Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CES Medicina Veterinaria y Zootecnia
On-line version ISSN 1900-9607
Ces. Med. Vet. Zootec. vol.10 no.1 Medellín Jan./June 2015
Caso clinico
Retinal degeneration related to hypoxic anaesthesia in a mare¤
Degeneración retiniana en una yegua relacionada a hipoxia anestésica
Degeneração da retina em uma égua relacionada àhipóxia anestésica
Sandra Acevedo Toro1*, MV, Esp Clin, MSc; Carlos Aparicio Ortiz2, MV, MSc;Felipe Graciano Echeverry3, MV, cEsp Clin.
¤Para citar este artículo: Acevedo Toro S, Aparicio Ortiz C, Graciano Echeverry F. Retinal degeneration related to hypoxic anaesthesia in a mare. Rev CES Med Zootec. 2015; Vol 10 (1): 57-63.
*Autor para correspondencia: Sandra Acevedo Toro, Facultad de Ciencias Agrarias, Universidad de Antioquia. Correo electrónico: crisalida18@hotmail.com.
1 Grupo Centauro, Coordinadora Unidad de oftalmología y anestesia, Facultad de Ciencias Agrarias Universidad de Antioquia, Medellín, Colombia
2 Director Servicio Oftalmológico Veterinario SOV; Coordinador Servicio de Oftalmología Veterinaria Clínica de Pequeños Animales, Universidad Nacional de Colombia. Bogotá, Colombia
3 Médico veterinario, Hospital veterinario Universidad de Antioquia, Medellín, Colombia
(Recibido: 10 de octubre, 2014; aceptado: 15 de abril, 2015)
Abstract
Anamnesis: a three years-old female Colombian Paso Fino equine presented visual impairment related to an anesthetic intervention with two alpha-2-adrenergic agents (xylazine 0.8 mg/kg + detomidine 10 ug/kg). Clinical and laboratory findings: ophthalmologic exam resulted in positive threat test with evidence of impaired obstacle circumvention due to failure in perception of distances and details of objects, resulting in unsteady gait and mount inability. The eye fundus presented horizontal bands on the nontapetal area and mild peripapillary edema. Scotopic electroretinogram (ERG) was performed under white light evidencing a decrease in amplitude and increase in A and B wave latencies for both eyes, compared with the reference. Hyperreflective tapetal zones and retinal folds are also observed. Leptospira tests were negative (Patoc: 1.25, Icterohaemorrhagiae: 1.25, Grippotyphosa: negative, Pomona: negative, Canicola: 1.50, Hardjo: 1.25, Ballum: negative, Bratislava: 1.25). Therapeutic approach: dexamethasone (0.2 mg/kg IM, for three days) and a vitamin supplement were prescribed. Conclusion: administration of two alpha2- adrenergic agonists may cause retinal ischemia with later development of retinal degeneration in horses.
Key words: Alpha2-adrenergic, equines, hypertension, retinal ischemia, sedation.
Resumen
Anamnesis: Equino hembra paso fino 36 meses de edad que presenta déficit visual relacionado a un evento anestésico con 2 agentes alfa-2-adrenérgicos (xilacina 0,8 mg/kg + detomidina 10 ug/kg). Hallazgos clínicos y de laboratorio: Al examen oftalmológico presenta prueba de amenaza positiva, al sorteo de obstáculos evidencia alteración en la percepción de distancias y detalle de objetos, resultando en una marcha insegura e imposibilidad de monta. El fondo de ojo se observan bandas horizontales en la zona no tapetal y leve edema peripapilar. Se realiza ERG bajo ambiente escotopico con luz blanca y se observa una disminución en la amplitud y aumento de latencias en ondas A y B de ambos ojos, comparado con la referencia. Posteriormente se visualizan zonas de hiperreflectividad tapetal y pliegues retínales. Se realizó prueba de Leptospira con resultado negativo: Patoc: 1.25, Icterohaemorrhagiae: 1.25, Grippotyphosa: negativo, Pomona: negativo, Canícola: 1.50, Hardjo: 1.25, Ballum: negativo, Bratislava: 1,25. Aproximación terapéutica: se formula dexametasona a razón de 0,2mg/kg IM durante 3 días y luego un suplemento vitamínico. Conclusión: La mezcla de dos agonistas alfa2-adrenérgicos puede causar un evento isquémico retinal con el posterior desarrollo de degeneración retiniana en caballos.
