Introduction
Several techniques have been used to determine volatile fatty acids (VFA) absorption in vivo, such as the collection of portal blood 1; intraruminal infusion of radioactive 2 or stable VFA isotopes 3,4; rumen evacuation and infusion of VFA into the washed ventral sac 5; continuous infusion of nonlabeled VFA into the intact ruminal digesta 6; and pulse-dose of valeric acid plus a marker of fluid passage 7 which may be mixed with the evacuated digesta 8 or mixed in locus 9. Although the last method has become common (infusion of markers in pulse-dose), there is a claim that this technique could alter the measurement of VFA clearance. If a large volume of marker solution is used to make homogenization easier 7, the higher volume of rumen fluid may decrease the clearance rate of VFA 5,8. Another problem is the poor fitness of regressions describing the decay of markers concentrations over time, which ultimately reflect an incomplete homogenization in locus 2. On the other hand, if the ruminal digesta is evacuated, the markers can be satisfactorily mixed with the rumen content 8, but it is supposed to destabilize the ruminal environment, throughout disruption of the mat and loss of digesta stratification, increase of oxygen concentration, decrease of temperature, and decrease of blood flow and rumen motility.
Due to these concerns Daniel, de Resende Júnior 10 have proposed an alternative approach for infusion and collection of markers. The new technique is based on the natural mixing of markers with ruminal digesta because of rumen motility. A complete homogenization may be reached sometime after the pulse-dosing. At this time ruminal fluid sampling series can be started. Nonetheless, it is necessary to validate this technique. Thus, the objective of this study was to compare the approach proposed by Daniel, de Resende Júnior 10 with a technique which infusing markers into the evacuated digesta for measurements of ruminal clearance of VFA 8. Another aim was to verify the sensibility of the techniques to the variation of VFA absorption and passage rates induced by nutritional plans.
Material and methods
Experimental design
This study was approved by the Committee of Ethics in Animal Experimentation of the Federal University of Lavras under protocol no. 001/2008.Three Jersey and one Nellore nonlactating and nonpregnant cows were surgically adapted with rumen cannula (10.2 cm diameter) and randomly allocated in a cross-over design, with 18-d periods. Animals were housed in a tie-stall barn with sand beds. Feeds, mineral-vitamin mix, and fresh water were available ad libitum. The four treatments comprised a combination of two factors arranged in split-plot:
100% forage diet (maize silage) and marker infusion to intact digesta.
100% forage diet and marker infusion to evacuated digesta.
50% forage plus 50% concentrate diet and marker infusion to intact digesta.
50% forage plus 50% concentrate diet and marker infusion to evacuated digesta.
Diets were whole-plots whereas techniques to measure VFA clearance were sub-plots. Diets were composed of maize silage and a commercial concentrate based on ground corn and soybean meal. Cows were fed once daily at 8:00 h and feed intake was determined by the difference between the amounts of feed offered and refused.
Measurements of VFA absorption
Measurements of VFA absorption were performed on days 16 and 18 of each period, 6 h after feeding (14:00 h). During the collection, water and feed were suspended to avoid interfering with rumen volume.
Marker solutions contained 340 g of valeric acid 8 and Cr-EDTA (14.2 g of Cr) 11, with pH adjusted to 6.5 in a total volume of 4 L. Solutions were stored in plastic bottles and kept refrigerated until usage. For measuring VFA clearance with intact digesta, marker solution was infused into the reticulorumen using a funnel attached to a rigid PVC tube (3/4 inch), with several holes in the distal third. Immediately before infusion, the solution was heated to 37 °C and the total volume was divided into four equal parts. One liter was infused into the cranial sac and other three liters were infused in different places of the rumen ventral sac 10.
For the standard technique, the ruminal digesta was manually evacuated and transferred to buckets (capacity of 50 L) for the addition and mixing of the marker solution, as proposed by 8. A representative sample of rumen content was collected for dry matter (DM) determination. During the evacuation, the solution of markers was intermittently added into the digesta to facilitate and ensure good homogenization. The digesta was weighted, intensely homogenized, and returned to the reticulorumen. Samples of approximately 100 mL of rumen fluid were collected from the ventral sac using the rigid PVC tube (7.2 cm in diameter) with several holes in the distal third coupled to a tube and a plastic bottle for suction. Immediately after collection, pH was determined and aliquots were frozen at -20 °C for determination of VFA and Cr concentrations.
