Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
CES Medicina Veterinaria y Zootecnia
versão On-line ISSN 1900-9607
Ces. Med. Vet. Zootec. vol.9 no.2 Medellín jul./dez. 2014
Sub-Acute Ruminal Acidosis and non-structural carbohydrates: a study model in nutritional immunology¤
Acidosis Ruminal Sub-Aguda y carbohidratos no estructurales: un modelo de estudio en inmunología nutricional
Acidose ruminal subaguda e carboidratos não estruturais: um modelo de estudo em imunologia nutricional
Luis Miguel Gómez 1,2,3, MVZ, MSc, cPhD, Sandra Lucía Posada 2, Zoot, MSc, PhD, Martha Olivera 3, MV, Dr. Sci. Agr 3*
¤To cite this article: Gómez LM, Posada SL, Olivera M. Sub-Acute Ruminal Acidosis and Non-Structural Carbohydrates: a study model in nutritional immunology. Rev CES Med Zootec. 2014; Vol 9(2): 295-306.
*Corresponding author: Martha.olivera@udea.edu.co. Profesora Titular, Facultad de Ciencias Agrarias, Universidad de Antioquia, AA 1226, Medellín, Colombia
1 Departamento de Investigación y Desarrollo, Grupo Nutri-Solla, Empresa Solla S.A. Carrera 42 No. 33-80 Itagui, Colombia.
2 Grupo de Investigación en Ciencias Agrarias-GRICA, Facultad de Ciencias Agrarias, Universidad de Antioquia UdeA, Calle 70 No. 52-21, AA 1226, Medellín, Colombia.
3 Grupo de Investigación Biogénesis, Facultad de Ciencias Agrarias, Universidad de Antioquia UdeA, Calle 70 No. 52-21, AA 1226, Medellín, Colombia.
(Recibido: 14 de julio, 2014; aceptado: 8 de septiembre, 2014)
Abstract
Nutritional immunology combines two areas of knowledge that did not interact until recently. One of the best examples studied to date is the bovine rumen. The symbiotic relationship between the host and rumen microorganisms can be altered causing a breakdown of immunological tolerance and imbalance of animal homeostasis. Dietary inclusion of supplements rich in non-structural carbohydrates is required for high yielding cows to meet their energy requirements. However, the use of those diets can lead to substantial changes in the rumen ecosystem, reducing the pH and promoting the development of subacute rumen acidosis. This generates lysis of gram-negative bacteria, release of lipopolysaccharides, breaking of immune tolerance, and activation of a cascade of inflammatory mediators with systemic effects that affect milk yield and quality. The gastrointestinal tract is the most important place where lipopolysaccharides are produced and its translocation mechanism from the rumen to peripheral circulation is still controversial. This review proposes a biological model integrating nutritional and immunological aspects of production, absorption, and mechanisms of action of lipopolysaccharides and its effects on milk production and compositional quality.
Key words: Immunogens, lipopolysaccharides, nonstructural carbohydrates, nutritional immunology, SARA.
Resumen
La inmunología nutricional combina dos áreas del conocimiento que no interactuaban hasta hace algunos años. Uno de los mejores ejemplos estudiados hasta la fecha lo constituye el rumen bovino. La relación simbiótica entre hospedero y microorganismos ruminales puede alterarse provocando una ruptura de la tolerancia inmunológica y un desequilibrio en la homeostasis del animal. Para cubrir los requerimientos energéticos de las vacas de alta producción lechera es necesario incluir en la alimentación suplementos de elevado contenido en carbohidratos no estructurales. Sin embargo, el uso de estas dietas puede provocar cambios sustanciales en el ecosistema ruminal, disminuyendo el pH y promoviendo el desarrollo de acidosis ruminal subaguda. Esto genera la lisis celular de las bacterias gram negativas, la liberación de lipopolisacáridos, la ruptura de la tolerancia inmunológica y la activación de una cascada de mediadores inflamatorios que tienen consecuencias sistémicas y afectan el rendimiento productivo del animal y la calidad composicional de la leche. El tracto gastrointestinal es el lugar más importante donde se producen los lipopolisacáridos, pero el mecanismo de translocación del rumen a la circulación periférica es aún controversial. En esta revisión de literatura se propone un modelo biológico que integra aspectos nutricionales e inmunológicos relacionados con la producción, absorción y mecanismos de acción de los lipopolisacáridos y los efectos sobre la producción y la calidad composicional de la leche.
Palabras clave: Carbohidratos no estructurales, inmunógenos, inmunología nutricional, lipopolisacáridos, SARA.
