antigen of Streptococcus equi. J. Immunol. 1985 Nov;135(5):3134-3137. [ Links ]
85. Hulting G, Flock M, Frykberg L, Lannergård J, Flock J, Guss B. Two novel IgG endopeptidases of Streptococcus equi. FEMS Microbiol. Lett. 2009 Sep;298(1):44-50. [ Links ]
86. Azevedo AF, J Galhardas, A Cunha, P Cruz, LMD Gonçalves, AJ Almeida. 2006. Microencapsulation of Streptococcus equi antigens in biodegradable microspheres and preliminary immunisation studies. Eur J Pharm Biopharm 64, 131-137. [ Links ]
87. Newton JR, Verheyen K, Talbot NC, Timoney JF, Wood JL, Lakhani KH, et al. Control of strangles outbreaks by isolation of guttural pouch carriers identified using PCR and culture of Streptococcus equi. Equine Vet. J. 2000 Nov;32(6):515-526. [ Links ]
88. Knowles EJ, Mair TS, Butcher N, Waller AS, Wood JLN. Use of a novel serological test for exposure to Streptococcus equi subspecies equi in hospitalised horses. Vet. Rec. 2010 Mar 6;166(10):294-297. [ Links ]
89. Andrew Waller, Carl Robinson, J. Richard Newton. Further thoughts on the eradication of strangles in equids. J Am Vet Med Assoc 2007c: 231(9): 1335-1336. [ Links ]
90. Prescott JF, Timoney JF. Could we eradicate strangles in equids? J Am Vet Med Assoc 2007;231 (3):377-378. [ Links ]
91. Rosanowski SM, Cogger N, Rogers CW. An investigation of the movement patterns and biosecurity practices on Thoroughbred and Standardbred stud farms in New Zealand. Prev Vet Med. 2013; 1;108(2-3):178-87. [ Links ]