Palabras clave: Alfa2-adrenérgicos, Equinos, Hipertensión, Isquemia de retina, Sedación
Resumo
Anamnesis: Equino fêmea passo fino 36 meses de idade que apresenta déficit visual relacionado a um evento anestésico com 2 agentes alfa-2-adrenérgicos (xilacina 0.8 mg/kg detomidina 10 ug/kg). Achados clínicos e de laboratório: Ao exame oftalmológico apresenta prova de ameaça positiva, ao sorteio de obstáculos evidência alteração na percepção de distâncias e detalhe de objectos, resultando numa marcha insegura e impossibilidade de monta. As faixas horizontais de fundo de olho são observadas no nontapetal área e edema peripapilar suave. Escotópica ERG foram realizados sob luz branca ambiente e uma redução na amplitude e aumento latências ondas A e B de ambos os olhos, em comparação com a referência é observado. Posteriormente áreas do tapete hyperreflectivity e dobras de retina são exibidos. Teste de Leptospira foi realizada com resultado negativo: 1,25 Patoc Icterohaemorrhagiae 1,25 Grippotyphosa: negativo, Pomona: negativo, canicola: 1.50, Hardjo: 1,25, Nes: negativo, Bratislava 1,25. Therapeutic abordagem: dexametasona é formulado a 0,2 mg / kg IM por 3 dias e, em seguida, um suplemento vitamínico. Conclusão: A mistura de dois agonistas alfa-2-adrenérgicos pode causar evento isquêmico da retina com o posterior desenvolvimento da degeneração da retina em cavalos.
Palavras-chave: Alfa2 -adrenérgicos, Equine, hipertensão, isquemia da retina, sedação.
Introduction
Vision loss in horses represents a major problem related to its importance in these animals, during work, riding and jumping. The causes for blindness in horses include equine recurrent uveitis, retinal detachment, corneal ulcer, retinal atrophy, retinal degeneration and optic neuritis (Gilger et al., 2011).
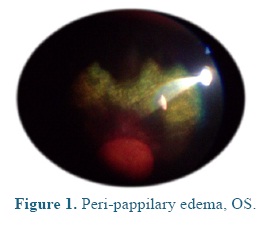
Retinal degeneration represents one of the less frequent blindness causes in horse, unlike what usually occurs in dogs (Nell et al., 2010; Cutler et al., 2000). It is related to different etiologies such as trauma, infectious diseases and ischemia (Nell et al., 2010). The typical changes of the ischemic retinal degeneration in the acute stage are sudden and irreversible blindness, presenting inflammatory changes such as generalized or diffuse peri-papillary corio-retinitis. In this phase additional peripapillary edema can be observed as sign of optic neuritis or optical nerve degeneration (Cutler et al., 2000). In late stages severe atrophy of the neuro-sensorial retina, transretinal pigment migration and glial cicatrization in the non tapetal fundus area are observed (Nell et al., 2010).
There are different causes of ischemia, including important blood loss, traumatic brain injury, septic embolism, carotid and palatine arteries ligation, and hemodynamically active anesthetic agents. The a-2 adrenergic agonists are the most commonly used in the equine practice, having important dose-dependent effect when higher doses or longer therapeutic times are applied, generating an important hypotensor effect (Muir WW et al., 2009), which is compensated with peripheral vaso-constriction, that, in the case of being prolonged, can affect retinal vessels, causing a pressure increasing and a tissue oxygen input reduction leading to a corioretinopathy and/or optic neuritis.
The main objective of this paper is to report a case of retinal degeneration in a 36-months- old Colombian Paso Fino mare, caused by the use of a combination of a-2 adrenergic agonist drugs.
Patient examination
Anamnesis
A 36-months-old Colombian Paso Fino mare, weighing 360 kg (determined by volumetric belt) and orangepeel color was taken to ophthalmological examination because of its visual loss. The owner reported that the patient was subjected throughout to profound sedation with a combination of two a-2 adrenergic agonists (xylazine 0.8 mg/kg, IV + detomidine 10 ug/kg, IV). After this procedure, the mare presented sedation effects during 2 days, and it passed 6 hours before the patient could raise its head. By this moment it was evident that the patient had an obvious visual deficit.