Resumo
A imunologia nutricional combina duas áreas de conhecimento que não interagiam até alguns anos atrás. Um dos melhores exemplos estudados até o presente consiste no rúmen bovino. A relação simbiótica entre hospedeiro e microrganismos do rúmen pode ser alterada causando uma quebra da tolerância imune e um desequilíbrio na homeostase do animal. Para satisfazer as necessidades energéticas de vacas de alta produção leiteira é necessário fornecer suplementos alimentares de elevado conteúdo em carboidratos não estruturais. No entanto, o uso dessas dietas pode provocar alterações importantes no ecossistema ruminal, reduzindo o pH e promovendo o desenvolvimento de acidose ruminal subaguda. Isto gera a lise de bactérias gram-negativas, a liberação de lipopolissacarídeos, a quebra da tolerância imune e a activação de uma cascata de mediadores inflamatórios que têm efeitos sistémicos e afetam o desempenho produtivo do animal e a composição do leite. O trato gastrointestinal é o lugar mais importante na produção dos lipopolissacarídeos, mas o mecanismo de translocação do rúmen para a circulação periférica é ainda controversa. Nesta revisão de literatura se propõe um modelo biológico que integra aspectos nutricionais e imunológicos relacionados com a produção, absorção e mecanismos de ação de os lipopolissacarídeos e os efeitos sobre a produção e composição do leite.
Palavras-chave: >Dieta das aves domestica, galinhas especializadas na produção de ovos, fosforo, vitamina D.
Introduction
The study of the interactions between nutrition and immunology provides a new perspective beyond the maximum performance, especially from the point of view of animal health and its consequences in longevity. In dairy cattle, there is a well-known example of this (relationship between non-structural carbohydrate and sub-acute ruminal acidosis) which can break the homeostasis with underlying economic consequences affecting the net profit. According to this, the study of the mechanisms by which nutrition components affects immune status, provide new insight into the nutritional management of dairy cow due to the immune system requires higher amounts of energy for its functioning that could be rechanneled into milk yield.
Nutritional immunology is an interdisciplinary field that has made possible the interaction of different specialties which traditionally do research independently. Such science focuses on the study of underlying mechanisms of the immune response modulation by active components supplied through the feedstuff. Thanks to this new discipline, new mechanisms of action, different from nutritional ones, have been discovered, such as the conjugated linoleic acid (CLA), linolenic acid, resveratrol and E, A, and D vitamins 37. Besides, new explanations have been found to modulate the immune response and therefore the health status of animals with diet-derived compounds 26.
The bovine rumen is a representative example of the host-microorganism symbiosis that might be altered causing a breakdown in immunological tolerance, leading to imbalance in homeostasis. Therefore, there is an interest in the study of such interaction to understand the immune-pathological mechanisms by which the host takes effective action against some immunogens generated after the feedstuff with high amounts of NSC, causing substantial changes in the ruminal ecosystem by decreasing rumen pH and developing Sub-Acute Ruminal Acidosis (SARA) 17, 27.
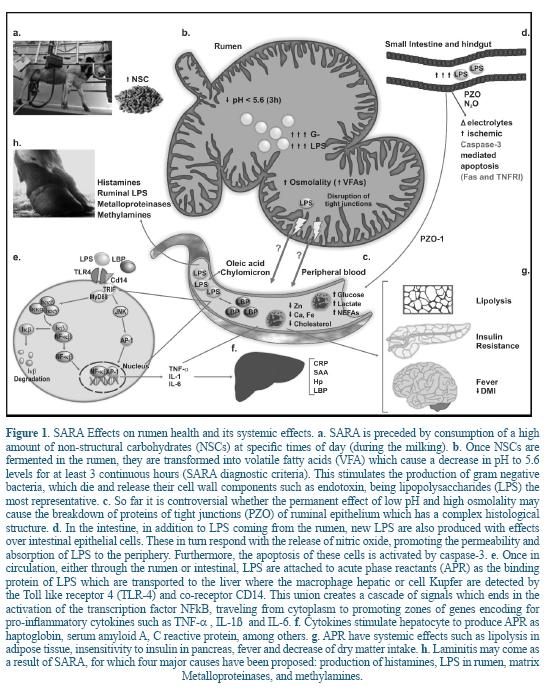
SARA is characterized by favoring proliferation of negative gram bacteria (i.e Escherichia coli) over positive gram bacteria which, once dead in the gastrointestinal tract, releases endotoxin such as lipopolysaccharides (LPS)38. However, the translocation mechanism of the LPS from the rumen to the peripheral circulation is still controversial and it seems that it would not be through the ruminal papillae but through the gut. LPS are immunogens classified as pathogen associated molecular patterns (PAMPs) which are able to stimulate pattern recognition receptors (PRR) of the innate immune system. Once the interaction PAMPs-PRR takes place, immunological tolerance is broken and molecular production is started with important local and systemic implications 1.