For the intact digesta, samples of rumen fluid were collected at 90, 105, 120, 150, 210, and 330 min after infusion. Sampling sequence started 90 minutes after infusion in order to wait the ruminal motility to perform the homogenization of the markers with the digesta, as proposed by Daniel, de Resende Júnior 10. For the evacuated digesta, the samples were collected at the following times: zero (immediately after the return of digesta to the rumen), 15, 30, 60, 120 and 240 min 8.
After thawing, rumen fluid was centrifuged (8.855 g for 15 min) to obtain the supernatant, which was analyzed for chromium by atomic absorption spectrophotometry (Varian Spectr AA-100, Varian Australia Pty Ltd, Victoria, Australia) and VFA by gas-liquid chromatography (CP-3800 Gas Chomatograph Varian, Varian Chomato-graphy Systems, California, USA). For VFA analysis, 250 μL of the supernatant were added with 150 μL of caproic acid as an internal standard (N Caproic Acid, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and 100 μL of distilled water. As external standard, a composed solution of acetic, propionic, butyric and valeric acid was used. The capillary column of composite phase had 25 m of length, 0.25 mm internal diameter and 2 mm thick film (CP-Wax 58 - FFAP- CB, Varian Analytical Instruments, California, USA). A temperature cicle was used in the column oven. It was 65 °C in the first 30 seconds; in the next three minutes it was increasing at 20 °C per minute up to 125 °C, and then the temperature was rising 50 °C per minute to reach 170 °C. The total time of analysis was 4.9 minutes.
Feed samples were also collected during sampling periods and oven-dried at 60°C for 72 h. Dried samples were ground through a 1-mm screen (Wiley mill) and sub-samples were analyzed for the following: DM in an air-forced oven at 105 °C for 24 h; crude protein (CP), ether extract (EE) and ash, and neutral detergent fiber (NDF). The nonfiber carbohydrates fraction was computed as: NFC = 100 - CP - NDF - EE - ash.
For the measurements with the evacuated digesta, the rumen fluid volume was estimated by multiplying the weight of evacuated digesta by moisture content. Another estimation of the rumen fluid volume was done by the dilution of Cr at time zero of collection, computed as the intercept of the curve describing the decay of Cr concentration over time. For the infusions with intact digesta, the rumen fluid volume was estimated only by the dilution of Cr 90 min after pulse-dosing which was considered as time zero.
A simple exponential equation describing the change in the valerate concentration over time was used to calculate the fractional clearance rate: Rt = R0 × e−k × t 8. The disappearance of valerate by passage with the rumen fluid was assumed equivalent to the ruminal fluid passage rate, determined by the exponential decay of Cr concentration over time. The fractional rate of valerate absorption was calculated by difference.
Statistical analysis was performed by the Mixed procedure of SAS 12 using the following model: Yijkl = μ+ Pi + Cj + Dk + C(D)j(k) + Il + DIkl + eijkl; where μ: overall mean, Pi: random effect of period (i = 1 or 2), Cj: random effect of cow (i = 1 to 4), Dk: fixed effect of diet (k = forage or forage plus concentrate), C(D)j(k): error term to test whole-plot effects, Il: fixed effect of infusion technique (l = intact or evacuated), DIkl: interaction of diet and infusion technique, eijkl: residual error. Feed intake and digesta weight were analyzed with period, cow, and diet effects in the model.
Results
The concentrate supplementation decreased the content of NDF and increased the content of NFC (Table 1). Due to the lower NDF content 13, the diet comprising forage plus concentrate increased food intake (+43%, P = 0.02).
Table 1 Chemical composition of experimental diets
| Nutrient (% dry matter) | 100% Maize silage | 50% Maize silage + 50% concentrate† |
|---|---|---|
| Dry matter, % as fed | 39.3 | 47.1 |
| Crude protein | 9.2 | 11.1 |
| Ether extract | 3.9 | 2.7 |
| Ash | 3.8 | 4.0 |
| Neutral detergent fiber | 47.0 | 36.8 |
| Nonfiber carbohydrates | 36.1 | 45.4 |
† Commercial concentrate based on ground corn and soybean meal.
The volume of rumen fluid was greater when cows fed only forage than for those fed forage plus concentrate when the calculation was based on the moisture content of rumen digesta (Figure 1). However, there was interaction between diet and calculation method, so when the calculation was done by the exponential curve of chrome dispersion in the rumen fluid the amount of rumen liquid was greater in the concentrate containing diet than in the forage diet.