Clinical findings
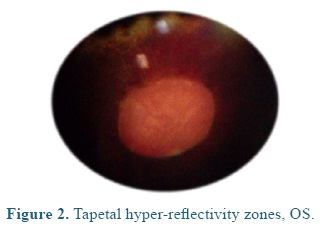
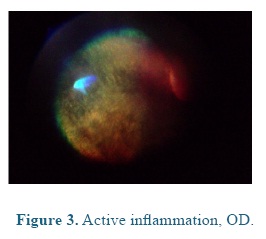
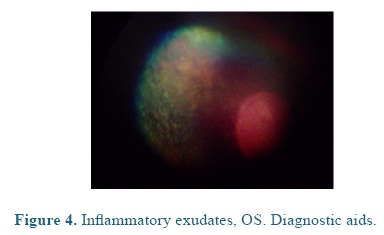
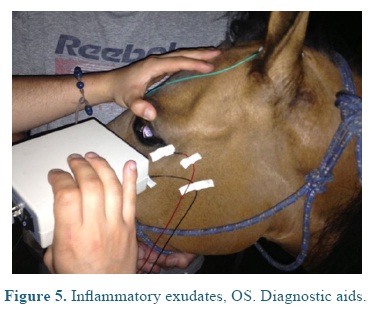
On admission the heart and respiratory rates, pulse, temperature, and capillary refill time were normal. At the ophthalmological examination the patient presented positive menace test, and altered distance perception and object details to the obstacle test, resulting in an unsecure, uncoordinated and hypermetric walking. The patient was anxious because of its proprioceptive difficulties, so it was impossible to be ridden. At the ocular fundus examination horizontal bands were observed in the nontapetal fundus area, as well as, mild peri-papillary edema (Figure 1), and tapetal hyper-reflectivity zones (Figure 2) with retinal folds and active inflammation (Figure 3) and inflammatory exudates (Figure 4), which guided the diagnosis of active corio-retinitis.
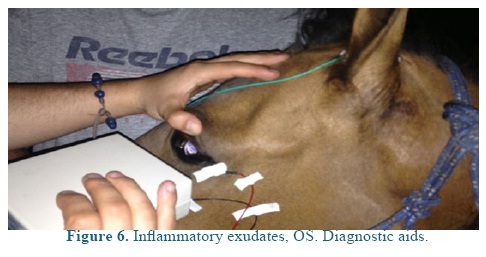
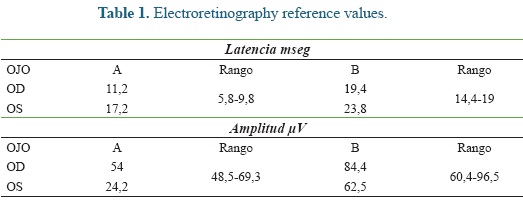
An electroretinography (ERG) was done under white light escotopic environment with palpebral electrode under pharmacologic sedation and mydriasis using Akonic System BioPC with three channels to register the evocated bio-potentials (Figure 5). An amplitude reduction, and A and B waves latency increase was observed in both eyes (Figure 6), compared to reference (Table 1). A leptospirosis test was performed (microagglutination test, MAT) and resulted negative, with no significant results: Patoc 1.25, Icterohaemorrhagiae 1.25, Grippotyphosa: negative, Pomona: negative, Canícola: 1.50, Hardjo: 1.25, Ballum: negative, Bratislava: 1.25.
Treatment approachThe retinal ischemia is an infrequent clinical entity, with no effective treatment because of the permanent and irreversible retinal cells injury or death, being one of the causes of blindness and visual incapacity. The treatment is focused in controlling the secondary effects of the inflammatory reactions such as uveitis, cataracts or glaucoma. In order to control these inflammatory effects dexametasone, at a dose of 0.2mg/kg, IM was administered for 3 days..