Non-structural carbohydrates (NSC) and sub-acute ruminal acidosis (SARA)
70% to 80% of dairy cows' diet are Carbohydrates 42 ; from which NSC (compounds of plants containing simple sugar or those more complex like starch) are the most used in Livestock. The main source of supplement in dairy cows is grains, as they are an effective-cost option of digestive energy to fulfill the high requirements of dairy cows that forages cannot meet 44. Feedstuffs with nutritionally dense diets increase the amount of volatile fatty acids and lactic acid in the rumen, which exceeds its absorption capacity and therefore the buffer power of the animal organism 30, 51. The combination of these factors creates a decrease in the ruminal pH for long periods during the day (pH <5,6 for more than 3 hours) creating SARA. This sub-clinic acidosis affects dry matter intake, milk yield, ruminal microflora, and digestion; it may cause diarrhea, damage in the mucosa of the gastrointestinal tract, laminitis and liver abscesses in dairy cows 30, 41, 51.
Production and translocation of endotoxin in dairy cows
There are three theories about the translocation of endotoxins to the circulation. The first is directly through the rumen mucosa; the second is in the gut; and the third by specific transporters. The ruminal epithelium is a stratified squamous type with four strata: basale, spinosum, granulosum, and corneum 50. The stratum basale is the cell layer adjacent to the basale lamina (that is in contact with the circulation) with a substantial amount of mitochondria and other organelles. The next strata are the spinosum and granulosum which do not have a clearly defined division. The epithelium looses mitochondria and evenness as stratum advances. Strata spinosum and granulosum contribute to most metabolic functions of tissues (i.e ketogenesis) and they are the reservoir of cells with the highest participation in energetic metabolism of the animal 7.
In the stratum granulosum, cells have tight junctions mediated by desmosomes (cell structures remaining attached to neighboring cells) that act as a diffusion barrier in the rumen wall. The cells in the stratum corneum, the outermost, are characterized because they have lost most of the organelles, although the presence of keratin is increased, allowing it to work as a natural barrier and becoming an innate defense mechanism in the rumen. The number of cells of the stratum corneum is highly dependent on the composition of the diet 20. Animals fed with diets high in concentrated reduce the ruminal pH and increase rate propionate-acetate and the molar proportions of butyrate, which results in a thick corneal layer with more than 15 cell layers. In the case of animals fed with diets high in forage, the stratum corneum consists of just 4 cell layers 20. Therefore, as stratum corneum layers advance from the outside inward, the integrity of desmosomes is missing and cells without such joints are seen. Tight junctions or occludens areas, which form an impermeable barrier, are located in the middle layers (strata granulosum and spinosum) and not in the outer layers or corneal layers 24.
The major source of endotoxin in dairy cows is the gastrointestinal tract, which contains a high amount of gram negative bacteria, especially when diets include high amounts of NSC, resulting in a potential source of LPS when entering to the organism through the gastrointestinal tract 38. Accordingly, the most important factor that increases the concentration of LPS in the rumen fluid, in the first third of lactation, is the abrupt shift to energetically denser diets at the beginning of lactation 2, 17, 27. LPS are subsequently released to the rumen fluid during lysis of gram negative bacteria. Several studies have shown that when the concentration of LPS in the lumen of the gastrointestinal tract increases after a diet rich in NSC or by oral administration 6 these are translocated into the systemic circulation 17, 27 (Figure 1). Such translocation leads to a failure in the permeability of the ruminal epithelium caused by high osmolality (the total number of particles of solute in a solvent kg) creating inflammation and rupture of the ruminal papillae 30.
Other studies have shown that the translocation of LPS from the gut to the peripheral circulation increases due to the inflammation of the intestinal epithelium, together with an excessive production of cytokines and tissue destruction, causing an increase in the enteric permeability 6, 14.
In vitro experiments have shown that endotoxins are transported from the gastrointestinal tract into the peripheral circulation, in particular through the ruminal wall at a greater speed than in the colon wall but at pH acids, increasing 5 to 6 times permeability through the colon and ruminal tissues and allowing the passage of large molecules 16.
Despite the evidence of LPS passing from the rumen and gut into the blood, the precise place of translocation is still controversial. There is no direct evidence that the LPS free from rumen and subsequent to SARA move through the ruminal walls into the bloodstream. It is also difficult to explain from the anatomical point of view because of the multilayer structure of the ruminal epithelium 7. In the study in vitro of Emmanuel et al. 16 it was demonstrated LPS pass through the rumen wall at pH 5.5. However, the LPS concentrations added to mucosal tissues were 500 µg/ml, one dose 50 times greater than the free LPS in the rumen when it is induced with SARA grain-based diet 17, 27. This could have caused a disruption in the structure and a failure in the barrier of the rumen epithelium, which is unlikely to happen with concentrations close to the physiological state 34.