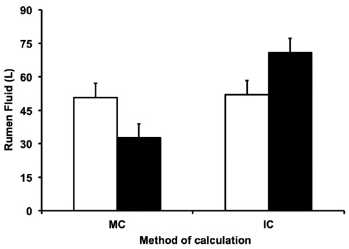
Figure 1 Volume of rumen fluid. Volume of rumen fluid estimated from the moisture content (MC) of the ruminal digesta or calculated from the exponential curve of chrome dispersion in ruminal fluid (IC) when the markers were added into evacuated digesta. Diets contained 100% forage (□) or 50% forage plus 50% concentrate (■). P = 0.02 for effect of calculation method; P = 0.96 for effect of diet; P = 0.03 for interaction of diet and calculation method.
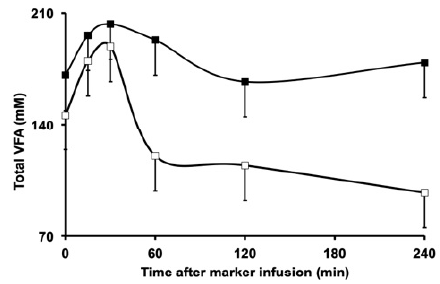
When evaluating the ruminal pH, we verified that the pH was higher in forage diet than in the concentrate containing diet (Figure 2). We also found that supplementation with concentrate resulted in higher concentration of total VFA, acetate, propio nate and butyrate (Figure 3).
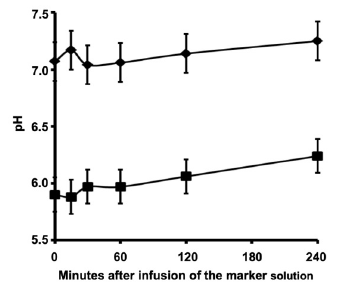
Figure 2 Rumen fluid pH during sampling. Diets contained 100% forage (♦) or 50% forage plus 50% concentrate (■). P = 0.01 for diet effect; P = 0.06 for collection time effect.
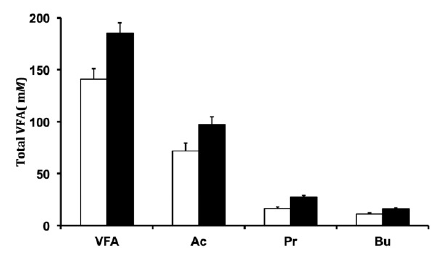
Figure 3 Total VFA. Acetate (Ac), propionate (Pr), and butyrate (Bu) concentrations in the rumen fluid when the marker solution was infused into the intact digesta diets contained 100% forage (□) or 50% forage plus 50% concentrate (■). P = 0.03 for the diet effect.
The fractional clearance rate of total rumen VFA (Table 2) was similar between the two methods of markers infusion. In the current study, the R2 of the regressions which described the decline in valerate and Cr concentration over time did not differ across methods of infusion and were satisfactory high (Table 2). The R2 of the regressions describing the decline in Cr concentration over time (Table 2) was high for both techniques and numerically (P = 0.22) higher in the technique of infusion of markers into intact digesta.
Table 2 Clearance of volatile fatty acids (VFA), volume of rumen fluid, and determination coefficient of the exponential regressions describing the decay of ruminal valerate (R2 Val) and chrome (R2 Cr) concentrations over time
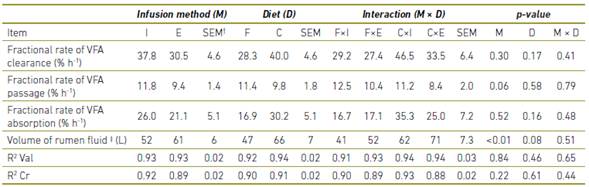
† Standard error of the mean.
‡ Volume of rumen fluid calculated from the intercept of the exponential regression describing the decay of chrome concentration over time.
The fractional rates of absorption of VFA (Table 2) were similar between the two infusion methods and the fractional rate of total clearance (P = 0.17) and of absorption (P = 0.16) tended to be higher for the concentrate containing diet (Table 2).
Over the sampling period, there was a pH increase (Figure 2) and VFA concentration decrease (Figure 4) in both diets, but with different magnitudes.