Discussion
The retina is the eye structure responsible of the vision capacity, connecting the information to the visual cortex through the optic nerve and the lateral geniculate body; is the most metabolically active tissue of the body, as its oxygen consumption indicates (Ofri et al., 2008). Its oxygen consumption is constant and both its excess and failing are highly damaging. The perfusion of the retinal vascular bed depends on certain level of autorregulation, which is based in two different systems (Conti et al., 2010). These are in part external to the eye and very sensitive to blood pressure and oxygen, carbon dioxide and pH partial pressure variations in the tissue. The possibilities of autorregulation consist in vasoconstriction in the case of arterial hypertension, oxygen tension increase or alkalosis, and, in the vasodilation in the case of arterial hypotension, carbon dioxide tension increase or acidosis (Santos et al., 2014).
It should be noted that at the corio-capilar level the blood circulation is more rapid and the choroids results to be the most perfused tissue in the whole organism. The reason for such a high circulatory velocity is attributed to the need to remove heat that accumulates from the light focused on the retina. An interruption in the choroid or retinal vasculature immediately causes ischemia and a serious and irreversible loss of function, despite the glycogen storing in Müller cells. Once hypoxia has started, death of retinal cells occurs rapidly, producing intra and extracellular edema, with neuronal elements disintegration, which finally leads to retinal atrophy and gliosis.
Equines have a paurangiotic vascularization in the retinal tissue, and its minuscule vessels are extended only a short distance from the disk (Ofri et al., 2008).
Sedatives had been widely used in veterinary medicine to chemically immobilize the patients before bloodless procedures. The most commonly used in domestic animals are the phenothiazine derivatives, benzodiazepines and α-2 adrenergic agonists. Within the family of α-2 adrenergic receptors, the α-2 adrenorreceptors are characterized by producing analgesic, sedative and anticonvulsants effects.
Alfa-2 receptors had been found in the central nervous system, gastrointestinal tract, uterus, kidneys and red blood cells. The sedative and analgesic effects produced by these receptors are similar to the ones induced by µ-opiates receptors, because both are located in similar regions of the brain and even in the same neurons (Caballero, Ahuma et al., 2002).
When µ-opiates or α-2 agonist drugs are united to their specific receptors, membrane- associated G-proteins are activated, which allows the opening of K+ channels causing the loss of this ion. The neuron stays hyperpolarized being incapable to respond to new stimulations. The pre-synaptic release of noradrenalin is interrupted, which inhibits the adrenergic neurons answer, leading to a depression of the central nervous system by a sympatholytic effect with loss of warning and surveillance functions. The α-2 adrenergic agonists can cause bradycardia, decreased cardiac output, sinoatrial blocks, first and second grade of atrio-ventricular block, atrio-ventricular dissociation, and marked sinus arrhythmias. In the most of the cases these situations are induced by an increasing of the vagal tone as a result of the post-synaptic activation of the α-2 receptors, located on vascular smooth muscle, developing peripheral vasoconstriction that results in an increase of arterial pressure which is offset through a reflex bradycardia. The hypertensive episode is short and is followed by a marked hypotension (Belda, Laredo, Escobar, Agut, Soler, Lucas et al., 2005).
When a patient is subjected to high doses or to a prolonged anesthesia, the hypertension is constant, taking it to a hypertensive retinopathy, as a consequence of the high arterial pressure. Eye lesions associated to the hypertension result in a vascular autorregulation failure. In response to the increases in pressure, the retinal arterioles are submitted to vasoconstriction, which conducts to compensatory hypertrophy and hyperplasia of the smooth muscle layer. With chronic vasoconstriction the smooth muscle layer cells had diminished their contractile strength, developing fibrotic changes and allowing the insidious leakage of plasma in the arteriolar wall, causing the hyalinization and necrosis of the smooth muscle. The diffuse arteriolar narrowing related to chronic hypertension is commonly seen (Brown et al., 2005).
The radius of the arterioles decreases approximately 2/3, leading to degenerative changes in the wall that can conduct to endothelial and muscle cells rupture, with blood and serum leaking, generating edema, hemorrhages and retinal detachment, characteristics of hypertensive retinopathy (Federica, Teresa, Clarke, Stefano, Brian, Michael et al., 2000).