In order to know the site of ruminal endotoxin absorption, a study was carried out in steers and Escherichia coli endotoxin were administered directly into their rumen labeled with 51Cr 4, 33. These studies showed that none of the steers experienced absorption through the lymph (thoracic duct) or blood (portal vein) or forage based-diets or diets with high inclusion of grains or acidosis itself. Khafipour et al. 28 showed that there was no correlation between the concentration of LPS in the rumen, the severity of SARA and the degree of inflammation in lactating Holstein dairy cattle. According to the above evidence, the rumen may be impermeable to endotoxins in physiological conditions, unless it suffers a considerable injury in the tissue integrity. Therefore, it is possible that the translocation of LPS occurs primarily from the gut.
The theory on which LPS pass through the gut is based on the fact that the intestinal epithelial of the bovine is a monolayer structure with tight junctions at the apical pole of the cell 47. Chin et al. 13 showed that an abnormal concentration of endotoxin in the intestinal lumen induced cell apoptosis, as a result, disruption of the tight junctions, specifically in the ZO-1 protein was initiated; the production of nitric oxide was increased, causing increased mucosal permeability and blood flow with influx of inflammatory cells into the area. Another study showed that the enterocyte regulatory system was altered in presence of LPS through the inhibition of proton pump under extracellular acidotic conditions, which resulted in a cytoplasmic acidification and alteration in cellular function 12.
The rumen is an immense source of LPS, when present in the gut, many of them are excreted through the bile salts 10; the remaining ones are displaced into the circulation. A small proportion of LPS consistently occurs in the gut for which immune tolerance in the host is developed, alleviating some of the responses resulting from the acute phase of inflammation 2. In this case the production of LPS is independent from pH but dependent on starches that are not degraded in the rumen. The overshoot of starches that reach the ileum and large intestine causes a change in the populations of micro-organisms and consequently an increase in the release occurs in the posterior tract of LPS 53. Finally, SARA is an entity which triggers the production of LPS because of the death of gram negative bacteria (due to the decrease in ruminal pH for three or more hours) subsequent to a diet rich in NSC, even though the translocation mechanism of LPS from either the rumen or the gut into the circulation is controversial 3.
The latest theory is that the LPS use transcellular and paracellular mechanisms by Toll- receptor 4 type (TLR-4) 40. Furthermore, Goshal et al. 22 showed that LPS can be transported into the blood by mechanisms provided by long chain fatty acids like oleic acid and formation of chylomicron from intestinal epithelial cells. The arrival of LPS into the systemic circulation, triggers the production of acute phase reactants (APR) of inflammation, such as the lipopolysaccharide binding protein (LBP), serum amyloid A (SAA), the C-reactive protein (CRP), and the haptoglobin (Hp), which stimulate the macrophages or hepatic Kupffer cells and other phagocytic cells mediated by the TLR-4 the release of pro-inflammatory cytokines such as the tumor necrosis factor alpha (TNF-α), the interleukin 1 (IL-1ß) and IL-6 17. The APRs are generated as response of the animal to disturbances in the homeostasis caused by infection, tissue injury, neoplastic growth or immunological disorders 19. The APR are responsible for carrying out the functions of systemic response, providing energy and substrates for defense against pathogens, preventing the transfer of metabolites needed for them and limiting and helping repair the damage of the infected tissue 19.
LBP function is to facilitate the removal of endotoxins from blood, either by carrying them to the macrophages when they are in low concentration or transporting with lipoprotein of high density 21. The Hp is macromolecular protein binding hemoglobin which occurs during acute inflammation 54. SAA is an apolipoprotein which is associated with high density lipoproteins during the acute response. Both HP and SAA are highly sensitive in bovine 36. The CRP is part of the innate immune system and belongs to the family of pentraxins. Its main function is to bind to phosphocholine, allowing the recognition of pathogens or phospholipid constituents of damaged cells after an inflammatory process. It also can activate the complement system and phagocytic cells 56.
During the acute phase response, the pro-inflammatory cytokines such as TNF-α, IL-1ß and IL-6 lead to changes in the stability of the mitochondria in various tissues, thereby affecting the animal metabolic profiles 15. One example is the important relationship between the glucose levels in blood and liver, which are regulated according to the needs of the immune system cells. In moderate inflammation, there is an increase in the uptake of glucose into these cells, generating a stimulus for hepatic glucose production and a corresponding increase in blood glucose. However, with a more intense acute response, there is a marked increase in plasma glucose levels 15.
Changes have also been described in the profile of minerals and other metabolites in dairy cows during inflammatory states such as SARA. Zebeli etal. 57 demonstrated an inverse association between plasma levels of Ca, Fe, cholesterol and consumption of barley diet beyond 45% dry matter. This study showed a positive relationship with lactate. Such alterations generate clues about the effects endotoxins may be generating in the host. For example, cholesterol is the main precursor for the synthesis of bile acids; thus the decrease at high grain diets could be associated with the need to increase bile secretions to detoxify high amounts of endotoxins present in the gastrointestinal tract 11. Plasma Ca has been involved in the detoxifying capability of endotoxin in the host, decreasing their detoxifying capacity when there are high concentrations of the mineral 46. Fe is an important mineral for the growing of microorganisms which is transferred from the peripheral blood to the reticular endothelial system, limiting both its availability and growth. Lactatemia is reported to be caused by an enhanced glycogenolysis and a reduced capacity of the extrahepatic tissues to utilize lactate 49.