Discussion
As expected, the increased in food intake showed that concentrate supplementation was effective to drive higher energy intake compared with the forage based diet.
The estimation of fluid volume from the moisture of rumen content includes the water contained inside the feed particles, it is expected that this method should provide a higher amount of fluid for the forage than for concentrate based diet, due to the concentrate retains less water inside of its particles. When the calculation was done from the dispersion curve of chromium, the water inside the particles is not considered. Actually, it was expected that the amount of fluid out of the food particles should be greater in the concentrate plus forage than the forage diet, mainly due to the higher production of VFA by rumen microorganisms which could lead to higher osmotic pressure of rumen content. Higher osmotic pressure was associated to increasing in rumen liquid content 14.
The ruminal pH was higher in forage diet than in the concentrate containing diet. This difference in pH was expected since the pH is inversely related to the concentrations of VFA present in the rumen 8.
The peak of VFA production occurs several hours after feed intake 15. The sampling series began 6 and 7.5 h after feeding and this moment was closer to the VFA production peak. Since feed was withdrawn during sampling period, VFA formation was lower than the clearance, which led to decreased concentrations of VFA across sampling. Thus, the pH was expected to rise during the 240 min of sampling.
The approach developed by Daniel, de Resende Júnior 10 seems to be reliable for estimating the clearance of VFA because its estimates are consistent with those obtained by the technique of adding markers into the evacuated digesta 8.
One of the advantages of the infusing of markers into evacuated digesta is the homogenization, because the rumen content can be easily manipulated out the rumen, allowing an excellent mixture of the markers. This was demonstrated by Júnior, Pereira 8, who achieved high values of R2 of the non-linear regressions that described the decay of markers over time. In the current study, the R2 of the regressions which described the decline in valerate and Cr concentration over time did not differ across methods of infusion and were satisfactory high. The homogenization of digesta by rumen motility is too efficient as that performed by hand in the evacuated digesta.
The fractional passage rate of ruminal fluid to the omasum tended to be higher when the marker was infused into intact digesta, probably because of the rumen environment was not destabilized, especially by not disrupting the mat, so that the rumen motility may be maintained without interruption. Thus, the infusion of markers into intact digesta seems to be more efficient than infusion into evacuated digesta, because the rumen physiology is better preserved. In this experiment, the lower fractional passage rate when the marker was infused into evacuated digesta was associated with higher rumen fluid volume, confirming what was reported by Júnior, Pereira 8 who found a negative correlation between the volume of rumen fluid and the fractional rate of passage.
The R2 of the regressions was high for both techniques and numerically higher in the technique of infusion of markers into intact digesta, providing more evidences that markers can be better homogenized by natural rumen motility than by hand in the evacuated digesta, indicating once again that the method proposed by Daniel, de Resende Júnior 10 seems to be superior to the method of adding markers to the evacuated digesta. Other authors used infusion of markers without rumen evacuation. However, they used larger volume of solution 7, which also interferes with the clearance of VFA, or they reported problems related to the homogenization 2. This demonstrates that the technique proposed by Daniel, de Resende Júnior 10 also appears to be superior to other techniques reported for infusion of tracers in pulse dose, without rumen evacuation.
The fractional rates of absorption of VFA were similar between the two infusion methods, demonstrating again that it is secure estimating fractional rates of clearance using the technique of infusion of markers into intact digesta, as proposed by Daniel, de Resende Júnior 10.
The fractional rate of total clearance and of absorption (P = 0.16) tended to be higher for the concentrate containing diet. This phenomenon has biological coherence whereas the areas of the rumen papillae were largest and the epithelium had higher mitotic index in animals fed forage plus concentrate (data not showed).
The presence of a higher amount of rapidly fermentable nutrients and higher VFA concentration stimulated the cell proliferation leading to the rumen adaptation to the larger amount of VFA as evidenced by the clearance and absorption rate when animals were fed the more energetic diet. Melo, Costa 16 found a direct association between ruminal epithelium surface and the fractional rate of VFA clearance and absorption, confirming what was reported by 17.
The infusion of markers into intact digesta proved to be effective for determining the clearance of ruminal VFA. The homogenization of the marker solution into the intact digesta promoted by rumen motility seems to be more efficient than the one done manually into the evacuated digesta. This result has direct applicability in improving the productive efficiency of livestock, since it is fast, low cost and requires no experience beyond that required by the commonly used techniques.