Clinical symptoms are the result of the anatomic disposition of corio-capillaries and the absence of choroid autorregulation. The blood-retina barrier which consists of tight junctions between the endothelial cells of the vasculature of the retina and the cell junctions between the pigmented retinal epithelium can be disrupted with the consequent leak of serum and blood cells in the retina and sub-retinal space (Kyhn, Warfvinge, Scherfig, Kiilgaard, Prause, Klassen, Young, Cour et al., 2009).
Internal ischemic retinal stains (fluffy stains) are focal areas of opacity inside the retina; they are irregular, white and spongy and, clinically evident. These are the neural debris resulting from the interruption of nerve axons due to the occlusion of arterioles (Osborne, Casson, Wood, Chidlow, Graham, Melena et al., 2004). The retinal edema can be generalized or local, mainly in the tapetal zone (Sharma, Kanaujia, Mishra, Agarwal, Tripathi et al., 2013). As a consequence of the compromised oxygen supply, an ischemic and hypoxic retinal process starts. This can take to cell death and blindness.
Often the veterinarians can find ophthalmological disorders in horses, involving retinal damage from recurrent uveitis, associated to infectious processes such as leptospira. Based on this, titles for this bacterium were measured (Icterohemorrhagiae: 1.25; Canícola: 1.50; Hardjo: 1.25; Bratislava 1.25), all considered negative (Gasque et al., 2008). In the other hand, the clinical signs in the patient are so acute and almost always related to an anesthetic event with no compatible symptoms with uveitis.
As a complementary method to the ophthalmologic examination, looking for the retinal functionality and ERG was performed. This test can provide valuable information about functional diseases of retina. The ERG as a graphic representation which consist in a fast negative wave, called "A", followed by a positive wave called "B", finishing with a very slow positive potential called "C". The depolarization of rods and cones represent the "A" wave, while "B" wave is represented by the activity of Müller cells and the repolarization of the photoreceptors, and "C" wave refers to pigmented epithelium (Maggs; 2000, Weichsler, Herrera et al., 2007).
Latency refers to the time it takes to each retinal cell to represent a maximum response, which is increased in "A" and "C" waves of both eyes. And the amplitude showing the electrical power of cellular responses can be altered for the "A" wave of the left eye. This confirms the retinal injury present in the patient of this report, with and altered activity of rods and cones, as well as, Müller cells, related to the anesthesia received previously by the patient and to the other signs already reported, indicating the retinal hypoxia suffered by the mare. All these findings confirm the diagnosis of visual alteration secondary to hypertension caused by the a-2 adrenergic agonists used.
Conclusions
Arterial hypertension can cause damage to retinal blood vessels. The higher blood pressure and higher the time it has been raised, the more likely the damage is severe. Some drugs such as α-2 adrenergic agonist agents or their combination administered for a prolonged period of time can produce a hypertensive syndrome, which could lead to a retinal ischemic case.
In the first instance, α-2 adrenergic agonists can cause bradycardia y lately cardiac output decreasing. Subsequently, vagal tone increase due to a post-synaptic activation of the α-2 receptors located in the vascular smooth muscle, developing peripheral vaso-constriction and arterial pressure increasing.
One of the most important causes which can lead to these blood pressure changes in the retina is the prolonged administration and/or high doses of α-2 adrenergic agonists, generating a severe hypertension syndrome, anxiety, headache y ischemia.
In the chronic hypertension the arteriolar narrowing is commonly seen and it conduces to compensatory hypertrophy and hyperplasia of vascular smooth muscle. Parallel to the chronic vaso-constriction, the vascular smooth muscle cells can decrease their contractile function; developing fibrotic changes and allowing the insidious leakage of plasma in the arteriolar wall, causing the hyalinization and necrosis of the smooth muscle, and then the retina is hypoxic and it cells suffer damage. The hypoxia is probably present in all retinal ischemic diseases. Some studies suggest that those processes that conduct to death are similar in the ischemia/reperfusion and hypoxia. It is known that in ischemia there is an imbalance between the demand and energy supply, as a consequence of the partial or complete alteration of the blood flow. The blood supply to the retina is complex and its reduction in certain vessels may induce retinal ischemia in some areas and not in others. It is possible that the cause of the symptoms and lesions in the different retinal ischemic diseases can be a combination of hypoxia, anoxia or oligohemia instead of complete ischemia.