Laminitis has a strong relationship with SARA and immunogens generated by the ingestion of high grain diets. Four key molecules have been involved in the development of laminitis. The first is histamine, the second are endotoxins, the third are metalloproteinases and the fourth are methylamines. One of the most controversial is histamine because Goth 23 injected histamine in cows and they did not develop any type of laminitis. One of the reasons for this result is the rapid degradation of histamine in the gastrointestinal tract, which do not occur with endotoxin or metalloproteinases. It is important to note that the metalloproteinases are enzymes activated by cell wall components of gram positive bacteria such as lipoteichoic acid (LTA). In a study in dairy cows, a vaccine was designed for oral administration only against Escherichia coli LPS or concomitantly with the blocking of LTA. When it was administered only against LPS, there was a decrease in the incidence of laminitis, and when blockers LPS and LTA were mixed, a greater decrease in incidence of laminitis was demonstrated 2. A diet based on high percentages of grain increases in number concentration of methylamine in rumen 2; when passing from the rumen to circulation it may cause damage in blood vessels in the hoof of cow through free radicals such as formaldehyde, hydrogen peroxide, and ammonia via amino oxidase sensitive to semi-carbazide 55.
Endotoxins and mammary glandin
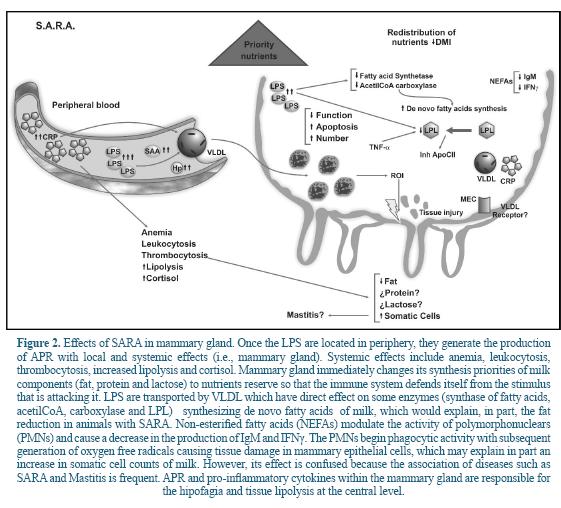
Although there are no exact details on how LPS and mediators of the inflammation of fat production affect milk, it is speculated that this effect may be explained by the release of cytokines such as TNF-α and IL-1ß, produced by the macrophage and released into the circulation of the blood 52. Based on the analysis of the literature the following model was built (Figure 2).
Several studies have suggested that intraperitoneal infusion of LPS or the administration of TNF-α or IL-1ß in peripheral blood reduces lipogenesis in mammary gland of lactating rats 5, 35. Also, it has shown that TNF-α inhibit the activity of lipoprotein lipase (LPL), a key enzyme in the release of glycerol and non-esterified fatty acids (NEFAs), limiting the supply of circulating lipids available for its metabolism in mammary gland 5.
Additionally it has been reported that some mediators generated by LPS inhibit the activity of key enzymes involved in de novo synthesis of fatty acids such as fatty acid synthase and acetyl-CoA carboxylase 35, 43. In this sense, some APR as CRP, are involved in lipid metabolism 29. Several studies have shown that administration of LPS affects the VLDL secretion with a marked dose-dependent effect. That is, at low doses of LPS the VLDL increases, whereas at higher doses, the removal rate of VLDL decreases 18. CRP is increased because of the presence of LPS from the rumen, which is also associated with VLDL (Figure 2). In cows, VLDL is the primary source of triglycerides which are then transferred to the mammary gland cells 9. Approximately 50% of fatty acids in ruminant milk are derived from blood triglycerides 9.
During lactation there is an increase in LPL activity of mammary tissue with a decrease in LPL of adipose tissue 25. The association between CRP and VLDL is a defense mechanism of the host to prevent activation of LPL by apoC-II during inflammatory states and reduce the removal of triglyceride-rich lipoproteins such as VLDL. In other words, the host keeps fat synthesis in the mammary gland to have it available to defend itself against an attack. This is evidenced by Barcia and Harris 8, who showed that triglyceride-rich lipoproteins are attached to LPS, promoting a protective response against LPS-induced toxicity and modulating the response of the host against bacterial toxins.
The effect of SARA on parameters like the milk protein is not yet clear, however, some changes occurring therein are due to an increase in digestible organic matter in the rumen that enhance the synthesis of microbial protein 39. Few studies have demonstrated a decrease in protein of milk in cows with SARA. Stone 51 evaluated 500 cows that had suffered SARA and whose rumen pH increased through diet. In response to an apparent pH recovery, milk production increased by 2.7 kg/day; fat, and protein in 0.3 and 0.1 percentage points. Another study in dairy cows showed that both the production and the fat content decreased in response to an increase in the amount of grains in the diet and their corresponding activation of the inflammatory response 27. According to some authors, there is a negative correlation between the content, production and production adjusted at 3.5% fat in milk and CRP levels in cows fed with incremental levels of barley grains (0%, 15%, 30% and 45%) 56. This same study reported a positive relationship between milk production and incremental levels of barley. While incremental levels of barley were associated with milk production, the energy efficiency of the same (calculated as the amount of milk fat per kg of dry matter intake) decreased 26% compared to cows that received 0% of barley.
A study in France in cows with SARA showed an association with both somatic cell counts and indicators of the negative energy balance (i.e. NEFAs). That relationship grew twice when comparing cows at risk of SARA from moderate to high (based on the protein fat ratio) 45. The explanation could be that the NEFAs have been associated with the regulation of some functions of the bovines PMNs, in particular with an increase in the respiratory burst associated with phagocytosis, reduction in cell viability and a marked increase in necrosis. Paradoxically, the NEFAs showed no effect on apoptosis 32, 48. It is clear that a relationship exists between lipo-mobilization, levels in plasma NEFAs and modulation of the bovine PMNs functions. However, more studies are needed to help understand the mechanisms by which this relationship occurs because it is not clear so far.
Concluding remarks
The intake of diets high in NSCs and development of SARA in dairy cows are well known from the perspective of animal nutrition. However, during this condition PAMPs molecules type are generated (e.g. LPS) which have the ability to activate the immune system generating impacts both locally and systemically. Recently, a new discipline has been growing, known as nutritional immunology, which has allowed understanding the cause and effect relationship between nutrients and the production of immune system molecules capable of altering homeostasis, animal welfare and the productive performance. The gastrointestinal tract is the most important source of PAMPs production in ruminants. However, the anatomical site in which the translocation occurs, for subsequently joining their respective receptors (receptors of the immune system such as TLRs) and then generating bioactive molecules (APRs and cytokines), is a fundamental and also controversial issue. So far, there is a greater biological plausibility (including histological structure) in which this translocation occurs from the gut and not from the rumen. The binding of PAMPs with their respective receptors triggers a number of responses mediated by APR in the host that explain, at least in part, SARA symptomatology such as the decline in dry matter intake, alteration in compositional quality of milk, the decrease in digestibility of the fiber, among others.
During the acute phase of inflammation, proinflammatory cytokines with systemic effect are also generated, which causes a greater energetic consumption by limiting the amount of energy available for production and maintenance functions. Locally, vaso-active molecules with inflammatory capacity are produced, generating other symptomatologies like laminitis. In the mammary gland there are alterations in nutrient availability, causing several changes, among them, the compositional quality of milk. The study of the immunological basis of SARA pathophysiology, together with a deep nutritional knowledge, will make possible to understand more integrally the role of each of its molecules and the impact on both the severity and prognosis of the disease. It will also allow developing new feedstuff strategies to improve host's homeostasis, animal welfare, and performance.
Acknowledgements
Sustainability 2013-2014 of the Biogenesis Group.
References
1. Akira S, Uematsu S, Takeuchi O 2006. Pathogen recognition and innate immunity. Cell 2006; 124:783-801 [ Links ]