The diagnosis of hypertensive and/or ischemic retinopathy is essentially a clinical matter and it is vital to determine both the primary process conditions of a hypertensive process and the drug interactions which can induce theses changes, considering that retina is one of the target organs affected during hypertensive processes.
References
1. Santos A, Rita A, Martins J, Ambrosio AF, Cavadas C. Emerging novel roles of neuropeptide in the retina: From neuromodulation to neuroprotection. Prog Neurobiol. 2014; 112: p. 70-79. [ Links ]
2. Belda E, Laredo FG, Escobar M, Agut A, Soler M, Lucas X. Agonistas a-2 adrenérgicos en sedación y anestesia veterinaria: An. Vet. Murcia 2005; 21: 23- 33. [ Links ]
3. Church ML, Norman JC. Electroretinogram responses of the normal thoroughbred horse sedated with detomidine hydrochloride. Vet Opth 2012; 15 s2: 77-83 [ Links ]
4. Cutler TJ, Brooks DE, Andrew SE, Denis HM, Biros DJ, Gelat KN, Komaromy AM, Kallberg M. Disease of the equine posterior segment. Vet Opht 2000; 3: 73-82. [ Links ]
5. Maggs DJ. Basic diagnostic techniques. In: DH Slatter. Veterinary ophathalmology. 4th Ed. St. Louis, Missouri: Saunders elsevier; 2008. p.81-106. [ Links ]
6. Caballero E, Ahuma F. SNC. Farmacos tranquilizantes. In: LM Botana. Farmacologia y terapéutica veterinaria. 1st Ed. Madrid: Mcgraw-hill interamericana; 2002. p. 158-168. [ Links ]
7. Federica M, Teresa C, Clarke EA, Stefano P, Brian CG, Michael GD. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695-702. [ Links ]
8. Conti F. Fisiología de la visión. In: F Conti. Fisiología médica. 1st Ed. Mcgraw-hill latinoamericana. 2010; 18: p.379-408. [ Links ]
9. Gilger BC. Equine ophthalmology. 2nd Ed. Missouri (USA): Saunders; 2011. [ Links ]
10. Hughes KJ. Ocular manifestations of systemic disease in horses. Equine Vet J Suppl 2010; 37: 89- 96. [ Links ]
11. Sharma K, Kanaujia V, Mishra P, Agarwal R, Tripathi A. Hypertensive retinopathy. Clinical queries: nephrology. Vol 2. 2013. p. 136-139. [ Links ]
12. Muir WW, Hubbell JA. Equine anesthesia. 2nd Ed. Missouri (USA): Elsevier; 2009. [ Links ]
13. Kyhn MV, Warfvinge K, Scherfig E, Kiilgaard JF, Prause JU, H Klassen, M Young, M Cour. Acute retinal ischemia caused by controlled low ocular perfusion pressure in a porcine model. Electrophysiological and histological characterisation. Exp Eye. [ Links ]
14. Res 2009; 88: P. 1100-1106. [ Links ]
15. Nell B, Walde I. Posterior segment diseases. Equine Vet J Suppl 2010; 37: 69-79. [ Links ]
16. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004; 23: 91–147 [ Links ]
17. Weichsler N, D Herrera. Electroretinografia: uso clínico. En: D Herrera. Oftalmologia clínica en animales de compañía. 1st Ed. Buenos Aires: Intermedica; 2007. p. 73-83. [ Links ]
18. Gasque R. Leptospirosis. En: Enciclopedia Bovina. Universidad Nacional Autónoma de México. 1st Ed. 2008; 18: p.168-173. [ Links ]
19. Ofri R. Retina. En: DH Slatter. Veterinary ophathalmology. 4th ed. St. Louis, Missouri: Saunders elsevier; 2008. p.285-317. [ Links ]
20. Brown SA. Pathophysiology of systemic hypertension. In SJ Ettinger, EC Feldman. Veterinary internal medicine. 6th Ed. St Louis, Missouri: Elsevier saunders; 2005. p. 472-476. [ Links ]