2. Ametaj BN, Koenig KM, Dunn SM, Yang WZ, Zebeli Q, Beauchemin KA. Backgrounding and finishing diets are associated with inflammatory responses in feedlot steers. J Anim Sci 2009; 87:1314-1320. [ Links ]
3. Ametaj BN, Zebeli Q, Summera I. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Brazilian J Anim Sci 2010; 39:433-444 [ Links ]
4. Anderson SD. Endotoxic and anaphylactic-type shock in steers from intravenous injection of Escherichia coli endotoxin and ruminal absorption of endotoxin. Thesis MSc Kansas State University, Manhattan, USA, 1984. [ Links ]
5. Argiles JM, López-Soriano FJ, Evans RD, Williamson DH.Interleukin-1 and lipid metabolism in the rat. Biochem J 1989; 259:673-678 [ Links ]
6. Aschenbach JR, Seidler T, Ahrens F, Schrodl W, Buchholz I, Garz B, Kruger M, Gabel G. Luminal salmonella endotoxin affects epithelial and mast cell function in the proximal colon of pigs. Scand J Gastroenterol 2003; 38:719-726 [ Links ]
7. Baldwin RL. Use of isolated ruminal epithelial cells in the study of rumen metabolism. J Nutr 1998;128:293-296 [ Links ]
8. Barcia AM, Harris HW. Triglyceride-rich lipoproteins as agents of innate immunity. Clin Infect Dis 2005; 41:S498-S503 [ Links ]
9. Bauman DE, Griinari JM. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livest Prod Sci 2001;70:15-29 [ Links ]
10. Bertok L. Effect of bile acids on endotoxin in vitro and in vivo (physicochemical defense). Bile deficiency and endotoxin traslocation. Ann N Y Acad Sci 1998; 851:408-410 [ Links ]
11. Bertok L. Bile acids in physico-chemical host defense. Pathophysiol 2004; 11: 139-45 [ Links ]
12. Cetin S, Dunklebarger J, Li J, Boyle P, Ergun O, Qureshi F, Ford H, Upperman J, Watkins S, Hackam DJ. Endotoxin differentially modulates the basolateral and apical sodium/proton exchangers (NHE) in enterocytes. Surgery 2004; 136:375-383 [ Links ]
13. Chin AC, Flynn AN, Fedwick JP, Buret AG. The role of caspase-3 in lipopolysaccharide-mediated disruption of intestinal tight junctions. Canadian J Physiol Pharmacol 2006; 84:1043-1050 [ Links ]
14. Drewe J, Beglinger C, Fricker G.Effect of ischemia on intestinal permeability of lipolysaccharides. Eur J Clin Invest 2001; 31:138-144 [ Links ]
15. Elsasser TH, Caperna TJ, Li CJ, Kahl S, Sartin JL. Critical control points in the impact of the proinflammatory immune response on growth and metabolism. J Anim Sci 2008; 86:E105-25 [ Links ]
16. Emmanuel DGV, Madsen KL, Churchill TA, Dunn SM, Ametaj BN. Acidosis and lipopolyssacharide from Escherichia coli 055: B5 cause hyperpermeability of rumen and colon tissues. J Dairy Sci 1997; 90:5552-5557 [ Links ]
17. Emmanuel DGV, Dunn SM, Ametaj BN. Feeding high proportions of barley grain stimulates an inflammatory response in dairy cows. J Dairy Sci 2008; 91:606-614 [ Links ]
18. Feingold KR, Staprans I, Memon RA, Moser AH, Shigenaga JK, Doerrler W, Dinarello CA, Grunfeld C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J Lipid Res 1992; 33:1765-1776 [ Links ]
19. Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340:448-454 [ Links ]
20. Gaebel G, Martens H, Suendermann M, Galfi P. The effect of diet intraruminal pH and osmolarity on sodium chloride and magnesium absortion from the temporarily isolated and washed retículo-rumen of sheep. Exp Physiol 1987; 72:501-511 [ Links ]
21. Gallay P, Heumann D, LeRoy D, Barras C, Glauser MP. Mode of action of anti-lipolysaccharide-binding protein antibodies for prevention of endotoxemic shock in mice. Proc Natl Acad Sci USA 1994; 91:7922-7926 [ Links ]
22. Goshal S, Witta J, Zhong W, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009; 50: 90-97 [ Links ]
23. Goth A. Goth´s Medical pharmacology. 7 ed St Louis CB Morsby Co USA; 1974. [ Links ]
24. Graham C, Simmons NL. Functional organization of the bovine rumen epithelium. Am J Physiol Regul Integr Comp Physiol 2005; 288:R173-R181 [ Links ]
25. Herrera E, Lasunción MA, Gómez-Coronado D, Aranda P, López-Luna P, Maier I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol 1988; 158:1575-1583 [ Links ]
26. Hontecillas R, Wannemeulher MJ, Zimmerman DR, Hutto DL, Wilson JH, Ahn DU, Bassaganya-Riera J. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J Nutr 2002; 132:2019-2027 [ Links ]
27. Khafipour E, Krause DO, Plaizier JC. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 2009; 92:1060-1070 [ Links ]
28. Khafipour E, Li S, Plaizier JC, Krause DO. Rumen microbiome composition determined using two nutritional models of subacute rumen acidosis. Appl Environ Microbiol 2009; 75:7115-7124 [ Links ]
29. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J Lipid Res 2004; 45: 1169-1196 [ Links ]
30. Kleen JL, Hooijer GA, Rehage J, Noordhuizen JP. Subacute ruminal acidosis (SARA): a review. J Vet Med A Physiol Pathol Clin Med 2003; 50:406-414 [ Links ]
31. Kushner I, Gewurz H, Benson MD. C-reactive protein and the acute phase response. J Lab Clin Med 1981; 97:739-749 [ Links ]
32. Lacetera N, Scalia D, Franci O, Bernabucci U, Ronchi B, Nardone A. Effects of nonesterified fatty acids on lymphocyte function in dairy heifers. J Dairy Sci 2004; 87:1012-1014 [ Links ]
33. Lassman BA. Release of endotoxin from rumen bacteria and endotoxin absorption from the rumen. Thesis MSc Kansas State University. Manhattan, USA, 1980. [ Links ]
34. Li S, Khafipour E, Krause DO, Kroeker A, Rodriguez-Lecompte JC, Gozho GN, Plaizier JC. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J Dairy Sci 2012; 95:294-303 [ Links ]
35. López-Soriano FJ, Williamson DH. Acute effects of endotoxin (lipopolysaccharide) on tissue lipid metabolism in the lactating rat. The role of delivery of intestinal glucose. Mol Cell Biochem 1994; 141:113-120 [ Links ]
36. Martínez-Subiela S, Tecles F, Parra MD, Cerón JJ. Proteínas de Fase Aguda: Conceptos básicos y principales aplicaciones clínicas en medicina veterinaria. An Vet 2001;17:97-114 [ Links ]
37. Monk JM, Hou TY,Chapkin RS. Recent advances in the field of nutritional immunology. Exp Rev Clin Immunol 2011; 7:747-749 [ Links ]
38. Nagaraja TG, Bartley EE, Fina LR, Anthony HD. Relationship of rumen gram-negative bacteria and free endotoxin to lactic acidosis in cattle. J Anim Sci 1978; 47:1329-1336 [ Links ]
39. National Research Council. Nutrient Requirement of Dairy Cattle 2001; 7th edition. National Academic Press Washington USA [ Links ]
40. Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR-4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 2006; 176:3070-3079 [ Links ]
41. Nocek JE. Bovine acidosis: implications on laminitis. J Dairy Sci 1997; 80:1005-1028 [ Links ]
42. Nocek JE, Russell JB. Protein and energy as an integrated system: Relationship of ruminal protein and carbohydrate availability to microbial synthesis and milk production. J Dairy Sci 1988; 71:2070-2107 [ Links ]
43. Pekala PH, Kawakami M, Angus CW, Lane MD, Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci USA 1983; 80:3743-3747 [ Links ]
44. Peyraud JL, Delagarde R. Managing variations in dairy cow nutrient supply under grazing. Animal 2013; 7 :57-67 [ Links ]
45. Raboisson D, Derville M, Herman N, Cahuzac E, Sans P, Allaire G. Herd-level and territorial-level factors influencing average herd somatic cell count in France in 2005-2006. J Dairy Res 2012; 79:324-332 [ Links ]
46. Rosen FS, Skarnes RC, Landy M,Shear MJ. Inactivation of endotoxin by a humoral component. III role of divalent catión and a dialyzable component. J Exp Med 1958; 108:701-711 [ Links ]
47. Rusu D, Loret S, Peulen O, Mainil J, Dandrifosse G. Immunochemical, biomolecular and biochemical characterization of bovine epithelial intestinal primocultures. BMC Cell Biol 2005; 6:42 [ Links ]
48. Scalia D, Lacetera N, Bernabucci U, Demeyere K, Duchateau L, Burvenich C. In vitro effects of nonesterified fatty acids on bovine neutrophils oxidative burst and viability. J Dairy Sci 2006; 89:147-154 [ Links ]
49. Steiger M, Senn M, Altreuther G, Werling D, Sutter F, Kreuzer M, Langhans W. Effect of a prolonged low dose lipopolysaccharide infusión on feed intake and metabolism in heifers. J Anim Sci 1999; 77:2523-2532 [ Links ]
50. Steven DH, Marshall AB. Organization of the rumen epithelium. In Physiology of digestion and metabolism in the ruminant (ed AT Phillipson), pp 80-100. Oriel Press, New Casttle-upon-tyne, UK; 1969. [ Links ]
51. Stone WC. Nutritional approaches to minimize subacute ruminal acidosis and laminitis in dairy cattle. J Dairy Sci 2004; 8:E13-E26 [ Links ]
52. Sweet MJ , Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol 1996; 60: 8-26 [ Links ]
53. Waldo DR. Extent and partition of cereal grain starch digestion in ruminants. J Anim Sci 1973; 37:1062-1074 [ Links ]
54. Young CR, Wittum TE, Stanker LH, Perino LJ, Griffin DD, Littledike ET. Serum haptoglobin concentrations in a population of feedlot cattle. Am J Vet Res 1996; 57:138-141 [ Links ]
55. Yu PH, Wright S, Fan EH, Lun ZR, Gubisne-Harberle D. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochimica et Biophysica Acta (BBA) -Mol Cell Biol Lipids 2003; 1647:193-199 [ Links ]
56. Zebeli Q, Ametaj BN. Relationship between rumen lipopolysaccharide and mediators of inflammatory response with milk fat production and efficiency in dairy cows. J Dairy Sci 2009; 92:3800-3809 [ Links ]
57. Zebeli Q, Dunn SM, Ametaj BN. Strong associations among rumen endotoxin and acute phase proteins with plasma minerals in lactating cows fed graded amounts of concentrate. J Anim Sci 2010; 88:1545-1553 [ Links ]